Abstract
The RET receptor tyrosine kinase is a member of the cadherin superfamily and plays a pivotal role in cell survival, differentiation and proliferation. Currently, 12 ret/ptc chimeric oncogenes, characterized by the fusion between the intracellular domain of RET and different activating genes, which can cause ligand-independent dimerization and constitutive activation, have been described. β-catenin is usually involved in the maintenance of cell-to-cell adhesion and mediates the Wnt/β-catenin pathway important during embryogenesis and in cellular malignant transformation. Recently, a novel mechanism of RET-mediated function through the β-catenin pathway has been reported in multiple endocrine neoplasia type 2 and in sporadic thyroid carcinomas. Here, we investigated the effects of the ZD6474, a small molecule RET-inhibitor, on RET/β-catenin interaction. We confirmed the ZD6474 mediated-inhibition of recombinant RET kinase and of growth of cells expressing RET/PTC. Interestingly, we firstly observed reduced cellular mobility and changed morphology of TPC1 treated cells suggesting that RET-inhibitor could affect β-catenin cellular distribution as resulted in its co-immunoprecipitation with E-cadherin. We further investigated this hypothesis showing that TPC1 treated cells displayed predominantly β-catenin cytosolic localization. Surprisingly, RET and β-catenin co-immunoprecipitated in both ZD6474-treated and untreated TPC1 cells, suggesting that RET/β-catenin interaction might not be affected by RET kinase inactivation. All together these results suggest that RET kinase activation is crucial for β-catenin stabilization (pY654), localization and its signaling pathway activation but not for β-catenin/RET physical interactions, in human papillary thyroid carcinomas. In conclusion, ZD6474, by inhibiting RET kinase, down-modulates β-catenin pathway leading its recruitment to the membrane by E-cadherin.
Keywords: RET, β-catenin, ZD6474, RET-inhibitor, protein interaction
Introduction
RET is a receptor tyrosine kinase characterized by an extracellular cadherin-like domain and an intracellular kinase domain interrupted by an inter-tyrosine kinase insert region. The c-ret proto-oncogene is involved in different mutations and chromosomal rearrangements. RET receptor is predominantly expressed in tissues of neuroectodermic derivation. In human embryos, RET is expressed in a cranial population of neural crest cells, and in the developing nervous and urogenital systems; while in adults RET is expressed in several neural crest-derived cell lines, spleen, thymus, lymph nodes, salivary glands, spermatogonia and in thyroid C cells. Its biological function is not well defined even if different studies demonstrate its involvement in embryonic development, cellular migration, proliferation and differentiation [1, 2].
It is possible to distinguish two classes of genetic disease caused by mutations in the c-ret proto-oncogene: Hirschsprung's disease, that is due to loss-of-function mutations, and three types of autosomal dominant cancer syndromes associated with activating point mutations [3]: multiple endocrine neoplasia type 2A and 2B (MEN -2A, -2B) and familial medullary thyroid carcinoma (FMTC).
In addition to specific point mutations, the c-ret proto-oncogene undergoes different gene rearrangements that lead to the fusion of the RET kinase domain to the 5'- terminal regions of heterologous genes, generating chimeric oncogenes designated ret/ptc. The hybrid protein generally is capable of ligand-independent dimerization, which results in constitutive activation of kinase function. To date, at least 12 different variants of RET/PTC have been described: RET/PTC 1-9, PCM1/RET, ELKS/RET, and RFP/RET isolated from sporadic and radiation-associated PTCs. In particular, RET/PTC1 originates from chromosome 10 inversion, inv (10)(q11.2q21.2), and results from the fusion of the RET tyrosine kinase domain and H4 (D10S170) gene, whose function appears to be related to DNA damage-induced apoptosis [4]. The H4/RET fusion incorporates 101 amino acids of H4, predicted to encode a leucine zipper domain responsible for RET/PTC1 oligomerization and constitutive tyrosine kinase activity; interestingly RET/PTC1 rearrangement has been found to be associated with post-Chernobyl PTC of long latency [1, 5, 6].
Ret/ptc rearrangements activate the transforming potential of RET by multiple mechanisms. First, by substituting its transcriptional promoter with those of the fusion partners, they allow the expression of RET in the epithelial follicular thyroid cells, where it is normally transcriptionally silent. Secondly, the rearranged constitutively active chimeric oncoproteins are distributed in the cytosolic compartment of the cell. More importantly, the RET/PTC kinases form dimers due to the presence of protein-protein interaction motifs in RET fusion partners.
