Abstract
Despite their long recognised pivotal roles in immunological responses, Janus kinases (JAKs) and signal transducers and activators of transcription (STATs) are now seen as important players in cancer development and progression. Indeed, mutations in the JAKs are often found in myeloproliferative disorders (MPDs) and leukaemia, and the constitutive phosphorylation of STATs is a common occurrence in many solid and blood cancer cell lines and primary tumour specimens. More recently, we have also shown that JAKs likely have additional roles in promoting drug resistance in several cancer cell types. JAKs and STATs are thus molecules that may serve as useful targets in the clinic. This review will summarise studies that support this notion.
Keywords: JAK, STAT, cytokines, growth factors, lung cancer, apoptosis
Janus kinase (JAK)/ signal transducer and activator of transcription (STAT) signalling
Janus kinases (JAKs) and signal transducers and activators of transcription (STATs) are essential molecules in cytokine signal transduction pathways, as shown by studies using mutant cell lines, knockout mice and humans with somatic mutations in these genes (reviewed in [1, 2]). The JAKs are commonly found associated with the intracellular domains of cytokine receptors providing the enzymatic activity that they do not possess intrinsically. Following the binding of a given cytokine to their cognate receptor JAKs auto- and/or trans-phosphorylate. These activating events lead to the phosphorylation of the receptor per se and thereby the generation of STAT docking sites. STATs get recruited to the receptor, undergo phosphorylation, dimerise and translocate into the nucleus where they initiate the transcription of target genes (Figure 1) (reviewed in [3]). They can also be phosphorylated, and presumably activated, downstream of several growth factors and onco-proteins [4].
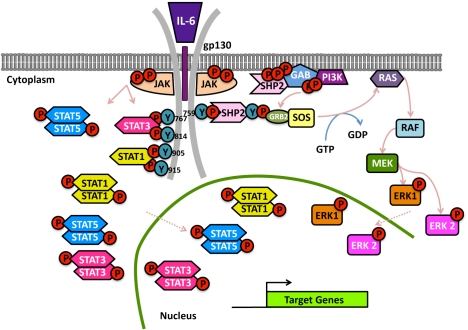
Figure 1.
Representation of JAK/STAT signal transduction pathways using IL-6 signalling as a paradigm. Ligand (IL-6) binding to the ligand binding unit (IL-6R) induces auto- and trans-phosphorylation of receptor pre-associated JAKs (JAK1, JAK2 and TYK2), phosphorylation of tyrosine motifs in the receptor signalling subunit (gp130), recruitment and re-arrangement of associated STATs (STAT1, STAT3 and, potentially, STAT5) which, upon tyrosine phosphorylation by the JAKs, are released, migrate to the nucleus and activate transcription. MAPKs are also activated via recruitment of SHP2/PTPN11 to tyrosine (Y) at position 759 on gp130, which leads to the transcription of additional target genes.
JAKs and STATs in normal homeostasis
Mammalian cells can express four different JAKs (JAK1, JAK2, JAK3 and TYK2) and seven STATs (STAT1-STAT6, including STAT5A and STAT5B). Some STATs have different splicing variants (STAT1, STAT3 and STAT4). These are found in three chromosomal clusters and can also arise post-translationally after, for example, proteolytic processing (STAT5A and STAT5B) (reviewed in [5]). STATs are 750-850 amino acids long and are ubiquitously expressed except for STAT4, which is restricted to myeloid cells, thymus and testis [6]. Their activity is regulated not only through tyrosine but also serine phosphorylation [7-9]. The structure of these molecules is depicted in Figure 2. Of note: the well-conserved SH2 domains are responsible for the association of STAT with tyrosine-phosphorylated motifs in the receptor [10] and for dimerisation with other tyrosine phosphorylated STATs [11, 12]. STAT1 and STAT3 are able to form homo- and heterodimers, as well as tetramers. Near the C-terminus is the transactivation domain (TAD). This domain has a serine residue (amino acids 727 in STAT1 and STAT3) that is necessary for maximal transcriptional activation of some genes [8, 11, 13-15]. Untreated cells have STAT1 and STAT3 randomly distributed in cytoplasm and nucleus [16]. After a few minutes of cytokine treatment, however, they become tyrosine phosphorylated in the cytoplasm and translocate to the nucleus [17]. In reality, it is not such a black and white picture and we now know that unphosphorylated STATs can also move into the nucleus and have biological impact (e.g. [18, 19] and reviewed in [3, 19]). When in the nucleus STATs can bind to specific sequences in the DNA and induce the transcription of many genes, globally termed as Interferon (IFN)-stimulated genes (ISGs) [2, 20]. Such genes are involved in diverse biological processes such as cell differentiation, proliferation and cell death.
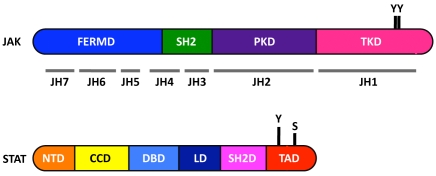
Figure 2.
Structure of JAKs and STATs. JAKs have 7 JAK-homology domains, named JH1-JH7. These form four main regions: the four-point-one, ezrin, radixin and moesin (FERM) domain (blue), the potential SRC homology 2 (SH2) domain (green), and the pseudokinase (purple) and tyrosine kinase domains (magenta). STATs have 6 domains, named N-terminal domain (orange), coil-coiled domain (yellow), DNA binding domain (turquoise), linker domain (navy), SH2 domain (pink) and transcriptional activation domain (red). Key phosphorylation tyrosine (Y) and serine (S) C-terminus residues are also depicted. (Not drawn to scale).
