Abstract
Breast cancer cells (BCCs) can remain quiescent for a long period, before detection and during remission. Mesenchymal stem cells (MSCs) exert both protective and growth support of BCCs. Intercellular interactions between MSCs and BCCs partly occur through membrane-bound CXCL12 (SDF-1α) and its receptor, CXCR4. MSCs can protect BCCs by suppressing immune cytotoxicity and concomitant induction of regulatory T-cells. This study investigated how the cellular interactions between MSCs and BCCs can be targeted to sensitize the BCCs to chemotherapy. Knockdown of CXCR4 and CXCL12 indicated that these molecules are involved in reduced proliferation of MDA-MB-231 and T47D BCCs. We therefore treated co-cultures of MSCs and BCCs with the CXCR4 antagonist, AMD3100, and showed that this treatment led to cycling of BCCs with increased sensitivity to carboplatin, although the effectiveness of carboplatin required the presence of AMD3100. Cytokine array analyses and transwell cultures indicated that AMD3100 caused an increase in BCC proliferation by inducing the production of IL-1α and IL-1β in MSCs after uncoupling from BCCs. The findings with cell lines were validated with primary BCCs from the blood of patients, and in nude BALB/ c mice. MDA-MB-231 was injected in the dorsal flank of mice. The tumors were treated with IL-1 receptor antagonist, AMD3100 and/ or carboplatin. The results verified a critical role for IL-1 in transitioning MSCs from protective to supportive with respect to BCC growth. The clinical significance of these studies was further highlighted in preliminary studies that detected circulating MSCs in obese, but not non-obese patients. Since obese breast cancer patients show poor outcome, these findings underscore that importance of MSCs in consideration for future development of efficient therapy.
Keywords: CXCR4, AMD3100, interleukin-1, mesenchymal stem cells, breast cancer
Introduction
Breast cancer (BC) survivors can develop metastasis after more than ten years of remission [1-4]. BC cells (BCCs) show preference for the bone marrow (BM) [5]. Thus, it is not a surprise that in many cases, the BM has been attributed as the source of BCCs during resurgence [5, 6]. The BM is a complex organ, which is home to hematopoietic (HSCs) and mesenchymal stem cells (MSCs). Both stem cells pose challenges to target BCCs in BM. In the case of HSCs, these cells are mostly located close to the endosteum where BCCs form gap junctional intercellular communication (GJIC) with stroma to achieve quiescence [7].
MSCs are multipotent cells that can differentiate along multiple lineages [8]. The untoward effect of MSCs in cancer biology is underscored by their “plastic” immune functions: exerting immune suppressor and enhancer functions [9]. MSCs can protect BCCs by suppressing NK functions, CTL responses and induce the expansion of regulatory T-cells [10]. The immune suppressor function could provide MSCs with an advantage to survive and establish dormancy.
Metastasis of BC can occur without a history of a primary tumor [11]. In addition, clinical detection of BC resurgence can occur after 10 years [12]. Thus, an understanding of how BCCs achieve quiescence could lead to therapeutic intervention to avoid tertiary metastasis and to prevent dormancy [13]. In BM, quiescent BCCs can be found close to the endosteum [14, 15]. BCC quiescence has been attributed to supportive functions of cells found within the BM microenvironment. These include cytokine production by the cancer and resident BM cells and also, the establishment of GJIC between BCCs and BM stroma [15, 16].
Although robust studies have not investigated the role of MSCs in quiescence of BCCs in BM, the functions of MSCs can be used to extrapolate how they could be involved in promoting dormancy of BCCs. As an example, BCCs and MSCs could interact by membrane-bound G-protein/ 7-transmembrane CXCR4 and the ligand, stromal cell-derived factor 1α (CXCL12) [17-22]. In BM, the entering BCCs can rapidly form a cellular complex with the endogenous MSCs since they are located at the abluminal side of the main blood vessel [17, 23, 24]. Here the immune suppressive effects of MSCs could provide an immediate advantage by protecting the BCCs for the eventual integration close to the endosteum [10, 15, 17, 22, 25]. The relevance of the study is not only limited to BM since MSCs have been implicated in the support of BCC growth at other regions [26-28].
Since MSCs are important for blood vessel integrity they are unlikely to be a safe target in cancer therapy [23]. Similarly, direct targeting of GJIC between BCCs and stroma could be toxic since gap junctions between stromal cells are important for hematopoietic support [29]. Instead, we propose that an understanding of the role of MSCs in the biology of BCCs could lead to therapeutic intervention. To this end, this study investigated the response of BCCs to carboplatin in the presence or absence of MSCs and compared this with a combined therapy of carboplatin and the CXCR4 antagonist, AMD3100. The studies with cell lines were validated with BCCs from patients. We showed a supporting role for MSCs on BCC proliferation once the cells were pharmacologically uncoupled with AMD3100. This uncoupling resulted in the production of IL-1 from MSCs. IL-1 was key to the proliferation of BCCs and, thus, responsiveness to carboplatin. The findings were validated in an animal model in which BCCs and MSCs were co-transplanted in the dorsal flank of mice. The outcome of BC could be worse in overweight individuals [30]. MSCs are not only enriched in adipose tissues, but are the source of adipocytes that can promote the invasion of BCCs [31, 32]. Therefore, this preclinical study with AMD3100 also has significance to the link between obesity and cancer.
Materials and methods
Cell lines
The following cell lines were purchased from American Type Culture Collection (ATCC; Manassas, VA) and cultured as per manufacturer's instructions: T47D; MDA-MB-231; hybridoma-producing CD3, CD4, CD8, CD5 and CD56 (HNK -1) mAb.
Reagents and antibodies
All tissue culture media were purchased from Gibco (Grand Island, NY), fetal calf serum (FCS) from Hyclone Laboratories (Logan, UT), IL-1α/ β siRNA, Ficoll-Hypaque and AMD3100 from Sigma (St. Louis, MO), propidium iodide, PE-cytokeratin mAb from BD Biosciences (San Jose, CA) and carboplatin from Teva Parenteral Medicines (Irvine, CA).
Goat anti-SDF-1α and anti-IL-1RI were purchased from R&D Systems (Minneapolis, MN), rabbit anti-CXCR4 from Affinity Bioreagents (Golden, CO), anti-CD32 mAb from AMAC (Westbrook, ME), rabbit anti-caspase-3 from BD Pharmingen (San Diego, CA), mouse anti-cytokeratin, -β-actin mAb, horseradish-peroxidase (HRP)-conjugated anti-rabbit, -goat and -mouse IgG were purchased from Sigma. CellTiter-Blue cell viability assay was purchased from Promega (Madison, WI) and CyQUANT Cell Proliferation kit from Invitrogen (Carlsbad, CA).
Hybridoma-producing antibodies were prepared from ascites, as described [33]. The optimal concentrations were tested by immunofluorescence with peripheral blood mononuclear cells (PBMCs) and cell depletion studies with PBMCs.
Culture of human MSCs
MSCs were cultured from BM aspirates as described [34, 25]. The use of human BM aspirates followed a protocol approved by the Institutional Review Board of The University of Medicine and Dentistry of New Jersey-Newark campus. Unfractionated BM aspirates were cultured in DMEM with 10% FCS (D10 media) in Falcon 3003 dishes. After 3 days, red blood cells and granulocytes were removed with Ficoll Hypaque. After four cell passages, the adherent cells were asymmetric, CD14-, CD29+, CD44+, CD34-, CD45-, SH2+, prolyl-4-hydroxylase- [25].