β-catenin plays an important role in the maintenance of cell-to-cell adhesion and mediates the Wnt/β-catenin signal transduction pathway, important during embryogenesis and in cellular malignant transformation. In particular, β-catenin can bind to E-cadherin thus connecting it to the actin cytoskeleton via β-catenin, while free β-catenin levels are regulated by activation of Wnt/β-catenin signaling pathway. In normal cells, in the absence of Wnt signal, free β-catenin is constantly targeted for degradation by a multiprotein complex consisting of Axin, the tumour suppressor adenomatous polyposis coli (APC), and the serine/threonine kinase glycogen synthase kinase 3 β (GSK3β) [7], which phosphorylates β-catenin on Ser/Thr residues. Phosphorylated β-catenin is then recognized and degraded by the proteasome, reducing the level of cytosolic β-catenin [8-10]. In the presence of Wnt ligand, the Wnt/ β-catenin pathway is up-regulated causing inhibition of the degradation complex. The net result is accumulation of cytosolic β-catenin. Stabilized β-catenin will then translocate into the nucleus and bind to members of the T-cell factor (Tcf)/Lymphoid enhancing factor (Lef) family of DNA binding proteins leading to transcription of Wnt target genes such as c-Myc and cyclin D1 [8-10].
Recently, a novel mechanism of RET-mediated function through the β-catenin signaling pathway has been reported in multiple endocrine neoplasia type 2 (MEN 2) and in sporadic thyroid carcinoma. In particular, a decrease of RET-mediated cellular proliferation, colony formation and tumor growth in nude mice was observed after down-regulation ofβ-catenin activity [11]. In addition, a cross talk between RET/PTC and Met kinase was described in terms of promotion of β-catenin transcriptional activity, leading to thyrocyte neoplastic transformation [12]. Moreover, it was demonstrated that in RET/PTC transfected cells different signaling events, such as PI3k/Akt and Ras/Erk pathways, downstream of RET/PTC, converge on β-catenin to stimulate cellular proliferation [13]. All together these data suggest that RET/PTC fusion proteins could mediate oncogenic transformation through the β-catenin signaling pathway in papillary thyroid carcinomas.
Here, we investigated the effects of compound ZD6474 on RET/β-catenin interaction. ZD6474 (Vandetanib, ZACTIMA™) is an anilinoquinazoline described by Carlomagno et al. [14] as an effective small molecule inhibitor of the transforming ability of RET oncoproteins in vitro and in nude mice and it is currently in phase II clinical trial for treatment of thyroid carcinomas.
Materials and methods
Antibodies and inhibitors
Anti RET (C-19) rabbit polyclonal antibody (Santa Cruz), anti b-catenin monoclonal antibody (clone 2H4A7, Upstate), E-cadherin antibody (Cell Signalling), anti JAK3 (Upstate) and mouse monoclonal anti-phosphotyrosine 4G10 antibody (anti-PY, Upstate). The horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies were obtained from GE Healthcare (Princeton, NJ). ZD6474, RET inhibitor was synthesized by our laboratory according to previously described protocol [15].
Cell lines
The human PTC cell lines TPC1 (expressing RET/PTC1) and NPA (negative for RET expression and carrying a BRAF(V600E) mutation were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% of bovine serum and 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine.
ELISA non-radioactive kinase assay
Recombinant His-tagged RET and ALK kinases were expressed in Baculovirus system and purified by Nickel-affinity chromatography as previously described [16, 17]. NuncImmuno plates (Nunc) were coated with peptide at 2.5 μg/well in 125 μl PBS 1× by incubation overnight at 27° C. Wells were washed with 200 μl of wash buffer (PBS-Tween 0.05%), 80 μl of BSA 4% was added by incubation for 2 h at 27°C, then wells were washed and stored at 4°C.
The kinase reaction was performed by incubating purified recombinant kinase (50 ng/well) in reaction buffer (50 mM Tris pH 7.5, 5 mM MnCl2, 5 mM MgCl2) containing 0.3 mM ATP, in a total volume of 100 μl/well at 27°C for 20 min. When testing inhibitors, the reaction mix was pre-incubated with the inhibitor or solvent control for 10 min at room temperature in a standard 96-well plate before transferring to the ELISA plate. The reaction was stopped by immediate washing. Phosphorylated peptide was detected using a monoclonal anti-phosphotyrosine antibody (4G10, Millipore; 100 μl/well) and a secondary antibody (anti-mouse IgG-HRP linked antibody, GE Healthcare). Then the plate was developed using 100 μl/well TMB (tetramethylbenzidine) Substrate Solution (Endogen) and 0.18 M H2SO4 stop solution. The absorbance was read at 450 nm using an Ultrospec® 300 microtiter plate reader (GE Healthcare). IC50 values were determined by GraphPad Prism software fitting the data using nonlinear regression.
Proliferation assay
Cells were plated (10,000 cells/well) in 96-well plates. At 24, 48, 72, and 96 h after addition of increasing concentration of RET-inhibitors, cells were labelled with 1 μCi/well [3H]thymidine (GE Healthcare) for 8 h. Cells were harvested onto glass fiber filters (Printed Filtermat, PerkinElmer Life Sciences) and [3H]thymidine incorporation was measured using a filter scintillation counter (1430 Micro-Beta, PerkinElmer Life Sciences).
Wound healing assay and cellular morphology
5 × 106 cells were plated in absence of bovine serum in 60 mm dishes, when cells were attached to the plate a wound has been performed using a p200 tip, then RET inhibitors or DMSO as control were added and cells have been visualized under inverted microscope at the T0 and at T18h of treatment.