Different cytokines activate distinct sets of JAKs and STATs and, consequently, have the potential to induce different gene expression programmes. Typically, type I IFNs activate JAK1 and TYK2 and induce transcription via a protein complex that is composed of STAT1, STAT2 and IFN-regulatory factor (IRF)-9 (also known as p48). Type II IFN (IFN-γ) leads to the activation of JAK1 and JAK2 and to the formation of STAT1 dimers. These different STAT complexes bind to different DNA consensus sequences and induce distinct sets of genes (with some overlap) [3, 17]. Other cytokines, such as those belonging to the interleukin (IL)-6 family, trigger the phosphorylation of JAK1, JAK2 and TYK2, the accumulation of STAT3 dimers and thus the transcription of STAT3 target genes (Figure 1). STAT1 and STAT5 can also be phosphorylated downstream of IL-6 but the significance of such “activation” is as yet unclear [21, 22]. STAT5 is usually triggered by ligands such as erythropoietin and prolactin and is thus involved in erythropoiesis, breast development and lactation [23]. STAT4 and STAT6 are activated by IL-12 and IL-4, respectively, and are therefore key molecules in regulating T helper cell (TH) 1 and 2 responses [24].
Studies using knockout mice and observations made in patients have largely confirmed initial work in cell lines generated by the Kerr and Stark groups, which were deficient in JAK/ STAT signalling components [25]. STAT1-deficient humans and mice are more susceptible to both viral and bacterial infections [26, 27]. STAT2-deficient mice are also more susceptible to viruses and, unsurprisingly, have defects in type I IFN responses [28]. STAT3 knockout mice are embryonic lethal but conditional knockouts have revealed essential roles for the acute-phase response in the liver and the control of IL-6 and IL-10 cytokine responses [22, 29, 30]. Mice that lack STAT4 have most IL-12-mediated responses severely impaired, including the induction of IFN-γ which is important to determine a TH1 response [31, 32]. IL-4, a TH2 cytokine, and IL-13 trigger STAT6 activation and, accordingly, STAT6-deficient animals lack a TH2 response [33, 34] and have defective IL-13 responses [35], respectively. Many cytokines and growth factors can lead to activation of STAT5A and STAT5B. Amongst these are erythropoietin, growth hormone, thrombopoietin, IL-2, IL-3, IL-5, IL-7, IL-9, IL-15 and GM-CSF (reviewed in [36]). STAT5A-deficient animals have pinpointed important roles in mammary gland development and lactation, whereas STAT5B-null animals suggest an important role in growth hormone biology.
Regulation of JAK/ STAT signalling and the consequences of dysregulated activation
Under normal conditions, STATs are active for minutes up to a few hours as these molecules are rapidly down-regulated. STAT activity can be inhibited by phosphatases [37-39], suppressors of cytokine signalling (SOCS, also named JAK-binding proteins and STAT-induced STAT inhibitors) ([40-43] and reviewed in [44]) and protein inhibitors of activated STATs (PIAS) ([45-48] and reviewed in [49]). The SOCS family has 8 members: SOCS1-7 and CIS. These molecules are characterized by an SH2 central domain and by a SOCS box. SOCS can act by distinct mechanisms: they can bind phosphorylated tyrosine residues on receptor chain and JAKs, or bind to STATs and block their recruitment to the ligand. In addition, the SOCS box can target STATs to degradation as it has E3 ubiquitin ligase activity [50]. STATs can also be inactivated directly by protein inhibitors of activated STAT proteins (PIAS) [45, 46]. PIAS can act on different levels: they can bind directly to STATs, thereby blocking protein-DNA interactions; induce degradation of the STATs, or alter STATs localisation [49]. The negative regulatory mechanisms controlling JAK/ STAT signalling thus ensure that STAT activation is cyclic and transient (further reviewed in [3]).
JAK deficiency can have severe consequences for an organism, as demonstrated by syndromes such as severe combined immunodeficiency (SCID) and autosomal recessive hyperimmunoglobulin E [51]. On the other hand, JAKs are often found mutated in myeloproliferative disorders (MPDs) and in many leukaemias. The most common mutation occurs on JAK2 on a valine residue on position 617, which is located on the regulatory pseudokinase domain (Figure 2). This mutation leads to constitutive activation of the kinase and thus constitutive phosphorylation (and, presumably, activation) of STAT3 and STAT5 [52-54]. These observations raise the possibility that JAK kinase inhibitors might prove useful in the management of MPDs and leukaemias bearing activating JAK mutations and several such inhibitors are currently being evaluated in clinical trials.
Interestingly, we have unravelled an additional role(s) for JAKs in drug resistance induced by signalling in response to fibroblast growth factor (FGF)-2 [55]. Many cancer patients have elevated levels of FGF-2 in their blood, which indicates a poor prognosis on univariate and multi-variate analyses [56-58]. In fact, FGF-2 is a potent mitogen and one of the many molecules capable of inducing drug resistance in cancer cells challenged with chemotherapeutic drugs [59-61]. Signalling through the FGF receptor triggers mitogen activated protein kinases (MAPKs) and leads to the assembly of a multi-protein complex, which contains protein kinase C (PKC) ε, v-raf murine sarcoma viral oncogene homologue (B-RAF) and p70 S6 kinase b (S6K2). As a consequence, the translation of a variety of anti-apoptotic genes is upregulated [60, 62, 63]. Such anti-apoptotic molecules thus arm the cell against the noxious effects of chemotherapeutic agents. As several growth factors including FGF-2 can activate JAK/ STAT signalling we asked if JAKs and/or STATs might contribute to this novel chemoresistance mechanism. We have discovered that FGF-2 induced phosphorylation of JAKs and their association with the multi-protein complex. Interestingly, downregulation of JAK1, JAK2 or TYK2 expression was sufficient to block FGF-2 survival signals thus leading to cell death of osteosarcoma cells in response to cisplatin [55]. However, to our surprise, silencing of STAT1, STAT3 or STAT5A/B did not impair FGF-2-mediated drug resistance. These observations provide an example of JAK signalling that is independent of STATs. A key question though is whether the effects seen are dependent on JAK kinase activity and/or structure. On-going work with selective JAK kinase inhibitors suggests that the kinase domain is important for transmitting FGF-2-induced drug resistance effects. It is therefore conceivable that JAK inhibitors could also be used to help reverse this form of multi-drug resistance which we have now found to be present in several common cancer types ([55, 61] and our unpublished data).
