Abstract
We investigated whether improved early visual processing on cognitive remediation (CR) exercises generalizes to visual and auditory learning and information manipulation in schizophrenia. Fourteen participants received neuropsychological testing before and after CR consisting of visual, auditory and cognitive control training. Achievement on visual training exercises was strongly and significantly correlated with improved visual learning, but not improved verbal learning or increased ability to manipulate visual information. Improvement in training, not training time, predicted cognitive gain. Implications for improving cognitive outcomes from CR include ensuring the trained task is learned and providing exercises of multiple modalities.
Keywords: cognitive remediation, schizophrenia, visual memory, computer-based cognitive remediation, sensory processing
1. Introduction
Multiple types of cognitive remediation (CR) improve many cognitive deficits in schizophrenia (McGurk et al., 2007; Wykes et al., 2011). It is not clear, however, whether specific features of CR programs are related to improvement in different cognitive domains. This issue is particularly relevant for visual learning and memory deficits, which consistently show less favorable outcomes than auditory verbal memory outcomes, even with computer-based CR (CCR) targeting the visual modality (Grynszpan et al., 2011).
One approach to determine the specificity of targeted CR is to analyze the relationship between achievement on individual components of CR training and improvement in different cognitive domains. This strategy has been informative in prior studies. Progress on specific aspects of visual attention training exercises predicted which patients improved on external measures of attention (Bell et al., 2009). Training of basic auditory processing correlated with improvements on auditory verbal learning and memory tasks and not visual learning tasks (Adcock et al., 2009; Fisher et al., 2009), suggesting that a ‘bottom-up’ training strategy, where early sensory processing is targeted with CCR, improves some but not other aspects of higher cognitive function (Adcock et al., 2009) and may be modality specific. A study in healthy elderly subjects showed a similar association in the visual modality: improvement on a trained visual perception discrimination task predicted improvement in a non-trained visual working memory task (Berry et al., 2010).
Given the limited generalization of CCR to visual memory to date, we examined the relationship between training measures captured from CCR and visual memory outcomes. We also studied the relationship of visual training measures to auditory verbal memory outcomes to evaluate cross-modality generalization. We hypothesized that improvement in visual task performance would correlate with improvement in pre-post measures of visual learning and visual memory but not with measures of auditory verbal learning or memory.
2. Experimental/Materials and Methods
2.1 Participants and Assessments
The Yale Human Investigation Committee approved all procedures. Fourteen adult outpatients with schizophrenia or schizoaffective disorder in an on-going randomized controlled trial of combining CCR with vocational rehabilitation at a community mental health center were included in this post-hoc analysis on the basis of completing at least 5 hours of visual CCR, with average visual training of 27.5 hours and standard deviation of 15.4 hours. All were clinically stable (no psychiatric medication changes, psychiatric hospitalizations, or changes in housing in the last month) and without history of head trauma, epilepsy, developmental delay, or substance abuse for 60 days. Demographic and psychiatric medication data are shown in Tables 1 and 2. Chlorpromazine equivalent doses were calculated using ratios from Woods (Woods, 2003).
Table 1.
Participant Demographics, Symptom Scales and Cognitive Measures at Baseline
| Variable | Mean | SD | Range |
|---|---|---|---|
| Age (years) | 47.5 | 10.8 | 25–62 |
| Education (years) | 13.6 | 3.5 | 11–25 |
| Gender | 8 Male | 6 Female | |
| Race | 7 Caucasian | 7 African American | |
| Global Assessment of Function | 41.2 | 4.7 | 35–50 |
| PANSS Total | 61.4 | 13.9 | 34–79 |
| Chlorpromazine Equivalents | 353.5 | 212.6 | 50–600 |
| HVLT scaled score | 38.0 | 8.0 | 29–57 |
| BVMT scaled score | 43.4 | 12.3 | 25–66 |
| LNS scaled score | 7.7 | 3.1 | 2–12 |
| SS scaled score | 45.1 | 10.0 | 27–59 |
Table 2.