Cellular morphology was analyzed on plated cells on a glass coverslips after May-Grunwald staining. Treated and untreated cells were then visualized under inverted microscope.
Immunoprecipitation and Western blotting
Cells (2 × 106) were collected by centrifugation, washed once with ice-cold phosphate-buffered saline, and lysed for 30 min in lysis buffer (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, 5 mM EGTA, 1 mM NaVO4, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml of pepstatin-A, leupeptin, and aprotinin). Lysates were clarified by centrifugation at 13,000 g for 15 min at 4 °C. Protein concentration was determined in the supernatant using the Bio-Rad Protein Assay (Bio Rad). Proteins (150 μg) were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. Primary and secondary antibodies were added in accordance to manufactory's instructions. Proteins were visualized by enhanced chemiluminescence (ECL) as recommended by the manufacturer (GE Healthcare). For immunoprecipitation experiment cell lysate were incubated overnight with primary antibody, then A/G beads (Santa Cruz) were added for 4 h and then after washing the unbound proteins were resolved by western blotting.
In vitro cold kinase assay
Different recombinant GST tagged b-catenin mutants, kindly supplied by Dr. A.M.L. Coluccia (National Nanotechnology Laboratory of CNR-INFM, University of Salento, Lecce, Italy) expressed in E.coli were purified using GST-agarose beads (GE Healthcare) according to the manufacturer's instructions. 2 μg of GST tagged β-catenin and 2 μg of recombinant RET kinase domain were incubated for 15 min at 30°C in the presence of ATP 100 μM. Phosphorylated proteins were analysed by SDS-PAGE and western blotting using anti-phosphotyrosine antibody.
Preparation of cytosolic and nuclear extract
Tripsinized cells were lysed in a digitonin buffer (1% digitonin, 150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 10 mM benzamidine-HCl, protease inhibitors). The lysates were centrifuged at 13000 rpm for 10 min, and supernatants representing cytosolic fractions were saved. The pellets representing nuclear components were lysed in RIPA buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS and 50 mM Tris pH 7.5). Same amount of protein was loaded on and analyzed by SDS-PAGE.
Confocal Microscopy analysis
TPC1 cells were cultured on glass coverslips and treated overnight with 5 μM of ZD6474 or DMSO as a control; then they were fixed with 4% paraformaldehyde, and treated briefly with 0,1 M Glycin in PBS (pH 7.4) followed by 0.3% Triton X-100 buffer. Cells were incubated overnight at 4 °C with rabbit anti-RET antibody (c-19, Santa Cruz Biotechnology) and mouse anti b-catenin antibody (BD Biosciences). They were then washed and stained with Alexa Fluor 488-conjugated donkey anti-rabbit (Invitrogen) and rhodamine-conjugated donkey anti-mouse (Rockland, Gilbertsville, PA) secondary antibody for 1 h at RT. Invitrogen TOTO-3 iodide (642/660) was used for nuclei staining. Cover-slips were washed and mounted with 95% glycerol in PBS.
Confocal microscopy was carried out on a Radiance 2100 laser scanning confocal microscope (Biorad Laboratories) equipped with a Krypton/ Argon laser and a red laser diode. In order to reduce bleed-through, confocal images were acquired sequentially. Noise reduction was achieved by “Kalman filtering” during acquisition.
Relative quantification by real-time RT-PCR of β-catenin target gene
Total RNA was isolated from TPC1 cells, cultured in presence or not of 5 μM ZD6474 for 18h, using TRIzol (Invitrogen) and cDNA were prepared using SuperScript II reverse transcriptase (Invitrogen) according to manufacturer's. Real time PCR was carried out using SYBR Green sequence detection reagents (Applied Biosystems) in a 25 μl volume containing l00 ng of cDNA, 12.5 μl of SYBR Green PCR Master Mix (Stratagene) and 0.1 μM each primer. Primers used for real time PCR were: c-Myc Fw 5'-CTG TAT GTG GAC GGC TTC TCG-3'; c-Myc Rev 5'-CTG CTG TCG TTG AGA GGG TAG-3'; cyclin D1 Fw 5'-GCT GGA GCC CGT GAA AAA GA-3'; cyclin D1 Rev 5'-CTC CGC CTC TGG CAT TTT G-3'; GAPDH Fw 5'-TGC ACC ACC AAC TGC TTA GC-3'; GAPDH Rev 5'-GGC ATG GAC TGT GGT CAT GAG-3'. Fluorescent signals were detected using an MxPro-3005 machine (Stratagene). Data analysis was performed normalizing Ct sample values (c-Myc and cyclin D1) on GAPDH values used as control and results of treated cells respect to not-treated TPC1 cells were presented in bar chart.