Unlike the JAKs, STATs are not found mutated in cancer cells but are inappropriately activated, presumably as a consequence of dysregulated cytokine/ growth factor signalling [4]. In particular, STAT3 and STAT5 are constitutively phos-phorylated not just in multiple cancer cell lines but also in many tumour specimens [64-68] As these molecules regulate the transcription of many genes that positively control cell proliferation and cell migration, as well as genes that negatively regulate apoptosis and immune recognition, they may participate directly in tumourigenesis [69]. Indeed, genes such as vascular endothelial growth factor (VEGF), c-MYC, BCL-xL, MCL-1, cyclin D1, JUNB, chemokines, proteases such as uPA and uPAR and, paradoxically, p21WAF1/CIP1, are known transcriptional targets of STAT3 ([70, 71] and reviewed in [72]). This transcriptional profile then explains why STATs have such a wide biological impact, with far-reaching consequences. Importantly, whereas STAT3 is a well-documented oncogene [73], STAT1 has long been seen as a tumour suppressor gene, capable of modulating the immune system and, under certain circumstances, directing it against tumour cells [74]. More recent reports, however, suggest that STAT1 is a double-edged sword and that it can also promote tumourigenesis [75].
Constitutive activation of STATs can be seen downstream of many receptor tyrosine kinases (RTKs), such as epidermal growth factor (EGF), hepatocyte growth factor (HGF) and platelet-derived growth factor (PDGF) receptors [72] and downstream of non-RTKs such as ABL [76] and SRC [77]. Interestingly, v-SRC transformation requires STAT3 tyrosine phosphorylation [77]. However, this is not true for all downstream oncogenes. Indeed, RAS requires the presence of unphosphorylated STAT3 in mitochondria [18], which shifts the cell metabolism towards fermentation in a manner that favours tumour cell growth. This raises interesting questions regarding the successful use of STAT3 inhibitors in the clinic [78, 79]. Indeed, it may be insufficient to simply interfere with phosphorylated, mainly nuclear-localised, STAT3 as this will not impair the effects of unphosphorylated STAT3 in mitochondria. Nevertheless, inhibiting molecules such as STAT3 remain worthwhile approaches in cancer treatment and the increasing understanding of how these molecules operate and are regulated will hopefully enable this soon.
JAK/ STAT signalling in lung cancer
Lung cancer, one of our research interests, causes more deaths per year than any other type of cancer in men [80, 81] and it is the second cause of death in women after breast cancer [82]. The classification of human lung cancer includes two major types: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC includes several sub-types of which adenocarcinoma is the most common [83]. Smoking is responsible for 80-85% of all lung cancer [83]. In 2008 about 1.52 million people around the world were diagnosed with lung cancer and approximately 1.31 million people died from the disease [81]. Lung cancer symptoms are detected in most patients too late [80, 81], which together with lack of effective treatments contribute to a poor prognosis for most patients. Indeed, regional or distant overt metastases are found in more than two-thirds of patients [81] and most of the remaining individuals will have occult metastatic disease. Consequently, most patients are ineligible for curative surgery and, as the remaining radiotherapy, chemotherapy and newer targeted treatment options are non-curative, novel therapeutic approaches and early disease markers are needed [81].
Cytokine responses are highly regulated and often transient. In cancer cells, however, cytokines responses can be dysregulated. For example, IL-6 secretion is often increased in cancer patients and in many cancer cell lines [84]. This can trigger dysregulation of several signalling components and like EGF it can lead to constitutively activated STAT3. STAT3 phosphorylation is, in fact, observed in around 50% primary NSCLC tumours and cancer cells lines derived from them [84-87]. This likely reflects mutations in EGF receptor, increased circulating IL-6, or activation of non-RTKs as discussed earlier.