Isolation of primary BCCs
Primary BCCs were isolated from peripheral blood of patients. Blood was obtained before chemotherapy, and before or after surgery. Three subjects were diagnosed with triple negative hormone receptors (n=4) and one with triple positive hormone receptors. The use of 5-10 mL human blood was approved by the Institutional Review Board of the University of Medicine and Dentistry of New Jersey-Newark Campus.
We enriched for cytokeratin (+) cells by negative selection. Immune cells were positively selected from peripheral blood mononuclear cells (PBMCs). The starting population, 106/ ml PBMCs was incubated with a cocktail of antibodies: CD3, CD4, CD8, CD32, CD56 and CD20, each at 1/ 200 final dilution. After one hour of incubation on ice, cells were washed with PBS and resuspended in 0.5 mL of PBS and 100 μL of Dynabead goat anti-mouse IgG (Invitrogen). The Dynabead-coupled cells were removed with a magnetic separator. The negative population was analyzed for cytokeratin by flow cytometry, by consecutive labeling with PE-murine anti-cytokeratin. The result indicated >90% cytokeratin (+) cells.
Flow cytometry
PBMCs were analyzed for BCCs by intracellular flow cytometry for cytokeratin. Cells were fixed in 4% formaldehyde for 15 min at 4°C, then permeabilized in 0.1% Triton X-100 for 30 min. Cells were washed in cold PBS and resuspended and incubated in 100 μl cold PBS containing 2% FBS and PE-conjugated mouse anti-cytokeratin (1:100 dilution) for 30 min at 4°C in the dark. Cells were washed in cold PBS, then immediately analyzed on the FACSCalibur (BD Biosciences).
Stable SDF-1α/ CXCR-4 Knockdown
The shRNA vector, pPMSKH1-SDF-1/ KC (wild-type and mutant), for CXCL12 was previously described [22]. pSUPER-CXCR4 (wild-type and mutant) shRNA vector for knockdown of CXCR4 was kindly provided by Dr. Si-Yi Chen (Baylor University) [35]. BCCs or MSCs were co-transfected with pTK-Hyg and pPMSKH1-SDF-1/KC or pSUPER-CXCR4 (both either wild-type or mutant) and then selected with hygromycin or G418. All knockdown cultures were maintained in media containing hygromycin. Levels of CXCL12 and CXCR4 protein expression were determined by western blot to validate knockdown.
Western analysis
Whole cell extracts from BCCs and MSCs were prepared as described [34] and 20 μg were analyzed by western blots using 4-20% SDS-PAGE (Bio-Rad, Hercules, CA). The proteins were transferred onto polyvinylidene difluoride membranes (Perkin Elmer Life Sciences, Boston, MA). Membranes were incubated overnight with primary antibodies and then detected the following day by 2 h incubation with HRP-conjugated IgG. All primary and secondary antibodies were used at final dilutions of 1/ 1000 and 1/ 2000, respectively. HRP was developed with chemiluminscence detection reagent (Perkin Elmer Life Sciences). The membranes were stripped with Restore Stripping Buffer (Pierce, Rockford, IL) for reprobing with other antibodies.
Proliferation/ viability
Cultures of BCCs and MSCs, alone or in co-culture, from each experimental setup were assayed for cellular proliferation and viability using the CyQuant Cell Proliferation Assay Kit (Molecular Probes; Eugene, OR) and CellTiter-Blue Cell Viability Assay (Promega), respectively, according to manufacturer's specific instructions.
Proliferation was determined by culturing cells in 96-well plates and then freezing the plates overnight at -80°C. The next day, thawed cells were incubated in CyQuant GR dye/ cell-lysis buffer for 5 min at RT, and examined using a fluorescence microplate reader at 480 nm excitation/ 520 nm emission. Proliferation was calculated from a standard curve of known numbers of BCCs and MSCs. To accurately assess BCC but not MSC proliferation in co-culture, proliferation of MSCs grown alone were subtracted from the total cellular proliferation recorded. Viability was assessed with CellTiter-Blue reagent, which was added to cells grown in 96-well plates. The plates were incubated for 4 h at 37°C and then read on a fluorescence microplate reader at 560 nm excitation/ 590 nm emission. Percent viability was calculated from a reference using untreated healthy cells, which were considered 100% viable, and cell-free wells containing reagent alone, which were considered 0% viable.
Cell cycle analyses
BCC and MSC co-cultures were incubated with anti-cytokeratin primary and FITC-anti IgG secondary antibodies to label the BCC fraction. Cells were then treated with RNase A (1 mg/ ml) and fixed with cold 70% ethanol. Cells were stained with 20 μg/ ml propidium iodide (PI) solution and transferred to round bottom tubes for DNA analysis by BD FACScan. Double positive cells identified only the desired cellular fraction, consisting of BCCs that incorporated the DNA dye. Cultures incubated with FITC anti-IgG alone were used as isotype controls. All analyses were performed using BD CellQuest software and percent statistics were given.
Transwell assay
BCCs (5 × 104) were added to the outer well of 24-well transwell cultures containing a 0.4 μM insert (BD Falcon). In parallel, BCCs were separated from co-cultures with MSCs by positive selection with anti-cytokeratin-conjugated; pan anti-mouse IgG Dynabeads (Invitrogen). MSCs from the negative fraction were then added to the inner wells of the transwell chamber in order to determine the effects of MSC-derived soluble factors on BCC proliferation. For neutralization of soluble IL-1α and IL-1β, anti-IL-1RI was titrated into the 24-well plates. Neutralization of BCC proliferation was observed at a concentration of 1 μg/ μl.
Cytokine array
Cytokine production by MSCs in the transwell assay was assessed using the Human Cytokine Antibody Array 5 (RayBiotech; Norcross, GA), as previously described [16]. Briefly, after 48 h of culture, the transwell was removed and the BCC growth media collected for cytokine determination. Background levels obtained with media alone were subtracted. The densities of spots were quantitated with UN-SCAN-IT densitometry software (Silk Scientific; Orem, UT). Cytokines demonstrating differential expression were normalized to internal positive controls and presented as fold change relative to an internal control, arbitrarily assigned a value of 1.
Transient transfection of IL-1α/ β siRNA
IL-1α and IL-1β siRNA duplexes (Sigma) were used to knockdown IL-1 production in BCCs, prior to culture with MSC transwell inserts. MDA-MB-231 (5 × 104) were seeded in 24-well plates, and after 24 h, 100nM IL-1α and IL-1β siRNA was delivered via DharmaFECT Transfection reagent (Dharmacon; Lafayette, CO). siRNA sequences were as follows: IL-1α |5'-guc auc aaa gga uga ugc u-3'|; IL-1β |5'-gau guc ugg ucc aua uga a-3'|. Knockdown was confirmed by PCR.