Results
ZD6474 inhibits RET kinase activity and transforming ability of TPC1 cells
First, we verified the activity of ZD6474 inhibitor (Table 1A) on the recombinant His-tagged RET kinase purified in our laboratory performing an in vitro ELISA non radioactive kinase assay. ZD6474 inhibited RET with an IC50 = 0.097 ± 0.006 μM, while it was much less active on ALK kinase, used as a specificity control (Table 1B). Then, we assessed the inhibitory effect of ZD6474 on different cell lines expressing RET/ PTC translocated forms of the RET kinase. In particular, ZD6474 inhibited proliferation ability of TPC1 cell line, expressing the RET/PTC1 rearranged form (IC50 = 0.1 ± 0.01 μM; Table 1C and Figure 1A) and of BaF3/PTC2 transfected cells expressing RET/PTC2 translocated oncogene (IC50 = 0.25 ± 0.03 μM). In contrast, ZD6474 did not inhibit the proliferation of NPA, a thyroid carcinoma cell line that does not harbour any RET rearrangement, and of non-transfected BaF3 cells used as controls. In addition, ZD6474 showed no effect on Karpas299, an NPM/ALK-transformed cell line (Table 1C). Therefore, TPC1 cell line is a good cellular model to assess the effects of ZD6474 on RET-dependent signaling pathways and in particular RET/β-catenin interaction.
Table 1.
Inhibitory effect of ZD6474 on RET kinase activity. A. Chemical structure of ZD6474 compound. B. In vitro non radioactive ELISA kinase assay on recom-binant purified His-tagged RET kinase domain and GST-ALK kinase domain as negative control. Results are presented as the mean ± S.D. (n = 3) of a single experiment, representative of three independent repeats. C. Inhibition of the proliferation ability in cells expressing different forms of RET (TPC1 with RET/PTC1 and BaF3 transfected cells with RET/PTC2) compared to negative controls. Results are presented as the mean ± S.D. (n = 6) of a single experiment, representative of three independent repeats.
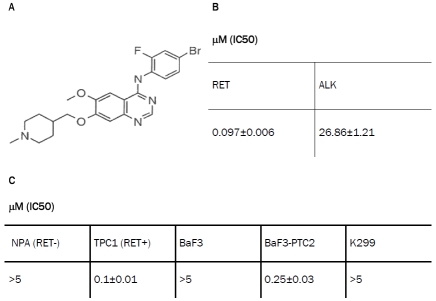 |
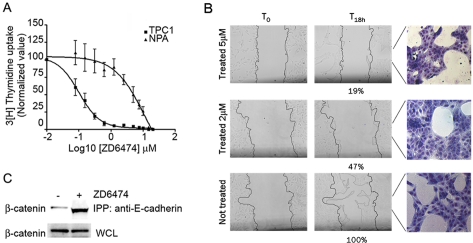
Figure 1.
ZD6474 affects the TPC1 cellular mobility and morphology regulating β-catenin distribution. A. Proliferation assay to determine ZD6474 inhibitor concentration for TPC1 cells compared to NPA cells used as negative control. Results are presented as the mean ± S.D. (n = 6) of a single experiment, representative of three independent repeats. B. Wound healing assay performed on TPC1 cells after treatment with 5 mM of ZD6474 or DMSO as control; percentages of migration are indicated below corresponding slide. On right treated and not-treated samples were stained with Giemsa in order to underline cellular morphology. Cellular samples were observed under optical microscope with a 10X objective. C. Immunoprecipitation with anti-E-cadherin antibody showing that major amount of β-catenin co-immunoprecipitated in TPC1 cells treated with 5 mM of ZD6474.
ZD6474 affects TPC1 cellular mobility and morphology by inhibiting nuclear localization of β-catenin
A wound healing assay was performed with TPC1 cells in the presence of 5 μM ZD6474, a concentration that assures complete suppression of TPC1 proliferation during the assay (Figure 1A), and a lower ZD6474 concentration (2 μM). TPC1 cells treated for 18 h with both 5 and 2 μM of ZD6474 displayed reduced cellular mobility compared to the untreated cells (Figure 1B, T18 h). In particular, the number of ZD6474 -treated cells that moved into the region of the wound was reduced from 100% to 19% at 5 μM ZD6474 and to 47% at 2 μM ZD6474 compared to non-treated sample (Figure 1B). Moreover, inhibitor treatment modified cellular morphology, as shown in the corresponding May-Grünwald staining (Figure 1B, on the right), in which treated cells appeared to be packed together with reduced branching formation ability that was evident in non-treated ones.
Changes in morphology and mobility suggested an involvement of adhesion molecules such as E-cadherin and β-catenin, therefore we performed an immunoprecipitation assay using anti E-cadherin antibody and then western blotting analysis with anti β-catenin antibody. Cells treated with ZD6474 compound displayed a higher amount of β-catenin bound to E-cadherin compared to the untreated control (Figure 1C). This result suggested that ZD6474 can influence β-catenin subcellular localization.
In order to assess this hypothesis we performed a confocal microscopy analysis to study β-catenin and RET/PTC cellular distribution (Figure 2A). While β-catenin showed a nuclear localization in untreated cells, it was mainly located in the cytosol and on the plasma membrane in cells treated with ZD6474 (Figure 2A left panels); while RET/PTC did not change its cellular distribution (Figure 2A middle panels).