The main transcription factor induced by IL-6-type cytokines, such as IL-6, oncostatin M (OSM) and leukaemia inhibitory factor (LIF), is STAT3. IL-6 is a pleiotropic cytokine that plays important roles in cell proliferation, differentiation, survival and apoptosis. IL-6 also takes part in immune responses, haematopoiesis and inflammation (reviewed in [21, 88]). In addition, IL-6 can control a variety of responses in many cell types and is a crucial regulator of the nervous system, endocrine system, bone metabolism, amongst others [89]. IL-6 induces transcription of the IL-6 gene via JAK2 and STAT3. This is thought to lead to increased autocrine production of this cytokine observed in different cancer cell lines. Thus, regulating JAK2 and/ or STAT3 could reduce IL-6 production, thereby impairing cell growth and enhancing their susceptiblity to other treatments [90]. Indeed, blockade of IL-6 signalling in lung cancer-derived cell lines was shown to be enough to inhibit cell growth [84, 91]. It was also shown that some tumours in mice are induced by ras, which can also stimulate secretion of IL-6 in different cell types. Ras-induced transformation in a variety of mouse models appears to require IL-6 and, consistent with that, IL-6 knockout animals were more resistant to ras-induced carcinogenesis ([92] and reviewed in [93]). Accordingly, knockdown, genetic ablation or antibody neutralization of IL-6 can limit tumour growth induced by ras [94]. Interestingly, some human lung adenocarcinomas also have RAS mutated [95]. Paradoxically, IL-6 can under certain circumstances decrease cell growth in some types of lung cancer cells [96]. For example, the growth of Lewis lung cancer carcinoma cells decreased after being transfected with IL-6 [97]. When these cells were treated with an anti -IL-6 antibody they did not proliferate, indicating that growth inhibition was not related to a direct autocrine effect of IL-6 [97]. In contrast, in other NSCLC cell lines, IL-6 caused an increase in growth (A549, Calu3, Calu6, and H23). In the presence of IL-6 antisense phosphorothioated oligonucleotides, cell proliferation was notably reduced. However, neither the presence of monoclonal neutralizing anti-IL-6 antibodies, nor exogenous IL-6, interfered with cell proliferation, or IL-6 synthesis. This probably reflects the now widely recognized importance of cellular background in determining cellular responses and biological outcome [22, 98]. Certainly in lung cancer patients, IL-6 appears to promote and sustain malignancy [71, 99-101]. For example, IL-6 has been found elevated in lung cancer patients and the autocrine production of IL-6 has been shown to lead to constitutive activation of STAT3 and promote lung adenocarcinoma and malignant pleural infusion. Increased levels of circulating IL-6 thus appear to be an adverse prognostic factor for lung cancer patients [100, 101].
In addition to JAKs and STATs, IL-6 signalling also involves activation of MAPKs (Figure 1). Hirano and colleagues [102] described several experiments carried out with gp130 receptor mutants in order to understand its regulation and the impact of the different pathways activated upon IL-6 signalling. They noted that there appeared to be a compensatory balance between the JAK/ STAT and MAPK pathways when either pathway was disturbed. In fact, sometimes gp130 can activate opposite signalling cues. This may explain why/ how IL-6 can have multiple functions in different types of cells [102].
PTPN11 (also known as SHP2) has a role not only as an enzyme but also as a protein adapter [103]. Receptor-bound PTPN11 (Y759 on gp130 for IL-6 and Y794 on LIF receptor) can bind GRB2 via two tyrosine residues in the C-terminal (tyrosine 542 and tyrosine 580) that are believed to establish interactions with the GRB2-son-of-sevenless (SOS) complex [10, 104-106], reviewed [21]) This complex, which forms a GDP/ GTP exchanger for RAS, results in MAPK cascade activation in response to IL-6 and LIF. In response to OSM, SH2- and collagen-homology-domain-containing protein (SHC) is recruited instead (Y861 on OSM receptor) and serves as the bridge that links gp130 signalling to MAPK cascades [107]. This also couples to MAPK cascades using GRB2.
Activation of MAPK signalling also involves GRB2-associated binding protein 1 (GAB1), another scaffold protein. This further increases the complexity of the response by allowing the recruitment of additional signalling pathways, such as phospholipase Cγ, phosphatidylinositol 3'-kinase (PI3K)/AKT and c-MET (reviewed in [21]). Furthermore, stress-activated kinases (additional MAPK family members) can also be activated by IL-6-type cytokines [108-110]. These molecules have also been implicated in cancer including lung cancer and thus exacerbated production of molecules such as IL-6 not only favour tumourigenesis via STAT activation but potentially also by virtue of inducing these well-known pro-survival and/ or mitogenic signals. This, in turn, suggests that successful therapies will likely involve targeting multiple molecules, which may be cross-activated but which elicit different arms of a mitogenic or anti-apoptotic response(s).
Concluding remarks
JAKs and STATs have been identified 20 years ago. Since then, our understanding of their molecular structures and biological functions, both in cellular homeostasis and pathogenesis, has greatly increased. With this knowledge comes the promise of being able to use them as useful and effective targets for the management of several diseases, including a variety of cancers.
For this purpose, our efforts to fully understand their cellular roles must continue as this will help to minimise off-targets effects in the clinic and thereby maximise potential benefits to patients.