Animal studies
Female nude BALB/ c mice (4 weeks) were obtained from Harlan Laboratories (Indianapolis, IN) and housed in a laminar flow hood at an AALAC-accredited facility for one week before being included in the studies. The use of mice was approved by the Institutional Animal Care and Use Committee, New Jersey Medical School (Newark, NJ). MDA-MB-231 (106), alone or in combination with MSCs (106), in 0.1 mL and equal volume of BD Matrigel (BD Biosciences, Bedford, MA) were injected subcutaneously in the dorsal flank of mice (Day 0, D0). When the tumors were 0.5 cm3, mice were injected intratumorally with AMD3100 alone or in combination with IL-1ra or vehicle (hereafter termed “treatment”). This was achieved with a Hamilton syringe at different sites within the tumors. Mice were given a second “treatment” after four days with carboplatin at 50 mg/ kg by intraperitoneal route. After two days, the mice were injected similarly with carboplatin. After one week the mice showed signs of distress due to a precipitous drop in total cell counts. The experiments were curtailed by euthanasia.
Statistical analysis
Statistical data analyses were performed with analysis of variance and Tukey-Kramer multiple comparisons test. p<0.05 was considered significant.
Results
CXCL12/ CXCR4 interaction in BCC quiescence
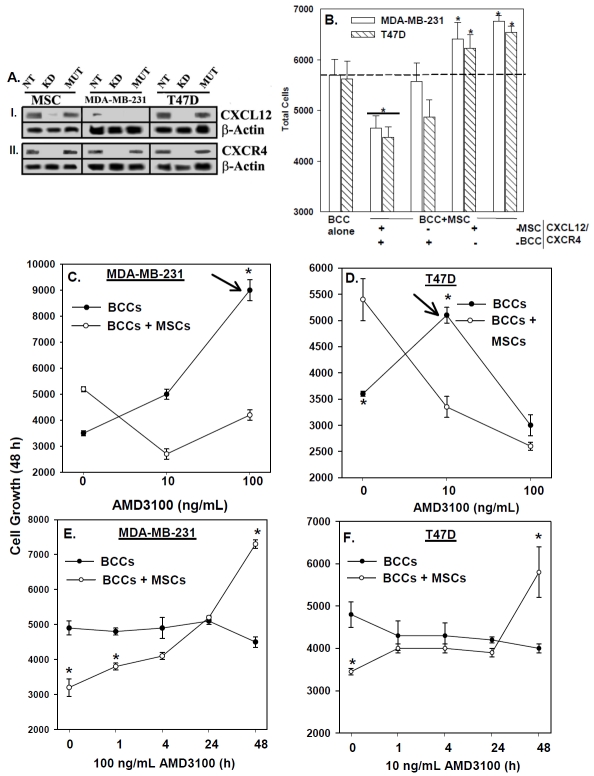
BCCs and MSCs interact through membrane-bound CXCL12 and CXCR4 [17]. We compared the proliferation of BCCs, in the presence or absence of MSCs, and then studied the role of CXCL12/ CXCR4 interaction. Co-cultures of BCCs and MSCs were studied in which the cells were untransfected or knockdown for CXCL12 and/or CXCR4 (Figure 1A).
Figure 1.
CXCL12-CXCR4 interaction between BCCs and MSCs in BCC proliferation. (A) Western blots were performed with extracts from MSCs and BCCs, stably knockdown (KD) for CXCL12 and CXCR4. Controls included mutant (mut) shRNA and non-transfectants (NT). Membranes were stripped and re-probed with β-actin for normalization. (B) MDA-MB-231 were pre-treated mitomycin C (5 μg/mL) to halt cell growth, and then cultured alone or in combination with MSCs for 7 days and cell proliferation measured to determine the effect of BCCs on MSC growth. (C) MSCs and BCCs, knockdown for CXCL12 or CXCR4 and controls were cultured alone or together for 48 h and then studied for proliferation. The results are presented as mean of 10 replicates in five different experiments, each with MSCs from a different donor (±SD). Dose-response curves were established for AMD3100 on BCC proliferation, in the presence or absence of MSCs in 48 h cultures (D and E). The results are presented as mean of ten replicates in five different independent experiments, each with MSCs from a different donor (±SD). Optimal AMD3100 (D and E) dose points were selected and then studied in proliferation responses at various times (F and G) and the results are presented as for D and E.*p≤0.05 vs. BCC alone.
We first optimized the method to ensure that we do not include MSC proliferation as part of the values for BCCs. Proliferation was studied with a dye-based method. We prevented the proliferation of BCCs by pre-treating 1.5 × 104 cells for 15 min with mitomycin C (5 μg/ mL), and then cultured alone or in combination with 2.5 × 103 MSCs for 7 days (Figure 1B). The total number of cells in the co-cultures (far right bar) when compared to BCCs cultured alone (middle bar) showed an increase proportional to the increase in MSCs cultured alone (far left bar). These findings indicated that BCCs do not significantly affect MSC growth. Therefore, we employed a similar subtractive method (# total cells in co-culture - # MSCs cultured alone = # BCCs in co-culture) throughout the study to determine the number of BCCs in co-culture, hereafter termed “BCC+MSC”.
Using a similar method, 2.5 × 103 untransfected BCCs and 103 untransfected MSCs were co-cultured in 96-well plates for 48 h (Figure 1C, second group from left) and the results showed significantly (p<0.05) reduced proliferation as compared to BCCs cultured alone (Figure 1C, Far left bars). The reduction in BCC proliferation in the untransfected co-cultures cannot be explained by cell death, since there was no significant (p>0.05) difference in cell viability (not shown).
We next performed similar studies with MSCs and BCCs, stably knockdown for CXCL12 and CXCR4. The knockdown of CXCL12 and/ or CXCR4 in both BCCs and MSCs (Figure 1C: far right set of bars) produced a significant (p<0.05) increase in proliferation in both MDA-MB-231 (open bar) and T47D (diagonal bar). There was no change in cell viability in the knockdown cells (not shown). These results indicate that the CXCL12/ CXCR4 axis is important in MSC-mediated regulation of BCC proliferation.
Pharmacological disruption of CXCL12/ CXCR4 in BCC proliferation
We next expanded on the molecular disruption of CXCL12-CXCR4 with a CXCR4 antagonist, AMD3100 [36]. We first examined the effects of AMD3100 on the proliferation of MDA-MB-231 and T47D, in co-culture with MSCs (Figures 1D and 1E), in the presence of two different concentrations of the antagonist (10 and 100 ng/ ml) for the 48-h culture. Parallel cultures contained vehicle. The antagonist promoted the proliferation of BCCs in co-cultures with MSCs (Figures 1D and 1E, open circles), as compared to BCCs alone (closed circles). MDA-MB-231 showed less sensitivity (100 ng/ ml) to AMD3100 as compared to T47D (10 ng/ ml).
The antagonist decreased the proliferation of BCCs when cultured alone (closed circles). In contrast to MDA-MD-231, T47D proliferation was inversely related to AMD3100 concentration (Figures 1D and 1E, closed circles). The effects cannot be explained by changes in viability (Figure S1), but instead may allude to an autocrine role for CXCL12/ CXCR4 in T47D proliferation which is impeded by administration of the antagonist at high dose. Repeat of studies at earlier time points verified 48 h as optimum for AMD3100-mediated proliferation (Figures 1F and 1G, open circles). The increase in proliferation, however, began at 1 h for MDA-MB-231, but later for T47D. There was no change in cell viability (Figure S2). These results indicated that pharmacological intervention with a CXCR4 antagonist is able to disrupt MSC-mediated BCC growth arrest at doses tolerable by the cells.