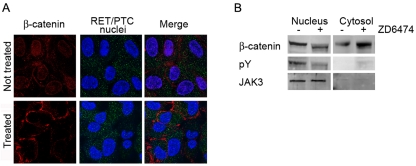
Figure 2.
ZD6474 affects the β-catenin cellular localization. A. Confocal microscopy analysis showing that TPC1 cells treated with 5 mM ZD6474 display cyto/membrane localization respect to nuclear localization of the untreated cells. Cellular samples were observed under confocal microscope with a 60X objective (zoom 2.5X). B. Cyto-nuclear extracts display that in the treated TPC1 cells β-catenin is predominantly in the cytosol, and not in the nucleus as in the untreated ones.
We confirmed confocal microscopy results by performing a western blotting analysis of sub-cellular fraction extracts. In this case, we observed that in the treated sample β-catenin was predominantly localized in the cytosol, while in the untreated one β-catenin displayed more nuclear localization (Figure 2B, first panel). Moreover, we evaluated tyrosine phosphorylation level of β-catenin in both extracts observing that only nuclear β-catenin was phosphorylated (Figure 2B middle panel). The purity of cellular extracts was controlled by western blotting analysis with JAK3 antibody that was predominantly in the nucleus (Figure 2B, nuclear extracts third panel).
ZD6474 inhibitor effect does not disrupt physical interaction between RET and β-catenin in TPC1 cells
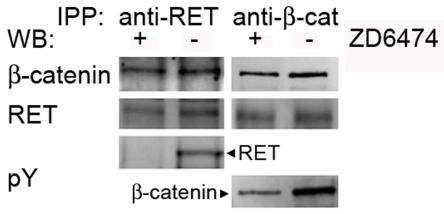
In order to determine the effect of ZD6474 on the physical interaction between RET and β-catenin, we performed a co-immunoprecipitation assay after treating cells with or without 5 μ, M of ZD6474 for 18 hours. Surprisingly, β-catenin and RET co-immunoprecipitated regardless of RET inhibition by ZD6474 (Figure 3). We verified the inhibitory effect of ZD6474 by analyzing tyrosine phosphorylation of RET and β-catenin, which was significantly diminished upon inhibitor treatment (Figure 3). Therefore ZD6474 inhibits RET activity and tyrosine phosphorylation, leading to a reduction of β-catenin tyrosine phosphorylation. However, the binding between the two proteins is not disrupted in TPC1 cells.
Figure 3.
ZD6474 inhibitor does not interfere with RET/β-catenin binding. Co-immunoprecipitation assay was performed on TPC1 cells treated and not treated with 5 mM of ZD6474, using anti-RET and anti-β-catenin antibodies and same antibodies for respectively western blotting as shown in the first and second panel. Western blotting analysis using anti-phosphotyrosine (pY) antibody was displayed in third and fourth panel respectively for RET and β-catenin.
Recombinant RET preferentially phosphorylates tyrosine-654 of GST-tagged β-catenin
Altogether, our results for the first time suggested a physical interaction between RET and β-catenin proteins in TPC1 cells, that is not affected by inhibition of RET catalytic activity. At this point, we asked which tyrosine residues could be directly phosphorylated by RET kinase. First, we systematically mutated tyrosine residues in GST-tagged β-catenin; in particular Y86, Y142 and Y654, which play an important role in β-catenin phosphorylation-mediated stability, were changed to phenylalanine [18]. We generated single and double mutants. Then we performed an in vitro cold kinase assay incubating recombinant RET kinase with WT and different mutants of GST- tagged β-catenin in the presence or absence of ATP and cofactors. Western blotting analysis of the kinase reaction products was then performed using anti-phosphotyrosine antibody (Figure 4 upper panel). In order to perform a quantitative evaluation of this result a densitometry analysis was performed normalizing signals on loading control. Interestingly, we observed a reduced level of tyrosine phosphorylation in the Y654F single mutant and Y86F-Y654F double mutant, about 25% and 30% respectively, compared to WT and other mutants, indicating that Y654 tyrosine residue may be preferentially phosphorylated by RET kinase (Figure 4 lower panel).
Figure 4.