Participants’ Intake Psychiatric Medications
| Medication or Medication Class | Number of Participants (14 total) |
|---|---|
| Atypical Antipsychotics | 14 |
| Aripiprazole | 2 |
| Clozapine | 3 |
| Olanzapine | 5 |
| Risperidone | 3 |
| Quetiapine | 2 |
| Ziprasidone | 1 |
| Typical Antipsychotics | 0 |
| Mood Stabilizers | 2 |
| Selective Serotonin Reuptake Inhibitors | 2 |
| Benzodiazepines | 1 |
| Anti-Parkinsonian Agents | 3 |
All subjects enrolled in a supported employment program for 12 months, including assignment to vocational specialists and weekly feedback groups about employment. Participants in the present analysis participated in up to 12 months of CCR, consisting of auditory (Brain Fitness Program), visual (Insight) and cognitive control exercises (Aristotle) developed by Posit Science. Once participants completed CCR, stopped participating in CCR or had participated for 1 year, they had follow-up assessments. For 12 of the 14 participants, auditory training was completed before visual CCR was begun. One participant did not do auditory CCR, and another began and completed auditory CCR while doing visual training. Participants were compensated $5 for each hour of CCR up to 10 hours per week and $50 for assessments before and after CCR.
Trained psychometricians administered the Positive and Negative Symptom Scale (PANSS) (Kay et al., 1989) and MATRICS Consensus Cognitive battery (Nuechterlein et al., 2008) before CCR and within 2 months of ending visual CCR. Two visual memory sub-tasks from the MATRICS battery, Brief Visual-Spatial Memory Test (BVMT) and Spatial Span, and analogous auditory verbal memory tasks, Hopkins Verbal Learning Test (HVLT) and Letter-Number Sequencing (LNS) were used for this analysis.
2.2 CCR and Performance Measures
The auditory exercises have been described previously (Fisher et al., 2009; Popov et al., 2011), and the cognitive control exercises were designed to improve executive function.
The visual program has four exercises. Three exercises (EX1, EX2, EX3) present two visual stimuli with a delay in between them, and require the user to identify characteristics of the stimuli. For EX1–3, the difficulty is adjusted to the user’s ability by adaptively tracking the duration of the two stimuli and the interstimulus delay to maintain 80% accuracy. As the user develops mastery on one set of stimuli for any given exercise, the stimulus configuration (SC) becomes more difficult by changing the nature of the stimuli and the background. EX1 presents two pairs of visual sweeps, which are two pairs of moving Gabor patterns in succession, and asks users to identify the directions of the sweeps’ movements, as previously described (Berry et al., 2010). SCs increase in difficulty by increasing the stimuli’s spatial frequency. EX2 presents the user with a target, a single bird. After a delay, the same bird must be located within a group of birds. The SCs become more challenging with more complex backgrounds and more similarity between the target bird and the others. In EX3, the user must focus on a central point to determine which of two possible vehicles are presented while simultaneously identifying the location of a road sign in the periphery. Difficult SCs have more distracters and greater peripheral demand. The fourth exercise (EX4) hides jewels behind objects. The objects move, and the user must recall which objects had jewels hidden behind them. The number of hidden jewels is adaptively tracked to maintain 80% accuracy. In EX4, later SCs have more background distracters, greater speed and duration of movement and decreased contrast between the moving objects and the background. For all exercises, a user who is training efficiently would be expected to complete more of an exercise’s content, as measured by SCs, than one who is struggling to attain mastery of the training material. Thus, users’ progression though SCs is a measure of achievement in the training exercise.
2.3 Statistical Analysis
To test the hypothesis that visual training achievement predicted improvement in visual memory and not verbal memory, Pearson correlations were performed between maximum SC reached in each of the four exercises and the change in scaled score on the four neuropsychological assessments, and relevant differences among the resulting correlations were evaluated using the Fisher r-to-z transformation. Additional correlations were calculated to determine whether training time independent of SC explained variance in outcomes.