AMD3100 in long-term BCC/ MSC co-cultures
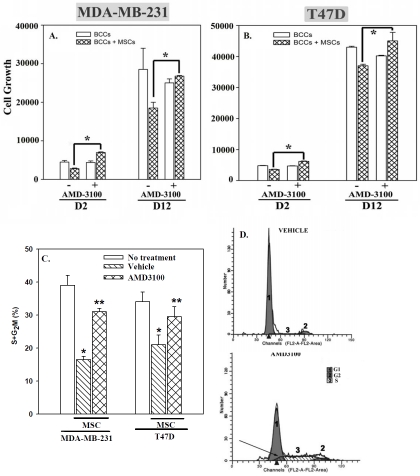
The subsequent studies determined the effect of AMD3100 when BCCs were cultured with MSCs for >48 h since this will recapitulate in situ when BCCs are expected to contact MSCs for long periods. BCCs were co-cultured with MSCs for 12 days (D12). Optimum AMD3100 (Figure 1) was added during the last 48 h of the cultures. Parallel cultures contained BCCs with vehicle (-) or 48 h exposure to AMD3100 (D2). For both cell lines, prolonged incubation with MSCs (D12 co-cultures) resulted in significant (p<0.05) decrease in proliferation as compared to BCCs cultured alone (Figures 2A and 2B). This decrease was significantly (p<0.05) reversed with AMD3100. The changes in proliferation could not be explained by differences in cell viability (Figure S3). In summary, the results, combined with those in Figure 1, indicated that disruption of MSC-mediated BCC growth arrest in short- and long-term co-cultures resulted in the BCCs transitioned into cycling cells.
Figure 2.
Effect of AMD3100 on BCC proliferation following extended co-culture with MSCs and on cell cycle. MDA-MB-231 (A) or T47D (B), alone or with MSCs, were treated with AMD3100 for either 2 (D2) or 12 (D12) days. Control cultures were untreated. The results are presented as the mean of ten replicates in five independent experiments, each with MSCs from a different donor (± SD). *p≤0.05 vs. BCC alone or 12-day cultures with MSCs were treated with AMD3100 for the last 48 h or untreated. At the end of the assay, cells were co-labeled with anti-cytokeratin and propidium iodide and then analyzed by flow cytometry. Results are presented as the mean percent cycling cells (S + G2/M phases) ± SD, n=3. Each experiment was performed with MSCs from a different donor (C). Representative histogram for MDA-MB-231 co-cultured with MSCs, with or without AMD3100. *p≤0.05 vs. BCCs alone; **p≤0.05 vs. BCCs with MSCs; no AMD3100. The histograms (D) represent non-cycling (1) and cycling cells (2, 3). Arrow shows the increased in cycling cells followingAMD3100 treatment.
AMD3100 in BCC cell cycle re-entry
We next investigated the cell cycle phase of BCCs in co-cultures with MSCs for 12 days, with or without AMD3100. The cultures were performed as described for Figures 2A and 2B. BCCs cultured alone served as control. DNA analyses by propidium iodide incorporation indicated significant (p<0.05) reduction in cycling cells (S/ G2/ M phase) with MSCs, as compared to BCCs alone (Figure 2C). Treatment with AMD3100 produced a significant (p<0.05) increase in cycling cells as compared to no treatment. Representative histograms (Figure 2D) showed the cycling (peaks 2 and 3) versus non-cycling (peak 1) phases for MDA-MB-231 co-cultured with MSCs, with (right) or without AMD3100 (left). The data indicated that AMD3100 is able to disrupt MSC-mediated BCC growth arrest by promoting cell cycle re-entry.
Soluble factors in re-entry of BCC into cycling
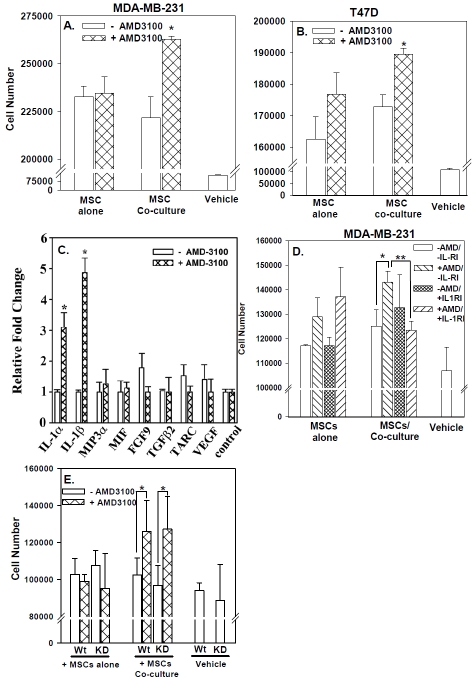
AMD3100 promoted proliferation and cell cycle re-entry (Figure 2) of BCCs, in co-culture with MSCs. In this set of studies we asked whether AMD3100-mediated proliferation of BCCs required direct contact between MSCs and BCCs. A contact-independent mechanism would suggest the involvement of soluble factors released by one or both cell types to induce proliferation. To address this question, we utilized a transwell assay in which “naïve” BCCs, which were not previously exposed to MSCs or AMD3100, were seeded in the outer wells. MSCs from co-cultures with AMD3100 or vehicle were added to the inner wells. In parallel, only vehicle was added to the inner wells. The results showed significant (p<0.05) increase in the proliferation of “naïve” BCC proliferation when the MSCs in the inner wells were from previous AMD3100-containing co-cultures (Figures 3A and 3B, middle hatched bars). Similar proliferation was not observed for antagonist-treated MSCs alone (left hatched bars). In summary, the results indicated that the “uncoupling” of BCCs from MSCs with AMD3100 resulted in the production of soluble factors from MSCs that supported the growth of BCCs.
Figure 3.
IL-1 in AMD3100-mediated BCC cycling. MSCs were co-cultured with BCCs for 12 days with, or without AMD3100. The MSCs were selected and then added to the inner wells of tran-swell cultures. Naïve BCCs were placed in the outer wells. After 48 h, BCCs were counted and the results presented as the mean of ten replicates in five independent experiments, each performed with MSCs from a different donor (±SD) (A and B). The media were analyzed for cytokine production with protein array and the normalized values presented as fold change relative to the internal control (C). The studies were repeated with anti-IL-1RI or control IgG (D); or with IL-1α/β knockdown MSCs (E). The results are presented as for A and B, n=5. *p≤0.05 vs. no AMD3100; **p≤0.05 vs. control IgG.
MSC-derived IL-1α in AMD3100-mediated BCC proliferation
We next performed studies to identify the factor (s) responsible for BCC proliferation (Figures 3A and 3B) in AMD3100-treated co-cultures of BCCs and MSCs. We collected media from representative (MDA-MB-231) transwell cultures and then analyzed with a cytokine/ growth factor microarray. The pro-inflammatory cytokines IL-1α and IL-1β (Figure 3C) were 3- and 5-fold greater, respectively, when MSCs were obtained from AMD3100-treated co-cultures.