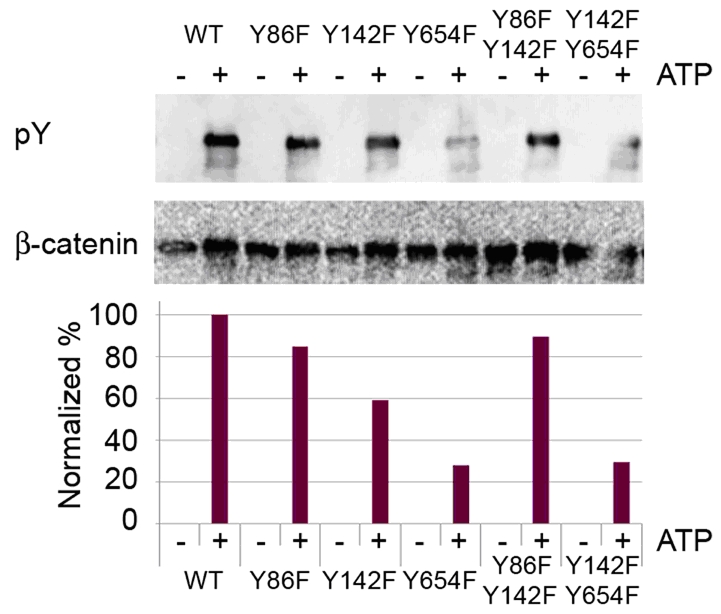
RET preferentially phosphorylates Y654 tyrosine residue of β-catenin. Cold non radioactive kinase assay was performed using 2 μg of each GST tagged β-catenin purified mutated proteins and recombinant His-RET in presence or not of ATP 100 mM. Western blotting analysis was performed using anti-phosphotyrosine (pY) antibody and anti β-catenin for loading control. Densitometry analysis was also performed using Image J program normalizing values considering corresponding loading and WT.
Effects of ZD6474 inhibitor on the RET/β-catenin interaction and signals
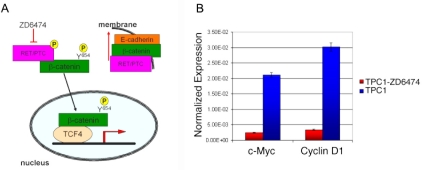
We present here a model of the ZD6474 effect on the interaction among RET, β-catenin and E-cadherin. In non-treated cells part of β-catenin may be phosphorylated on Y654 and stabilized by RET/PTC kinase, thus being able to migrate into the nucleus and turn target genes transcription on. When RET/PTC-expressing cells are treated with ZD6474 inhibitor, RET/PTC is inactivated, β-catenin does not enter the nucleus and is recruited by E-cadherin to the membrane, thus causing a change in morphology and migration ability of the cells (Figure 5A). Moreover, β-catenin target genes are shut off. Indeed, realtime PCR analysis showed suppression of c-Myc and cyclin D1 expression by ZD6474, in contrast to the expression of Jun B that is not a target of β-catenin and was unaltered (Figure 5B).
Figure 5.
Model describing the effect of ZD6474 inhibitor on RET/β-catenin interaction. A. When Ret/PTC expressing cells are treated with ZD6474 inhibitor, Ret/PTC is inactivated, therefore more β-catenin can be recruited by E-cadherin to the membrane changing morphology and migration ability of cells. B. Real Time PCR analysis of normalized expression of c-Myc and cyclin D1, β-catenin target genes in TPC1 cells treated with 5 mM ZD6474 (red) and not-treated cells (blue). Jun B, that is not a β-catenin target gene, was used as control. Results are normalized to endogenous GAPDH expression used as control and are presented as the mean ± S.D. (n = 3) of a single experiment, representative of three independent repeats.
Discussion
Papillary thyroid carcinomas are characterized by different fusion proteins generated by chromosomal rearrangements that fuse the intracellular kinase domain of RET to various heterologous gene partners. The RET/PTC1 rearrangement, involving the H4 (D10S170) fusion partner, is one of the most frequently detected in PTCs. Approximately 90% of RET/PTC1-positive papillary thyroid tumors have lost the expression of the normal H4 (D10S170) allele. This might result in a growth advantage in tumor progression [5, 19, 20].
β-Catenin plays an important role in the canonical Wnt pathway where it is tightly regulated by serine-threonine phosphorylation [7-9, 21]. In addition, β-catenin has a structural function at cell-cell adhesion sites, acting as a bridge between the cytoplasmic domain of cadherins and the actin-containing cytoskeleton [22, 23]. β-Catenin complex with the cytoplasmic tail of E-cadherin is mostly regulated by phosphorylation of the Y654 tyrosine residue, which induces β-catenin dissociation from E-cadherin [24].
Gujral et al. published a study focused on multiple endocrine neoplasia type 2 (MEN2) and sporadic thyroid carcinoma demonstrating that nuclear localization of β-catenin was frequent in thyroid tumours and their metastases from MEN2 patients and also that down-regulation of β-catenin activity decreased RET-mediated cellular proliferation, colony formation and tumor growth in nude mice. In this study for the first time a close relationship between RET and β-catenin proteins was described [11]. Additional studies described RET and β-catenin pathways in PTCs [12, 13]. However, the effects of RET inhibition on β-catenin function have not yet been investigated. Here we focused our attention on the inhibition of RET/PTC1 kinase activity using ZD6474, a potent RET inhibitor that is being tested in clinical trials for the treatment of thyroid cancer [14, 25, 26].
First, we verified the inhibitory effect of RET kinase activity using purified recombinant His-tagged RET-kinase domain and then in order to validate our model we performed proliferation assay on TPC1 cell line and other cell types. Our results confirmed that ZD6474 compound was able to inhibit RET kinase activity and to selectively block RET-dependent proliferation in TPC1 thyroid cancer cell line derived from papillary thyroid cancer (Table 1).