Acknowledgments
APC-P and MJS are funded by Cancer Research UK and by Cancer Treatment and Research Trust. MJS is also supported by a Department of Health funded Experimental Cancer Medicine Centre Grant and the Imperial College Biomedical Research Centre. NAB is funded by a PhD studentship (SFRH/ BD/ 61857/ 2009) from the Foundation for Science and Technology (FCT) (Lisbon, Portugal).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Akira S. Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells. 1999;17:138–146. doi: 10.1002/stem.170138. [DOI] [PubMed] [Google Scholar]
- 2.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 3.Santos CI, Costa-Pereira AP. Signal transducers and activators of transcription-from cytokine signalling to cancer biology. Biochim Biophys Acta. 2011;1816:38–49. doi: 10.1016/j.bbcan.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Bromberg JF. Activation of STAT proteins and growth control. Bioessays. 2001;23:161–169. doi: 10.1002/1521-1878(200102)23:2<161::AID-BIES1023>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Pellegrini S, Dusanter-Fourt I. The structure, regulation and function of the Janus kinases (JAKs) and the signal transducers and activators of transcription (STATs) Eur J Bio-chem. 1997;248:615–633. doi: 10.1111/j.1432-1033.1997.00615.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Z, Wen Z, Darnell JE., Jr Stat3 and Stat4: members of the family of signal transducers and activators of transcription. Proc Natl Acad Sci U S A. 1994;91:4806–4810. doi: 10.1073/pnas.91.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuai K, Ziemiecki A, Wilks AF, Harpur AG, Sadowski HB, Gilman MZ, Darnell JE. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993;366:580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Blenis J, Li HC, Schindler C, Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995;267:1990–1994. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- 9.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 10.Stahl N, Farruggella TJ, Boulton TG, Zhong Z, Darnell JE, Jr, Yancopoulos GD. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 11.Shuai K, Horvath CM, Huang LH, Qureshi SA, Cowburn D, Darnell JE., Jr Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell. 1994;76:821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- 12.Heim MH, Kerr IM, Stark GR, Darnell JE., Jr Contribution of STAT SH2 groups to specific interferon signaling by the Jak-STAT pathway. Science. 1995;267:1347–1349. doi: 10.1126/science.7871432. [DOI] [PubMed] [Google Scholar]
- 13.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 14.Kovarik P, Mangold M, Ramsauer K, Heidari H, Steinborn R, Zotter A, Levy DE, Muller M, Decker T. Specificity of signaling by STAT1 depends on SH2 and C-terminal domains that regulate Ser727 phosphorylation, differentially affecting specific target gene expression. Embo J. 2001;20:91–100. doi: 10.1093/emboj/20.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varinou L, Ramsauer K, Karaghiosoff M, Kolbe T, Pfeffer K, Muller M, Decker T. Phosphorylation of the Stat1 transactivation domain is required for full-fledged IFN-gammadependent innate immunity. Immunity. 2003;19:793–802. doi: 10.1016/s1074-7613(03)00322-4. [DOI] [PubMed] [Google Scholar]
- 16.Meyer T, Vinkemeier U. Nucleocytoplasmic shuttling of STAT transcription factors. Eur J Biochem. 2004;271:4606–4612. doi: 10.1111/j.1432-1033.2004.04423.x. [DOI] [PubMed] [Google Scholar]
- 17.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 18.Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheon H, Stark GR. Unphosphorylated STAT1 prolongs the expression of interferoninduced immune regulatory genes. Proc Natl Acad Sci U S A. 2009;106:9373–9378. doi: 10.1073/pnas.0903487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 21.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa-Pereira AP, Tininini S, Strobl B, Alonzi T, Schlaak JF, Is'harc H, Gesualdo I, Newman SJ, Kerr IM, Poli V. Mutational switch of an IL-6 response to an interferon-gamma-like response. Proc Natl Acad Sci U S A. 2002;99:8043–8047. doi: 10.1073/pnas.122236099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, Akira S. STAT family of transcription factors in cytokine-mediated biological responses. Cytokine Growth Factor Rev. 2000;11:199–207. doi: 10.1016/s1359-6101(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 25.Pellegrini S, John J, Shearer M, Kerr IM, Stark GR. Use of a selectable marker regulated by alpha interferon to obtain mutations in the signaling pathway. Mol Cell Biol. 1989;9:4605–4612. doi: 10.1128/mcb.9.11.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, Yang K, Chapgier A, Eidenschenk C, Eid P, Al Ghonaium A, Tufenkeji H, Frayha H, Al-Gazlan S, Al-Rayes H, Schreiber RD, Gresser I, Casanova JL. Impaired response to interferon-alpha/ beta and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 27.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 28.Park C, Li S, Cha E, Schindler C. Immune response in Stat2 knockout mice. Immunity. 2000;13:795–804. doi: 10.1016/s1074-7613(00)00077-7. [DOI] [PubMed] [Google Scholar]