In the next set of studies we investigated the involvement of IL-1α and IL-1β in the proliferation of BCCs in the outer wells (Figures 3A and 3B). This was addressed by repeating the co-culture assay in the presence of neutralizing anti-IL-1RI (Figure 3D). The anti-IL-1R1 significantly (p<0.05) impeded the proliferation of BCCs (middle group, far right bar) as compared to non-immune IgG (middle group, second bar from left). Anti-IL-1RI had no effect on the proliferation of “naïve” BCCs incubated with MSCs previously cultured alone (left group of bars).
We next identified whether MSCs and/ or BCCs are the sources of IL-1α and IL-1β, by repeating the transwell assay. Instead, we added “naïve” BCCs, knockdown for IL-1α and IL-1β, or wild-type (Figure 3E). If BCCs rather than MSCs are the source of IL-1, its knockdown should have a similar effect on proliferation as the neutralizing antibody. Addition of AMD3100-treated MSCs from co-cultures (middle group of bars) to the inner wells produced no discernible difference on proliferation of wild-type and knockdown BCCs. These results indicated that MSCs and not BCCs are the source of IL-1. In summary, the data indicated that treatment of MSC/ BCC co-cultures with AMD3100 caused the release of IL-1α and IL-1β from MSCs, which in turn induces BCC cell cycle re-entry and proliferation.
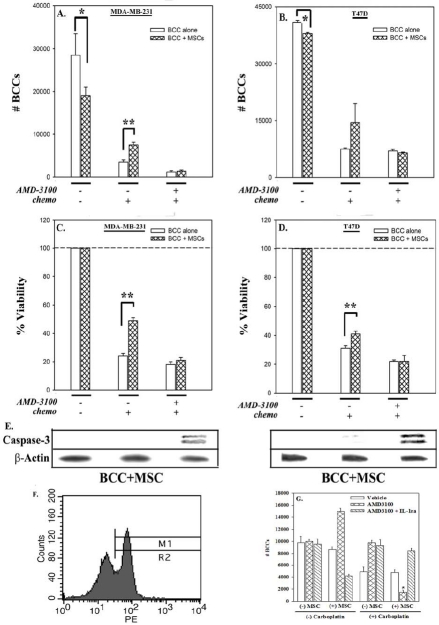
AMD3100-mediated susceptibility of BCC (lines and primary) to carboplatin
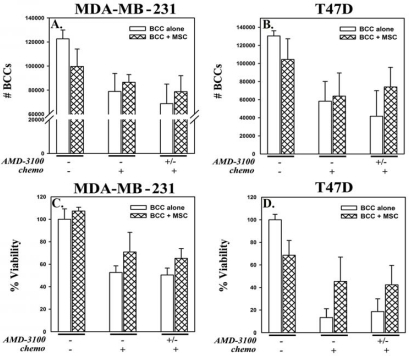
This section determined the clinical relevance of AMD3100 as potential therapy for BC. We examined the ability of AMD3100 to promote susceptibility to the chemotherapy, carboplatin, in the presence of MSCs. BCCs, in co-culture with MSCs (Figures 4A-4D; hatched bars) were chemoresistant (middle sets of bars) to carboplatin as compared to BCCs cultured alone (open bars). This resistance was reversed when carboplatin was added with AMD3100 (right sets of bars). The decrease in cell viability correlated with an increase in caspase-3 immunore-activity (Figure 4E) in BCCs, demonstrating apoptosis by AMD3100 and carboplatin.
Figure 4.
Treatment of co-cultured BCCs-MSCs with carboplatin and AMD3100. BCCs, cultured alone or with MSCs for 12 days, were treated with AMD3100, or untreated. After 48 h, all cultures were exposed to carboplatin, with re-added AMD3100 in cultures that had initial AMD3100. After 72 h, the viable cells were counted (A and B), and presented (C and D). The results are presented as the mean of ten replicates in five independent experiments, each with MSCs from a different donor (±SD). Whole cell extracts from these experiments were analyzed by immunoblot for caspase-3 (E). Membranes were stripped and re-probed with β-actin for normalization. Representative blots are shown for three independent experiments, each performed with MSCs from a different donor. (F) Cytokeratin (+) cells were detected in peripheral blood of BC patients by flow cytometry. (G) Cytokeratin-positive cells were negatively selected and then cultured alone or in combination with MSCs for 12 days as above. *p≤0.05 vs. BCCs alone; **p≤0.05 vs. BCCs + MSCs.
To examine the clinical relevance of the studies with cell lines, we isolated cytokeratin-positive cells from the peripheral blood of BC patients (Table S1). In untreated patients, approximately 20-30% of cells were found to be positive for this epithelial marker. Representative flow cytometry for cytokeratin (+) cells are shown in Figure 4F. Cytokeratin (+) cells were isolated from PBMCs by negative selection and the above study repeated with 5 × 103 primary BCCs (n=4) and 2.5 × 103 MSCs (Figure 4G). Interestingly, the results were similar to the studies with cell lines (Figure 2), underscoring the sensitivity of BCCs to carboplatin when MSCs are uncoupled with AMD3100.
An interesting observation with the timeline treatment by carboplatin and AMD3100 indicated that when co-cultures were first pre-treated with AMD3100 for 48 h, followed by carboplatin in the absence of AMD3100, the effect on cell proliferation and viability was lost (Figure 5; right sets of hatched bars). In summary, the findings indicated that AMD3100 needed to be included together as a cocktail to effectively target BCCs, in the presence of MSCs. In this light, AMD3100 may serve as an adjuvant treatment to chemotherapy in treating BC.
Figure 5.
Resistance to chemotherapy in non-combinatorial, AMD3100-treated MSC/BCC cocultures. BCCs cultured in the presence or absence of MSCs for 12 days were treated for 48 h with AMD3100, or untreated. Following 48 h, treated and untreated cells were exposed to carboplatin for 72 h to induce cell death. The treated cells did not receive chemotherapy in combination with a second dose of AMD3100 (as illustrated by +/-). At the end of this regiment cell proliferation (A and B) and viability (C and D) were assessed. The results are presented as the mean of ten replicates in five different experiments, each with MSCs from a different donor (± SD).
In vivo treatment of BCCs with carboplatin and AMD3100
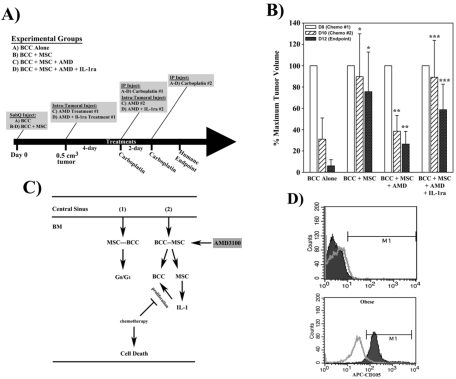
The in vitro findings, above, were validated in vivo, with female nude BALB/ c mice. BALB/ c mice were injected subcutaneously in the dorsal flank with 106 MDA-MB-231 in matrigel, alone or in combination with 106 MSCs (Figure 6A). Mice were divided into the following experimental groups (n=5 per group): (A) BCC injected alone; (B) BCC injected in combination with MSCs; (C) group B plus AMD3100 treatment; (D) group C plus IL-1ra co-treatment. All groups were treated with carboplatin as outlined in Figure 6A. After one week of the second injection of carboplatin the mice showed evidence of distress due to precipitous drop in cell counts and were therefore euthanized for humane reasons.