Next, we assessed the effects of RET inhibitor on TPC1 cells motility and morphology (Figure 1B). In order to be sure that cellular proliferation was totally inhibited during the wound healing assay, we used 5 μM of ZD6474 compound, as demonstrated by the proliferation assay (Figure 1A). Anyway we observed similar results using 2 μM of ZD6474 (Figure 1B). The effect caused by ZD6474 RET inhibitor suggested for the first time a direct involvement of E-cadherin and β-catenin [24]. Therefore, we performed immunoprecipitation assay to show that, in cells treated with the inhibitor, more β-catenin was bound to E-cadherin compared to untreated cells (Figure 1C). Moreover, we performed confocal microscopy analysis and cell fractionation to verify the effect of ZD6474 on β-catenin cellular localization (Figure 2). We observed that cells treated with inhibitor displayed a marked cytosolic and membranous β-catenin distribution, suggesting that RET/PTC1 inhibition might cause destabilization of β-catenin that is no longer able to migrate into the nucleus. Consequently, more β-catenin is available to be recruited to the membrane by E-cadherin. Interestingly, our results showed that ZD6474 inhibited RET kinase activity without interfering with the RET/ β-catenin binding, suggesting that this interaction is independent of RET activity.
In order to assess which tyrosine residues of β-catenin are phosphorylated by RET kinase, we mutagenized three tyrosine residues of a GST-tagged β-catenin protein, generating single and double mutants, and run an in vitro kinase assay using recombinant His-tagged RET kinase domain. Western blotting analysis supported by densitometry analysis showed that Y654 tyrosine residue was directly phosphorylated by RET kinase.
Altogether our results suggest that in the absence of RET inhibitor, β-catenin phosphorylation on Y654 by RET/PTC kinase leads to β-catenin migration into the nucleus and activation of target genes (Figure 5A). When RET/PTC is inactivated by ZD6474, the lack Y654 phosphorylation causes β-catenin recruitment by E-cadherin to the membrane. This β-catenin accumulation to the membrane triggers a change in the cells concerning morphology and migration ability. Moreover, as shown by real-time PCR analysis of c-Myc and cyclin D1 expression, β-catenin target genes are dowregulated in ZD6474-treated cells, confirming loss of β-catenin transcriptional activity in these cells (Figure 5B). Surprisingly, however, ZD6474 slightly reduces but does not abolish RET/ β-catenin interaction. This indicates that RET catalytic activity is not required for RET/β-catenin association.
Investigating the effects of ZD6474, as a RET inhibitor, has improved our knowledge of direct interaction between RET, β-catenin and E-cadherin proteins. We demonstrated that ZD6474 interferes with β-catenin phosphorylation and localization without affecting its binding to RET.
Acknowledgments
We are grateful to Dr. Addolorata Maria Luce Coluccia (National Nanotechnology Laboratory of CNR-INFM, University of Salento, Lecce, Italy) for different recombinant GST tagged β-catenin mutants expressed in E.coli.
This sdudy was supported by PRIN-MIUR; Ministry of Health; Italian Association for Cancer Research (AIRC-IG-4637) and Cariplo Foundation.
References
- 1.Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005;16:441–467. doi: 10.1016/j.cytogfr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Pierotti MA, Vigneri P, Bongarzone I. Rearrangements of RET and NTRK1 tyrosine kinase receptors in papillary thyroid carcinomas. Recent Results Cancer Res. 1998;154:237–247. doi: 10.1007/978-3-642-46870-4_15. [DOI] [PubMed] [Google Scholar]
- 3.Porter AC, Vaillancourt RR. Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene. 1998;17:1343–1352. doi: 10.1038/sj.onc.1202171. [DOI] [PubMed] [Google Scholar]
- 4.Merolla F, Pentimalli F, Pacelli R, Vecchio G, Fusco A, Grieco M, Celetti A. Involvement of H4(D10S170) protein in ATM-dependent response to DNA damage. Oncogene. 2007;26:6167–6175. doi: 10.1038/sj.onc.1210446. [DOI] [PubMed] [Google Scholar]
- 5.Celetti A, Cerrato A, Merolla F, Vitagliano D, Vecchio G, Grieco M. H4(D10S170), a gene frequently rearranged with RET in papillary thyroid carcinomas: functional characterization. Oncogene. 2004;23:109–121. doi: 10.1038/sj.onc.1206981. [DOI] [PubMed] [Google Scholar]
- 6.Alberti L, Carniti C, Miranda C, Roccato E, Pierotti MA. RET and NTRK1 proto-oncogenes in human diseases. J Cell Physiol. 2003;195:168–186. doi: 10.1002/jcp.10252. [DOI] [PubMed] [Google Scholar]