3. Results
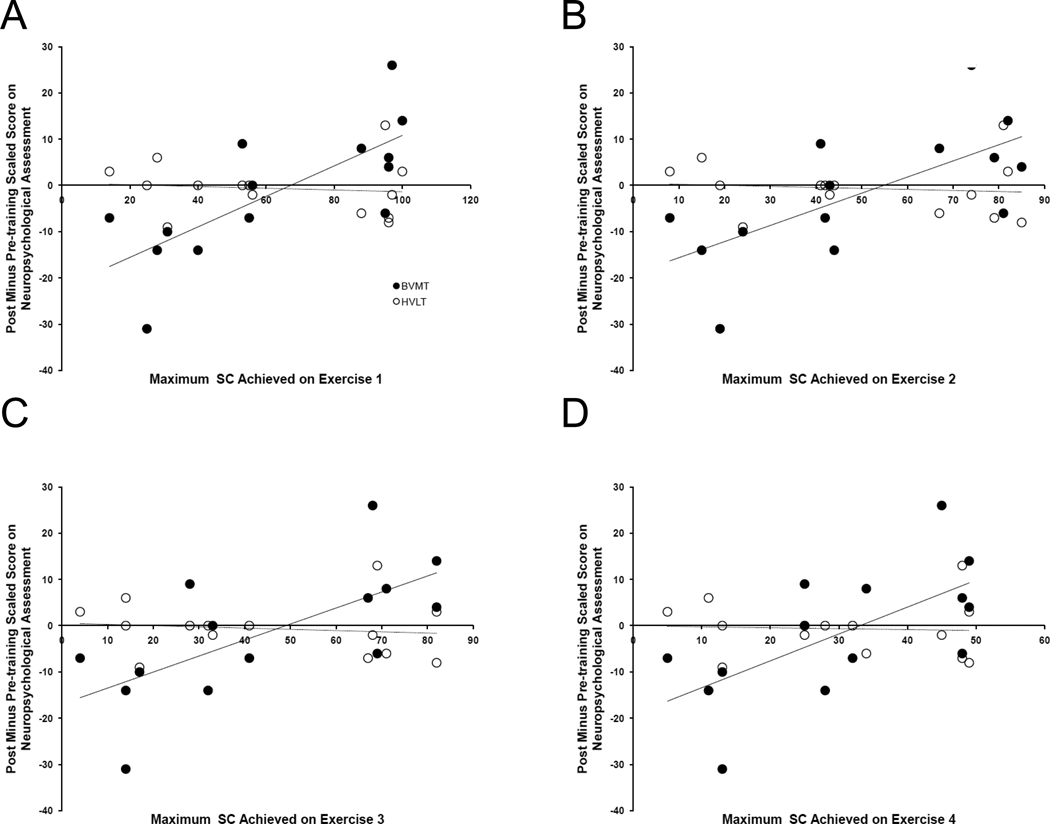
The baseline assessments are summarized in Table 1, and demonstrate the participants were symptomatic and cognitively impaired. SC for all four exercises strongly correlated with improvement in BVMT, but not with improvement in any of the other three neuropsychological outcomes (Figure 1 and Table 3). The correlations of SC on EX1 and EX2 with BVMT outcome were statistically significantly higher than correlations of EX1 and EX2 SC with outcome on HVLT. Training time was highly correlated with SC on each exercise, but not with change on BVMT (Table 4).
Figure 1. Relationship of Achievement in Visual Training to Visual and Verbal Learning Neuropsychological Outcomes.
Change in BVMT (closed circles), not HVLT (open circles), performance with training is correlated with the amount of visual training content completed in each of the four visual exercises. Regression lines are solid for BVMT and dashed for HVLT.
Abbreviations: BVMT = Brief Visual Memory Test; HVLT = Hopkins Verbal Learning Test; SC = stimulus configuration
Table 3.
Pearson Correlations of Best Performances in Visual Exercises and Change in Neuropsychology Assessment Scores, Brief Visual Spatial Memory Test (BVMT), Hopkins Verbal Learning Test (HVLT), Spatial Span task (SS) and Letter Number Sequencing (LNS) with Training
| Exercise | BVMT, r (p) |
HVLT, r (p) |
z-score (BVMT & HVLT) |
P (BVMT & HVLT) |
SS, r (p) |
LNS, r (p) |
z-score (SS & LNS) |
P (SS & LNS) |
|---|---|---|---|---|---|---|---|---|
| EX1 | 0.739b | −0.100 | −2.46a | 0.014a | 0.124 | −0.147 | −0.64 | 0.522 |
| (0.003) | (0.733) | (0.672) | (0.617) | |||||
| EX2 | 0.673b | −0.104 | −2.16a | 0.031a | 0.093 | −0.167 | −0.61 | 0.542 |
| (0.008) | (0.723) | (0.751) | (0.569) | |||||
| EX3 | 0.677b | −0.125 | −2.23a | 0.028a | 0.074 | −0.220 | −0.70 | 0.484 |
| (0.008) | (0.670) | (0.801) | (0.449) | |||||
| EX4 | 0.646a | −0.053 | −1.93 | 0.054 | 0.071 | −0.188 | −0.61 | 0.542 |
| (0.013) | (0.858) | (0.810) | (0.519) |
p (two-tailed) < 0.05
p (two-tailed) < 0.01
Table 4.