Figure 6.
Effects of AMD3100 and carboplatin on tumor volume. The method by which mice were treated is shown in (A). (B) Female BALB/c (n=5) were injected subcutaneously with matrigel and 106 MDA-MB-231 alone or in combination with 106 MSCs. The mean volumes at the first injection (day 8, D8) are normalized to 100% and the change in volumes at D10 and D12 are presented as mean±SD. (C) Schematic depicting mechanism of AMD3100 action in mediating BCC chemotherapeutic susceptibility following interaction with MSCs. BCCs that have metastasized from the primary tumor site into the systemic circulation enter the central sinus of the BM where they extravasate across the endothelium and come into contact with resident MSCs. Interaction between CXCL12, secreted by MSCs and/or BCCs, and CXCR4, expressed on both cell types, facilitates BCC integration and dormancy within the BM (1). Treatment with AMD3100 (2) breaks this chemokine ligand-receptor interaction and triggers the MSCs to release the inflammatory cytokines, IL-1α and IL-1β. Having been released from the MSCs, the BCCs begin to cycle and proliferate, thus conferring susceptibility to chemotherapeutic treatment. (D) Representative histogram illustrating circulating CD105(+) cells in blood from the patients studied in Figure 5G.
Mice injected with BCC alone (Figure 6B, far left group of bars) showed a drastic reduction in mean tumor volume (solid bar) at the end of the studies. This reduction was significantly (p<0.05) impeded by co-injection with MSCs (second group of bars from left). Preconditioning the tumors with AMD3100 significantly (p<0.05) reversed the chemoprotective effect of MSCs (second group of bars from right), although co-treatment with IL-1ra negated this response (far right group of bars). In summary, the results, using an in vivo tumor model, validated our in vitro studies (Figures 3 and 4), and indicated that AMD3100 disabled the chemoprotective effect of MSC through an IL-1-dependent mechanism.
CD105(+) cells in the circulation of obese BC patients
The in vivo model was intended to recapitulate the relevance of IL-1 in the tumors developed by MSCs and BCCs (Figure 6A-6C). The studies were performed by subcutaneous injection of the cells in the dorsal flank. Since the injection of BCCs did not allow for systemic circulation we could not perform survival studies. However, the clinical significance of the studies was important. We therefore analyzed the blood of patients studied in Figure 4G for circulating CD105 (+) cells as an indicator of MSCs. Only the two obese patients show detectable CD105 (+) cells in the circulation (Figure 6D).
Discussion
MSCs could pose a significant clinical dilemma for BC treatment, particularly the immune suppressive functions of MSCs, including inhibition of BCC proliferation [10]. In the absence of proliferation, the BCCs will have a seemingly dormant phenotype that could be refractory to most anti-cancer agents. New therapies are required to reverse the dormant BCCs into proliferating cells for targeting. In this study, we showed CXCL12 and its receptor, CXCR4, as mediators in the interactions between MSCs and BCC, leading to growth arrest (Figure 6C, part 1). Pharmacological disruption with AMD3100, (part 2) stimulated BCC proliferation through MSC-derived IL-1α and IL-1β, thereby rendering the actively cycling BCCs susceptible to carboplatin. The clinical significance of the studies was determined in two sets of studies: I) Testing the model with primary BCCs from patients (Figure 4G); II) and identifying MSCs in the circulation of obese patients. Since these patients are documented with worse outcome [30], this report could be the basis for further research in obesity and cancer.
MDA-MB-231 seems to be more responsive to AMD3100 than T47D (Figure 1). This might be due to a single interaction between CXCL12 and CXCR4 among MSCs and MDA-MB-231, since this cell line is null for CXCL12 [37]. Cell cycle arrest of BCCs depended on CXCL12-CXCR4 interaction; and re-entry into cycling by AMD3100 required the presence of MSCs since the antagonist alone showed no change (Figure 2D). Both the triple hormone receptor positive and negative BCCs (cell lines and primary cells) showed comparable outcome with AMD3100 and carboplatin (Figure 4). Further studies are required to determine if the hormone status is irrelevant to AMD3100 treatment. The 12-day co-culture is significant because clinically, it is expected that BCCs will be in contact with MSCs for prolonged periods; which is consistent with studies in which targeting of CXCR4 prevents metastasis of BC to the lung [38].
Interestingly, AMD3100 alone reduced the growth of the BCC cultured alone (Figures 1D and 1E). We surmised that CXCR4 might be activated through autocrine and/ or paracrine stimulation to stimulate cell growth [39], which would be blocked by AMD3100. In contrast, the antagonist “uncoupled” MSCs from BCCs to “free” the MSCs for support of BCC growth (Figure 6C).
The studies are significant to BC dormancy in any organ, but specifically for BM. In addition to acting as an antagonist to CXCR4, AMD3100, also known as Plerixafor, is a macrocyclic compound which is an allosteric agonist of CXCR7 [40]. Our studies do not indicate that AMD3100 activated CXCR7 for the proliferation of BCCs since alone, BCC growth was suppressed with AMD3100 (Figure 1). The data favored MSC-mediated factors in the growth of BCCs by AMD3100. Given the outcome of this study, it is not a surprise that AMD3100 can decrease primary and metastatic BC in mice, but with no effect on the overall survival for experimental lung metastases [38]. The studies add to the indication for AMD3100 [36].
Indeed, an interesting and unexpected observation is the need for continued presence of AMD3100 during treatment with carboplatin. This suggested that the interaction between BCCs and MSCs is rapid. Pre-treatment with the antagonist for 48 h prior to chemotherapy did not induce significant cell death (Figure 5) as compared to co-treatment (Figure 4). The clinical significance of this result is that therapies targeting CXCR4 to promote re-cycling of dormant BCCs may need to be delivered as an adjuvant to chemotherapy to achieve efficiency.
We determined that for the highly aggressive MDA-MD-231, treatment of co-cultures with AMD3100 promoted the release of IL-1α and IL-1β from MSCs (Figure 3C). This finding warrants caution, since in BM, MSCs regulate the immune response to prevent exacerbated inflammation [41], which could be detrimental to BM functions, with consequence to immune cell development and viral clearance [25, 41-43]. Due to humane endpoint, we could not prolong the studies beyond five days of chemotherapy since the mice showed evidence of distress with low blood counts. However, further studies are required to determine how targeting of CXCR4 in an animal model of MSC-mediated BC growth arrest in BM could be explored with other chemotherapies that would allow for long-term studies.
AMD3100 can mobilize HSCs. This poses some concerns if this agent is used in patients to target BM-resident BCCs. AMD3100, in combination with chemotherapy, could show untoward effects by mobilizing HSCs. Additional antagonists for CXCR4 should be studied for those that can facilitate targeting of BCCs but minimize toxicity to resident HSCs.