- 7.Helmbrecht K, Kispert A, von Wasielewski R, Brabant G. Identification of a Wnt/beta-catenin signaling pathway in human thyroid cells. Endocrinology. 2001;142:5261–5266. doi: 10.1210/endo.142.12.8554. [DOI] [PubMed] [Google Scholar]
- 8.Moon RT. Wnt/beta-catenin pathway. Sci STKE. 2005;2005 doi: 10.1126/stke.2712005cm1. cm1. [DOI] [PubMed] [Google Scholar]
- 9.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Barker N. The canonical Wnt/beta-catenin signalling pathway. Methods Mol Biol. 2008;468:5–15. doi: 10.1007/978-1-59745-249-6_1. [DOI] [PubMed] [Google Scholar]
- 11.Gujral TS, van Veelen W, Richardson DS, Myers SM, Meens JA, Acton DS, Dunach M, Elliott BE, Hoppener JW, Mulligan LM. A novel RET kinase-beta-catenin signaling pathway contributes to tumorigenesis in thyroid carcinoma. Cancer Res. 2008;68:1338–1346. doi: 10.1158/0008-5472.CAN-07-6052. [DOI] [PubMed] [Google Scholar]
- 12.Cassinelli G, Favini E, Degl'Innocenti D, Salvi A, De Petro G, Pierotti MA, Zunino F, Borrello MG, Lanzi C. RET/PTC1-driven neoplastic transformation and proinvasive phenotype of human thyrocytes involve Met induction and betacatenin nuclear translocation. Neoplasia. 2009;11:10–21. doi: 10.1593/neo.08916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellone MD, De Falco V, Rao DM, Bellelli R, Muthu M, Basolo F, Fusco A, Gutkind JS, Santoro M. The beta-catenin axis integrates multiple signals downstream from RET/papillary thyroid carcinoma leading to cell proliferation. Cancer Res. 2009;69:1867–1876. doi: 10.1158/0008-5472.CAN-08-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlomagno F, Vitagliano D, Guida T, Ciardiello F, Tortora G, Vecchio G, Ryan AJ, Fontanini G, Fusco A, Santoro M. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002;62:7284–7290. [PubMed] [Google Scholar]
- 15.Hennequin LF, Stokes ES, Thomas AP, Johnstone C, Ple PA, Ogilvie DJ, Dukes M, Wedge SR, Kendrew J, Curwen JO. Novel 4- anilinoquinazolines with C-7 basic side chains: design and structure activity relationship of a series of potent, orally active, VEGF receptor tyrosine kinase inhibitors. J Med Chem. 2002;45:1300–1312. doi: 10.1021/jm011022e. [DOI] [PubMed] [Google Scholar]
- 16.Mologni L, Sala E, Riva B, Cesaro L, Cazzaniga S, Redaelli S, Marin O, Pasquato N, Donella-Deana A, Gambacorti-Passerini C. Expression, purification, and inhibition of human RET tyrosine kinase. Protein Expr Purif. 2005;41:177–185. doi: 10.1016/j.pep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Gunby RH, Tartari CJ, Porchia F, Donella-Deana A, Scapozza L, Gambacorti-Passerini C. An enzyme-linked immunosorbent assay to screen for inhibitors of the oncogenic anaplastic lymphoma kinase. Haematologica. 2005;90:988–990. [PubMed] [Google Scholar]
- 18.Tominaga J, Fukunaga Y, Abelardo E, Nagafuchi A. Defining the function of beta-catenin tyrosine phosphorylation in cadherin-mediated cell-cell adhesion. Genes Cells. 2008;13:67–77. doi: 10.1111/j.1365-2443.2007.01149.x. [DOI] [PubMed] [Google Scholar]
- 19.Lanzi C, Cassinelli G, Nicolini V, Zunino F. Targeting RET for thyroid cancer therapy. Biochem Pharmacol. 2009;77:297–309. doi: 10.1016/j.bcp.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Grieco M, Cerrato A, Santoro M, Fusco A, Melillo RM, Vecchio G. Cloning and characterization of H4 (D10S170), a gene involved in RET rearrangements in vivo. Oncogene. 1994;9:2531–2535. [PubMed] [Google Scholar]
- 21.Akiyama T. Wnt/beta-catenin signaling. Cytokine Growth Factor Rev. 2000;11:273–282. doi: 10.1016/s1359-6101(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 22.Aberle H, Schwartz H, Kemler R. Cadherincatenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 23.Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107(Pt 12):3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- 24.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 25.Morabito A, Piccirillo MC, Falasconi F, De Feo G, Del Giudice A, Bryce J, Di Maio M, De Maio E, Normanno N, Perrone F. Vandetanib (ZD6474), a dual inhibitor of vascular endothelial growth factor receptor (VEGFR) and epidermal growth factor receptor (EGFR) tyrosine kinases: current status and future directions. Oncologist. 2009;14:378–390. doi: 10.1634/theoncologist.2008-0261. [DOI] [PubMed] [Google Scholar]
- 26.Sherman SI. Early clinical studies of novel therapies for thyroid cancers. Endocrinol Metab Clin North Am. 2008;37:511–524. doi: 10.1016/j.ecl.2008.02.005. xi. [DOI] [PubMed] [Google Scholar]