- 29.Alonzi T, Maritano D, Gorgoni B, Rizzuto G, Libert C, Poli V. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation [correction of activation] in the liver. Mol Cell Biol. 2001;21:1621–1632. doi: 10.1128/MCB.21.5.1621-1632.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maritano D, Sugrue ML, Tininini S, Dewilde S, Strobl B, Fu X, Murray-Tait V, Chiarle R, Poli V. The STAT3 isoforms alpha and beta have unique and specific functions. Nat Immunol. 2004;5:401–409. doi: 10.1038/ni1052. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 32.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 33.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 34.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, Doherty PC, Grosveld G, Paul WE, Ihle JN. Lack of IL-4- induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 35.Takeda K, Kamanaka M, Tanaka T, Kishimoto T, Akira S. Impaired IL-13-mediated functions of macrophages in STAT6-deficient mice. J Immunol. 1996;157:3220–3222. [PubMed] [Google Scholar]
- 36.Ferbeyre G, Moriggl R. The role of Stat5 transcription factors as tumor suppressors or oncogenes. Biochim Biophys Acta. 2011;1815:104–114. doi: 10.1016/j.bbcan.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Haspel RL, Salditt-Georgieff M, Darnell JE., Jr The rapid inactivation of nuclear tyrosine phosphorylated Stat1 depends upon a protein tyrosine phosphatase. Embo J. 1996;15:6262–6268. [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer T, Hendry L, Begitt A, John S, Vinkemeier U. A single residue modulates tyrosine dephosphorylation, oligomerization, and nuclear accumulation of stat transcription factors. J Biol Chem. 2004;279:18998–19007. doi: 10.1074/jbc.M400766200. [DOI] [PubMed] [Google Scholar]
- 39.ten Hoeve J, de Jesus Ibarra-Sanchez M, Fu Y, Zhu W, Tremblay M, David M, Shuai K. Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol Cell Biol. 2002;22:5662–5668. doi: 10.1128/MCB.22.16.5662-5668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto A, Masuhara M, Mitsui K, Yokouchi M, Ohtsubo M, Misawa H, Miyajima A, Yoshimura A. CIS, a cytokine inducible SH2 protein, is a target of the JAK-STAT5 pathway and modulates STAT5 activation. Blood. 1997;89:3148–3154. [PubMed] [Google Scholar]
- 42.Naka T, Matsumoto T, Narazaki M, Fujimoto M, Morita Y, Ohsawa Y, Saito H, Nagasawa T, Uchiyama Y, Kishimoto T. Accelerated apoptosis of lymphocytes by augmented induction of Bax in SSI-1 (STAT-induced STAT inhibitor-1) deficient mice. Proc Natl Acad Sci U S A. 1998;95:15577–15582. doi: 10.1073/pnas.95.26.15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 44.Hilton DJ, Richardson RT, Alexander WS, Viney EM, Willson TA, Sprigg NS, Starr R, Nicholson SE, Metcalf D, Nicola NA. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci U S A. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 46.Liu B, Liao J, Rao X, Kushner SA, Chung CD, Chang DD, Shuai K. Inhibition of Stat1-mediated gene activation by PIAS1. Proc Natl Acad Sci U S A. 1998;95:10626–10631. doi: 10.1073/pnas.95.18.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ungureanu D, Vanhatupa S, Kotaja N, Yang J, Aittomaki S, Janne OA, Palvimo JJ, Silvennoinen O. PIAS proteins promote SUMO-1 conjugation to STAT1. Blood. 2003;102:3311–3313. doi: 10.1182/blood-2002-12-3816. [DOI] [PubMed] [Google Scholar]
- 48.Rogers RS, Horvath CM, Matunis MJ. SUMO modification of STAT1 and its role in PIAS-mediated inhibition of gene activation. J Biol Chem. 2003;278:30091–30097. doi: 10.1074/jbc.M301344200. [DOI] [PubMed] [Google Scholar]
- 49.Shuai K. Regulation of cytokine signaling pathways by PIAS proteins. Cell Res. 2006;16:196–202. doi: 10.1038/sj.cr.7310027. [DOI] [PubMed] [Google Scholar]
- 50.Croker BA, Kiu H, Nicholson SE. SOCS regulation of the JAK/ STAT signalling pathway. Semin Cell Dev Biol. 2008;19:414–422. doi: 10.1016/j.semcdb.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kota J, Caceres N, Constantinescu SN. Aberrant signal transduction pathways in myeloproliferative neoplasms. Leukemia. 2008;22:1828–1840. doi: 10.1038/leu.2008.236. [DOI] [PubMed] [Google Scholar]
- 53.Oku S, Takenaka K, Kuriyama T, Shide K, Kumano T, Kikushige Y, Urata S, Yamauchi T, Iwamoto C, Shimoda HK, Miyamoto T, Nagafuji K, Kishimoto J, Shimoda K, Akashi K. JAK2 V617F uses distinct signalling pathways to induce cell proliferation and neutrophil activation. Br J Haematol. 2010;150:334–344. doi: 10.1111/j.1365-2141.2010.08249.x. [DOI] [PubMed] [Google Scholar]
- 54.Chen E, Beer PA, Godfrey AL, Ortmann CA, Li J, Costa-Pereira AP, Ingle CE, Dermitzakis ET, Campbell PJ, Green AR. Distinct clinical phenotypes associated with JAK2V617F reflect differential STAT1 signaling. Cancer Cell. 2010;18:524–535. doi: 10.1016/j.ccr.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carmo CR, Lyons-Lewis J, Seckl MJ, Costa-Pereira AP. A Novel Requirement for Janus Kinases as Mediators of Drug Resistance Induced by Fibroblast Growth Factor-2 in Human Cancer Cells. PLoS One. 2011;6:e19861. doi: 10.1371/journal.pone.0019861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graeven U, Andre N, Achilles E, Zornig C, Schmiegel W. Serum levels of vascular endothelial growth factor and basic fibroblast growth factor in patients with soft-tissue sarcoma. J Cancer Res Clin Oncol. 1999;125:577–581. doi: 10.1007/s004320050319. [DOI] [PubMed] [Google Scholar]
- 57.Ruotsalainen T, Joensuu H, Mattson K, Salven P. High pretreatment serum concentration of basic fibroblast growth factor is a predictor of poor prognosis in small cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1492–1495. [PubMed] [Google Scholar]
- 58.Salven P, Teerenhovi L, Joensuu H. A high pretreatment serum basic fibroblast growth factor concentration is an independent predictor of poor prognosis in non-Hodgkin's lymphoma. Blood. 1999;94:3334–3339. [PubMed] [Google Scholar]