The findings on CXCL12/ CXCR4 axis warrant further investigation as a potential druggable target to combat BC metastasis to BM, and other organs. Future studies will use animal models to assess whether CXCR4 antagonists can induce re-cycling of BCCs that interact with MSCs within the BM, as well as within the tumor (Figure 6). These studies will provide the information necessary to assess whether the BCC/ MSC interaction is a viable therapeutic target and provide information regarding safety and toxicity. The studies are particularly relevant to the BM, where MSCs reside around blood vessels and the trabeculae [23, 44].
The immune responses of MSCs, as described in this report, are important in designing therapies for patients with BC. In addition, MSCs are in clinical trials for inflammatory disease, which would establish crosstalk with the implanted MSCs. If the host receiving MSCs have undiagnosed cancer or is in remission, the immune functions of MSCs will be fundamental in decisions for stem cell therapy. MSCs should be considered as immune cells and the responses need to be central to future treatments for cancer as well as other fields for the safe and effective therapies.
Acknowledgments
This work was supported by the New Jersey Commission on Cancer Research and the Department of Defense (W81XWH-0810561 and W81XWH-0610689).
Conflict of interest
None of the authors have any conflict of interest.
SUPPLEMENTAL DATA
Table S1.
Demographics of breast cancer subjects
| Subjects | Stage | ER/PR | HER2 | Lymph Nodes | Obese |
|---|---|---|---|---|---|
| S1 | IIIB | − | − | − | No |
| S2 | III | − | − | − | No |
| S3 | IIA | + | + | + | Yes |
| S4 | III | + | − | − | Yes |
ER: Estrogen receptor; PR: Progesterone receptor
Shown are the demographics of BC patients. Subjects with >30 BMI were considered obese.
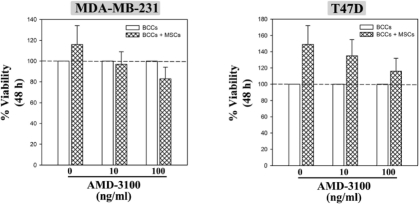
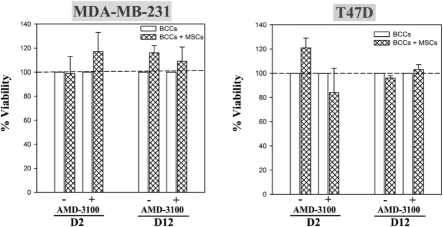
Figure 1S.
Dose-response with AMD3100 on BCC viability. BCCs cultured in the presence or absence of MSCs were treated with various doses of the CXCR4 antagonist, AMD3100, or untreated. After 48 h, viability was assessed. Results are presented as the mean ± SD, na=3. *p≤0.05 vs. BCC alone
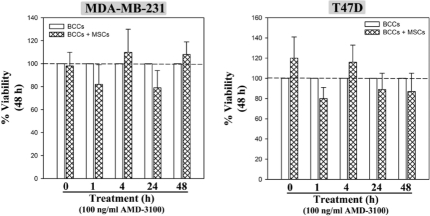
Figure 2S.
Time-course studies with AMD3100 on BCC viability. BCCs cultured in the presence or absence of MSCs were treated for various times with a fixed dose of AMD3100, or untreated. At the appropriate endpoint, viability was assessed. Results are presented as the mean ± SD, na=3. *P≤0.05 vs. BCC alone
Figure 3S.
Effect of AMD3100 on BCC viability in extended co-culture with MSCs. BCCs cultured in the presence or absence of MSCs for either 2 (D2) or 12 (D12) days were treated for 48 h with AMD3100, or untreated. Following treatment, viability was assessed. Results are presented as the mean ± SD, na=3. *P≤0.05 vs. BCC alone
References
- 1.Braun S, Naume B. Circulating and Disseminated Tumor Cells. J Clin Oncol. 2005;23:1623–1626. doi: 10.1200/JCO.2005.10.073. [DOI] [PubMed] [Google Scholar]
- 2.Gluck S. Autologous transplantation for patients with advanced breast cancer with emphasis on bony metastasis. Can J Oncol. 1995;5(Suppl 1):58–62. [PubMed] [Google Scholar]
- 3.Habeck M. Bone-marrow analysis predicts breast-cancer recurrence. Mol Med Today. 2000;6:256–257. doi: 10.1016/s1357-4310(00)01733-0. [DOI] [PubMed] [Google Scholar]
- 4.Mansi JL, Berger U, McDonnell T, Pople A, Rayter Z, Gazet JC, Coombes RC. The fate of bone marrow micrometastases in patients with primary breast cancer. J Clin Oncol. 1989;7:445–449. doi: 10.1200/JCO.1989.7.4.445. [DOI] [PubMed] [Google Scholar]
- 5.Patel SA, Heinrich AC, Reddy BY, Srinivas B, Heidaran N, Rameshwar P. Breast cancer biology: the multifaceted roles of mesenchymal stem cells. J Oncol. 2008;2008:425895. doi: 10.1155/2008/425895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naume B, Zhao X, Synnestvedt M, Borgen E, Russnes HG, Lingjaerde OC, Stromberg M, Wiedswang G, Kvalheim G, Karesen R, Nesland JM, Borresen-Dale AL, Sorlie T. Presence of bone marrow micrometastasis is associated with different recurrence risk within molecular subtypes of breast cancer. Mol Oncol. 2007;1:160–171. doi: 10.1016/j.molonc.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, Greco SJ, Bryan M, Patel PS, Rameshwar P. Gap Junction-Mediated Import of MicroRNA from Bone Marrow Stromal Cells Can Elicit Cell Cycle Quiescence in Breast Cancer Cells. Cancer Res. 2011;71:1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 8.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends in Molecular Medicine. 2001;7:259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 9.Helmy KY, Patel SA, Silverio K, Pliner L, Rameshwar P. Stem cells and regenerative medicine: accomplishments to date and future promise. Ther Deliv. 2010;1:693–705. doi: 10.4155/tde.10.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal Stem Cells Protect Breast Cancer Cells through Regulatory T Cells: Role of Mesenchymal Stem Cell-Derived TGF-{beta} J Immunol. 2010;184:5885–5894. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- 11.Katz D, Aharoni D. Lytic Lesions in Breast Cancer. N Engl J Med. 2004;351:2850. doi: 10.1056/NEJMicm040763. [DOI] [PubMed] [Google Scholar]
- 12.Talmadge JE. Clonal Selection of Metastasis within the Life History of a Tumor. Cancer Res. 2007;67:11471–11475. doi: 10.1158/0008-5472.CAN-07-2496. [DOI] [PubMed] [Google Scholar]
- 13.Dai H, van't Veer L, Lamb J, He YD, Mao M, Fine BM, Bernards R, van de Vijver M, Deutsch P, Sachs A, Stoughton R, Friend S. A Cell Proliferation Signature Is a Marker of Extremely Poor Outcome in a Subpopulation of Breast Cancer Patients. Cancer Res. 2005;65:4059–4066. doi: 10.1158/0008-5472.CAN-04-3953. [DOI] [PubMed] [Google Scholar]
- 14.Corcoran KE, Patel N, Rameshwar P. Stromal Derived Growth Factor-1{alpha}: Another Mediator in Neural-Emerging Immune System through Tac1 Expression in Bone Marrow Stromal Cells. J Immunol. 2007;178:2075–2082. doi: 10.4049/jimmunol.178.4.2075. [DOI] [PubMed] [Google Scholar]
- 15.Moharita AL, Taborga M, Corcoran KE, Bryan M, Patel PS, Rameshwar P. SDF-1{alpha} regulation in breast cancer cells contacting bone marrow stroma is critical for normal hematopoiesis. Blood. 2006;108:3245–3252. doi: 10.1182/blood-2006-01-017459. [DOI] [PubMed] [Google Scholar]