Relationship of Training Time with Achievement in Training and with Improvement in Visual Memory Outcome in the Brief Visual Memory Test (BVMT)
| Exercise Training Time |
EX1 SC (p) |
EX2 SC (p) |
EX3 SC (p) |
EX4 SC (p) |
Change in BVMT (p) |
|---|---|---|---|---|---|
| EX1 | 0.658a | 0.665b | 0.581b | 0.694b | 0.305 |
| (0.010) | (0.009) | (0.029) | (0.006) | (0.288) | |
| EX2 | 0.836c | 0.844c | 0.790c | 0.870c | 0.500 |
| (0.000) | (0.000) | (0.001) | (0.000) | (0.068) | |
| EX3 | 0.756b | 0.775b | 0.708b | 0.798b | 0.299 |
| (0.002) | (0.001) | (0.005) | (0.001) | (0.299) | |
| EX4 | 0.873c | 0.887c | 0.847c | 0.912c | 0.453 |
| (0.000) | (0.000) | (0.000) | (0.000) | (0.104) |
p < 0.05
p < 0.01
p < 0.001
4. Discussion
As hypothesized, achievement in visual training strongly and specifically predicts visual memory improvement. Improved basic visual processing does not appear to generalize to all memory tasks: it does not predict improvement on visual working memory tasks requiring significant manipulation of the presented information (such as Spatial Span) or auditory verbal learning and memory. While training time on visual processing exercises is associated with improvement on the exercises themselves, training time is only weakly and in this sample non-significantly associated with improvement in visual memory outcome measures. Gaining mastery of the content is more robustly associated with cognitive improvement than is the amount of time training.
This analysis helps explain the limited overall benefits of CCR on visual learning outcomes seen in this study (Figure 1a–d) and others (Grynszpan et al., 2011; McGurk et al., 2007; Wykes et al., 2011); participants who do not improve on the trained exercises are unlikely to improve on cognitive outcomes.
The present analysis was post-hoc with a small sample size and subjects received CCR consisting of visual, auditory and cognitive control exercises, so we cannot exclude the possibility that the auditory or cognitive training improved BVMT scores. The limited effect of CR on visual memory reported by Grynszpan et al and the strong correlations we found between visual training measures and BVMT suggest the visual training was responsible for the improvement on visual learning. As Berry et al found for healthy elderly subjects, in schizophrenia patients improved visual perception predicts improved visual memory.
This analysis has implications for improving CCR. Exercises improving basic visual processing do improve visual memory, and should be done, but outcomes involving manipulation of visual information may require different training. Progress should be evaluated during training to troubleshoot lack of training on trained exercise, as without improvement on the trained task, generalization to other tasks is unlikely to occur. Cross-modal training benefits may be limited in patients with schizophrenia.
Acknowledgement
We thank Jason K. Johannesen who kindly reviewed the analyses.
Role of Funding Source
This study is supported by funding from NIMH (NCT00339170) and a VA RR&D Senior Research Career Scientist Award both to Dr. Bell. These sponsors play no role in this study or publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. Wexler was a paid consultant for Posit Science Corporation at the time some of the data reported were collected and continues to have stock options in this company. Posit Science makes and plans at some time to sell the computer programs used for cognitive training in the study. All other authors declare that they have no conflicts of interest.
Contributors
Drs. Bell and Wexler designed the study and wrote the protocol, Drs. Surti and Corbera performed the statistical analyses, and Dr. Surti wrote the first draft of the paper. All authors contributed to and have approved the final manuscript.
Contributor Information
Toral S. Surti, Email: toral.surti@yale.edu.
Silvia Corbera, Email: silvia.corbera@yale.edu.
Morris D. Bella, Email: morris.bell@yale.edu.
Bruce E. Wexler, Email: bruce.wexler@yale.edu.
References
- Adcock RA, Dale C, Fisher M, Aldebot S, Genevsky A, Simpson GV, Nagarajan S, Vinogradov S. When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophr Bull. 2009;35(6):1132–1141. doi: 10.1093/schbul/sbp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MD, Fiszdon JM, Bryson G. Attention training in schizophrenia: differing responses to similar tasks. J Psychiatr Res. 2009;43(4):490–496. doi: 10.1016/j.jpsychires.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Clapp WC, Hardy JL, Delahunt PB, Mahncke HW, Gazzaley A. The influence of perceptual training on working memory in older adults. PLoS One. 2010;5(7):e11537. doi: 10.1371/journal.pone.0011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynszpan O, Perbal S, Pelissolo A, Fossati P, Jouvent R, Dubal S, Perez-Diaz F. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study. Psychol Med. 2011;41(1):163–173. doi: 10.1017/S0033291710000607. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl. 1989;(7):59–67. [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Popov T, Jordanov T, Rockstroh B, Elbert T, Merzenich MM, Miller GA. Specific cognitive training normalizes auditory sensory gating in schizophrenia: a randomized trial. Biol Psychiatry. 2011;69(5):465–471. doi: 10.1016/j.biopsych.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]