- 59.Song S, Wientjes MG, Gan Y, Au JL. Fibroblast growth factors: an epigenetic mechanism of broad spectrum resistance to anticancer drugs. Proc Natl Acad Sci U S A. 2000;97:8658–8663. doi: 10.1073/pnas.140210697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pardo OE, Lesay A, Arcaro A, Lopes R, Ng BL, Warne PH, McNeish IA, Tetley TD, Lemoine NR, Mehmet H, Seckl MJ, Downward J. Fibroblast growth factor 2-mediated translational control of IAPs blocks mitochondrial release of Smac/ DIABLO and apoptosis in small cell lung cancer cells. Mol Cell Biol. 2003;23:7600–7610. doi: 10.1128/MCB.23.21.7600-7610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pardo OE, Wellbrock C, Khanzada UK, Aubert M, Arozarena I, Davidson S, Bowen F, Parker PJ, Filonenko VV, Gout IT, Sebire N, Marais R, Downward J, Seckl MJ. FGF-2 protects small cell lung cancer cells from apoptosis through a complex involving PKCepsilon, B-Raf and S6K2. EMBO J. 2006;25:3078–3088. doi: 10.1038/sj.emboj.7601198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pardo OE, Arcaro A, Salerno G, Raguz S, Downward J, Seckl MJ. Fibroblast growth factor-2 induces translational regulation of Bcl-XL and Bcl-2 via a MEK-dependent pathway: correlation with resistance to etoposide-induced apoptosis. J Biol Chem. 2002;277:12040–12046. doi: 10.1074/jbc.M109006200. [DOI] [PubMed] [Google Scholar]
- 63.Pardo OE, Arcaro A, Salerno G, Tetley TD, Valovka T, Gout I, Seckl MJ. Novel cross talk between MEK and S6K2 in FGF-2 induced proliferation of SCLC cells. Oncogene. 2001;20:7658–7667. doi: 10.1038/sj.onc.1204994. [DOI] [PubMed] [Google Scholar]
- 64.Kirito K, Nagashima T, Ozawa K, Komatsu N. Constitutive activation of Stat1 and Stat3 in primary erythroleukemia cells. Int J Hematol. 2002;75:51–54. doi: 10.1007/BF02981979. [DOI] [PubMed] [Google Scholar]
- 65.Weber-Nordt RM, Egen C, Wehinger J, Ludwig W, Gouilleux-Gruart V, Mertelsmann R, Finke J. Constitutive activation of STAT proteins in primary lymphoid and myeloid leukemia cells and in Epstein-Barr virus (EBV)-related lymphoma cell lines. Blood. 1996;88:809–816. [PubMed] [Google Scholar]
- 66.Fernandes A, Hamburger AW, Gerwin BI. ErbB-2 kinase is required for constitutive stat 3 activation in malignant human lung epithelial cells. Int J Cancer. 1999;83:564–570. doi: 10.1002/(sici)1097-0215(19991112)83:4<564::aid-ijc20>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 67.Lin TS, Mahajan S, Frank DA. STAT signaling in the pathogenesis and treatment of leukemias. Oncogene. 2000;19:2496–2504. doi: 10.1038/sj.onc.1203486. [DOI] [PubMed] [Google Scholar]
- 68.Watson CJ, Miller WR. Elevated levels of members of the STAT family of transcription factors in breast carcinoma nuclear extracts. Br J Cancer. 1995;71:840–844. doi: 10.1038/bjc.1995.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 70.Alvarez JV, Frank DA. Genome-wide analysis of STAT target genes: elucidating the mechanism of STAT-mediated oncogenesis. Cancer Biol Ther. 2004;3:1045–1050. doi: 10.4161/cbt.3.11.1172. [DOI] [PubMed] [Google Scholar]
- 71.Eickelberg O, Pansky A, Mussmann R, Bihl M, Tamm M, Hildebrand P, Perruchoud AP, Roth M. Transforming growth factor-beta1 induces interleukin-6 expression via activating protein-1 consisting of JunD homodimers in primary human lung fibroblasts. J Biol Chem. 1999;274:12933–12938. doi: 10.1074/jbc.274.18.12933. [DOI] [PubMed] [Google Scholar]
- 72.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 73.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 74.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 75.Kovacic B, Stoiber D, Moriggl R, Weisz E, Ott RG, Kreibich R, Levy DE, Beug H, Freissmuth M, Sexl V. STAT1 acts as a tumor promoter for leukemia development. Cancer Cell. 2006;10:77–87. doi: 10.1016/j.ccr.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 76.Danial NN, Pernis A, Rothman PB. Jak-STAT signaling induced by the v-abl oncogene. Science. 1995;269:1875–1877. doi: 10.1126/science.7569929. [DOI] [PubMed] [Google Scholar]
- 77.Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, Jove R. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–83. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 78.Turkson J, Ryan D, Kim JS, Zhang Y, Chen Z, Haura E, Laudano A, Sebti S, Hamilton AD, Jove R. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J Biol Chem. 2001;276:45443–45455. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]
- 79.Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert Opin Investig Drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spiro SG, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med. 2005;172:523–529. doi: 10.1164/rccm.200504-531OE. [DOI] [PubMed] [Google Scholar]
- 81.Lopes Pegna A, Picozzi G. Lung cancer screening update. Curr Opin Pulm Med. 2009;15:327–333. doi: 10.1097/MCP.0b013e32832b98be. [DOI] [PubMed] [Google Scholar]
- 82.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 83.Shivapurkar N, Reddy J, Chaudhary PM, Gazdar AF. Apoptosis and lung cancer: a review. J Cell Biochem. 2003;88:885–898. doi: 10.1002/jcb.10440. [DOI] [PubMed] [Google Scholar]
- 84.Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, Bromberg JF. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haura EB, Zheng Z, Song L, Cantor A, Bepler G. Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res. 2005;11:8288–8294. doi: 10.1158/1078-0432.CCR-05-0827. [DOI] [PubMed] [Google Scholar]