- 16.Oh HS, Moharita A, Potian JG, Whitehead IP, Livingston JC, Castro TA, Patel PS, Rameshwar P. Bone Marrow Stroma Influences Transforming Growth Factor-{beta} Production in Breast Cancer Cells to Regulate cmyc Activation of the Preprotachykinin-I Gene in Breast Cancer Cells. Cancer Res. 2004;64:6327–6336. doi: 10.1158/0008-5472.CAN-03-3122. [DOI] [PubMed] [Google Scholar]
- 17.Corcoran KE, Trzaska KA, Fernandes H, Bryan M, Taborga M, Srinivas V, Packman K, Patel PS, Rameshwar P. Mesenchymal Stem Cells in Early Entry of Breast Cancer into Bone Marrow. PLoS ONE. 2008;3:e2563. doi: 10.1371/journal.pone.0002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dennis JE, Charbord P. Origin and differentiation of human and murine stroma. Stem Cells. 2002;20:205–214. doi: 10.1634/stemcells.20-3-205. [DOI] [PubMed] [Google Scholar]
- 19.Helbig G, Kent W, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. NF-kB Promotes Breast Cancer Cell Migration and Metastasis by Inducing the Expression of the Chemokine Receptor CXCR4. Journal of Biological Chemistry. 2003;278:21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 20.Muller-Sieburg CE, Deryugina E. The stromal cells' guide to the stem cell universe. Stem Cells. 1995;13:477–486. doi: 10.1002/stem.5530130505. [DOI] [PubMed] [Google Scholar]
- 21.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Rao G, Patel PS, Idler SP, Maloof P, Gascon P, Potian JA, Rameshwar P. Facilitating Role of Preprotachykinin-I Gene in the Integration of Breast Cancer Cells within the Stromal Compartment of the Bone Marrow: A Model of Early Cancer Progression. Cancer Res. 2004;64:2874–2881. doi: 10.1158/0008-5472.can-03-3121. [DOI] [PubMed] [Google Scholar]
- 23.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 24.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Experimental Cell Research. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-Like Activity of Mesenchymal Stem Cells: Functional Discrimination Between Cellular Responses to Alloantigens and Recall Antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein RH, Reagan MR, Anderson K, Kaplan DL, Rosenblatt M. Human Bone MarrowGÇôDerived MSCs Can Home to Orthotopic Breast Cancer Tumors and Promote Bone Metastasis. Cancer Res. 2010;70:10044–10050. doi: 10.1158/0008-5472.CAN-10-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-Associated Fibroblast-GÇôLike Differentiation of Human Mesenchymal Stem Cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klopp AH, Spaeth EL, Dembinski JL, Woodward WA, Munshi A, Meyn RE, Cox JD, Andreeff M, Marini FC. Tumor Irradiation Increases the Recruitment of Circulating Mesenchymal Stem Cells into the Tumor Microenvironment. Cancer Res. 2007;67:11687–11695. doi: 10.1158/0008-5472.CAN-07-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foss B, Hervig T, Bruserud O. Connexins are active participants of hematopoietic stem cell regulation. Stem Cells Dev. 2009;18:807–812. doi: 10.1089/scd.2009.0086. [DOI] [PubMed] [Google Scholar]
- 30.Boehmer U, Mertz M, Timm A, Glickman M, Sullivan M, Potter J. Overweight and Obesity in Long-Term Breast Cancer Survivors: How Does Sexual Orientation Impact BMI? Cancer Invest. 2011;29:220–228. doi: 10.3109/07357907.2010.550664. [DOI] [PubMed] [Google Scholar]
- 31.Mosna F, Sensebe L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user's guide. Stem Cells Dev. 2010;19:1449–1470. doi: 10.1089/scd.2010.0140. [DOI] [PubMed] [Google Scholar]
- 32.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 33.Rameshwar P, Denny TN, Stein D, Gascon P. Monocyte adhesion in patients with bone marrow fibrosis is required for the production of fibrogenic cytokines. Potential role for interleukin-1 and TGF-beta. J Immunol. 1994;153:2819–2830. [PubMed] [Google Scholar]
- 34.Greco SJ, Zhou C, Ye JH, Rameshwar P. An Interdisciplinary Approach and Characterization of Neuronal Cells Transdifferentiated from Human Mesenchymal Stem Cells. Stem Cells and Development. 2007;16:811–826. doi: 10.1089/scd.2007.0011. [DOI] [PubMed] [Google Scholar]
- 35.Lapteva N, Yang AG, Sanders DE, Strube RW, Chen SY. CXCR4 knockdown by small interfering RNA abrogates breast tumor growth in vivo. Cancer Gene Ther. 2004;12:84–89. doi: 10.1038/sj.cgt.7700770. [DOI] [PubMed] [Google Scholar]
- 36.Drugs R D. 2007;8:113–119. Plerixafor: AMD 3100, AMD3100, JM 3100, SDZ SID 791. [Google Scholar]
- 37.Mirisola V, Zuccarino A, Bachmeier BE, Sormani MP, Falter J, Nerlich A, Pfeffer U. CXCL12/ SDF1 expression by breast cancers is an independent prognostic marker of disease-free and overall survival. Eur J Cancer. 2009;45:2579–2587. doi: 10.1016/j.ejca.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Smith MCP, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 Regulates Growth of Both Primary and Metastatic Breast Cancer. Cancer Res. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 39.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 Pathway in Cancer. Clinical Cancer Research. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 40.Kalatskaya I, Berchiche YA, Gravel Sp, Limberg BJ, Rosenbaum JS, Heveker N. AMD3100 Is a CXCR7 Ligand with Allosteric Agonist Properties. Molecular Pharmacology. 2009;75:1240–1247. doi: 10.1124/mol.108.053389. [DOI] [PubMed] [Google Scholar]
- 41.Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P. Antigenpresenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-{gamma} Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang HS, Habib M, Chan J, Abavana C, Potian JA, Ponzio NM, Rameshwar P. A paradoxical role for IFN-gamma in the immune properties of mesenchymal stem cells during viral challenge. Exp Hematol. 2005;33:796–803. doi: 10.1016/j.exphem.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Tang KC, Trzaska KA, Smirnov SV, Kotenko SV, Schwander SK, Ellner JJ, Rameshwar P. Down-Regulation of MHC II in Mesenchymal Stem Cells at High IFN-{gamma} Can Be Partly Explained by Cytoplasmic Retention of CIITA. J Immunol. 2008;180:1826–1833. doi: 10.4049/jimmunol.180.3.1826. [DOI] [PubMed] [Google Scholar]
- 44.Sakaguchi Y, Sekiya I, Yagishita K, Ichinose S, Shinomiya K, Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood. 2004;104:2728–2735. doi: 10.1182/blood-2003-12-4452. [DOI] [PubMed] [Google Scholar]