- 86.Mukohara T, Kudoh S, Yamauchi S, Kimura T, Yoshimura N, Kanazawa H, Hirata K, Wanibuchi H, Fukushima S, Inoue K, Yoshikawa J. Expression of epidermal growth factor receptor (EGFR) and downstream-activated peptides in surgically excised non-small-cell lung cancer (NSCLC) Lung Cancer. 2003;41:123–130. doi: 10.1016/s0169-5002(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 87.Seki Y, Suzuki N, Imaizumi M, Iwamoto T, Usami N, Ueda Y, Hamaguchi M. STAT3 and MAPK in human lung cancer tissues and suppression of oncogenic growth by JAB and dominant negative STAT3. Int J Oncol. 2004;24:931–934. [PubMed] [Google Scholar]
- 88.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/ Jak/ STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sehgal PB, Wang L, Rayanade R, Pan H, Margulies L. Interleukin-6-type cytokines. Ann N Y Acad Sci. 1995;762:1–14. [PubMed] [Google Scholar]
- 90.Huang WL, Yeh HH, Lin CC, Lai WW, Chang JY, Chang WT, Su WC. Signal transducer and activator of transcription 3 activation upregulates interleukin-6 autocrine production: a biochemical and genetic study of established cancer cell lines and clinical isolated human cancer cells. Mol Cancer. 2010;9:309. doi: 10.1186/1476-4598-9-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bihl M, Tamm M, Nauck M, Wieland H, Perruchoud AP, Roth M. Proliferation of human non-small-cell lung cancer cell lines: role of interleukin-6. Am J Respir Cell Mol Biol. 1998;19:606–612. doi: 10.1165/ajrcmb.19.4.3247. [DOI] [PubMed] [Google Scholar]
- 92.Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 93.Ancrile BB, O'Hayer KM, Counter CM. Oncogenic ras-induced expression of cytokines: a new target of anti-cancer therapeutics. Mol Interv. 2008;8:22–27. doi: 10.1124/mi.8.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schafer ZT, Brugge JS. IL-6 involvement in epithelial cancers. J Clin Invest. 2007;117:3660–3663. doi: 10.1172/JCI34237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for Translational Therapeutics. Cancer. 2007;110:1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 97.Porgador A, Tzehoval E, Katz A, Vadai E, Revel M, Feldman M, Eisenbach L. Interleukin 6 gene transfection into Lewis lung carcinoma tumor cells suppresses the malignant phenotype and confers immunotherapeutic competence against parental metastatic cells. Cancer Res. 1992;52:3679–3686. [PubMed] [Google Scholar]
- 98.Kerr IM, Costa-Pereira AP, Lillemeier BF, Strobl B. Of JAKs, STATs, blind watchmakers, jeeps and trains. FEBS Lett. 2003;546:1–5. doi: 10.1016/s0014-5793(03)00411-3. [DOI] [PubMed] [Google Scholar]
- 99.Song L, Turkson J, Karras JG, Jove R, Haura EB. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150–4165. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 100.Yanagawa H, Sone S, Takahashi Y, Haku T, Yano S, Shinohara T, Ogura T. Serum levels of interleukin 6 in patients with lung cancer. Br J Cancer. 1995;71:1095–1098. doi: 10.1038/bjc.1995.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yeh HH, Lai WW, Chen HH, Liu HS, Su WC. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene. 2006;25:4300–4309. doi: 10.1038/sj.onc.1209464. [DOI] [PubMed] [Google Scholar]
- 102.Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 103.Hof P, Pluskey S, Dhe-Paganon S, Eck MJ, Shoelson SE. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92:441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 104.Lu W, Gong D, Bar-Sagi D, Cole PA. Sitespecific incorporation of a phosphotyrosine mimetic reveals a role for tyrosine phosphorylation of SHP-2 in cell signaling. Mol Cell. 2001;8:759–769. doi: 10.1016/s1097-2765(01)00369-0. [DOI] [PubMed] [Google Scholar]
- 105.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in antiapoptosis. Immunity. 1996;5:449–460. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 106.Schiemann WP, Bartoe JL, Nathanson NM. Box 3-independent signaling mechanisms are involved in leukemia inhibitory factor receptor alpha- and gp130-mediated stimulation of mitogen-activated protein kinase. Evidence for participation of multiple signaling pathways which converge at Ras. J Biol Chem. 1997;272:16631–16636. doi: 10.1074/jbc.272.26.16631. [DOI] [PubMed] [Google Scholar]
- 107.Hermanns HM, Radtke S, Schaper F, Heinrich PC, Behrmann I. Non-redundant signal transduction of interleukin-6-type cytokines. The adapter protein Shc is specifically recruited to rhe oncostatin M receptor. J Biol Chem. 2000;275:40742–40748. doi: 10.1074/jbc.M005408200. [DOI] [PubMed] [Google Scholar]
- 108.Bode JG, Ludwig S, Freitas CA, Schaper F, Ruhl M, Melmed S, Heinrich PC, Haussinger D. The MKK6/ p38 mitogen-activated protein kinase pathway is capable of inducing SOCS3 gene expression and inhibits IL-6-induced transcription. Biol Chem. 2001;382:1447–1453. doi: 10.1515/BC.2001.178. [DOI] [PubMed] [Google Scholar]
- 109.Wang Y, Robledo O, Kinzie E, Blanchard F, Richards C, Miyajima A, Baumann H. Receptor subunit-specific action of oncostatin M in hepatic cells and its modulation by leukemia inhibitory factor. J Biol Chem. 2000;275:25273–25285. doi: 10.1074/jbc.M002296200. [DOI] [PubMed] [Google Scholar]
- 110.Radtke S, Wuller S, Yang XP, Lippok BE, Mutze B, Mais C, de Leur HS, Bode JG, Gaestel M, Heinrich PC, Behrmann I, Schaper F, Hermanns HM. Cross-regulation of cytokine signalling: pro-inflammatory cytokines restrict IL-6 signalling through receptor internalisation and degradation. J Cell Sci. 2010;123:947–959. doi: 10.1242/jcs.065326. [DOI] [PubMed] [Google Scholar]