Abstract
In vitro and animal studies suggest a possible role for the tetracycline class of drugs in the inhibition of non-enzymatic protein glycation. We conducted a 3-month, randomized placebo-controlled pilot clinical trial of conventional sub-gingival debridement, (periodontal therapy) combined with either a three month regimen of sub-antimicrobial-dose doxycycline (SDD), a two week regimen of antimicrobial-dose doxycycline (ADD), or placebo in 45 patients with long-standing type 2 diabetes (mean duration 9 years) and untreated chronic periodontitis. Subjects were taking stable doses of oral hypoglycemic medications and/or insulin. Treatment response was assessed by measuring hemoglobin A1c (HbA1c),plasma glucose, and clinical periodontal disease measures. At one-month and three-month follow-up, clinical measures of periodontitis were decreased in all groups(data to be presented elsewhere). At three months, mean HbA1c levels in the SDD group were reduced 0.9% unitsfrom 7.2% units ± 2.2(±SD), to 6.3% units ±1.1, which represents a 12.5% improvement. In contrast, there was no significant change in HbA1c in the ADD (7.5%± 2.0 to 7.8%± 2.1) or placebo (8.5%± 2.0 to 8.5%± 2.6) groups. Mean HbA1c change from baseline was significantly greater in the SDD group compared with the ADD group (p=0.04) but not placebo (p=0.22). Moreover, a larger proportion of subjects in the SDD group experienced improvement (p<0.05) compared to the ADD or placebo groups. Mean plasma glucose levels were not significantly different between or within the groups. The results of this pilot study suggest that the treatment of periodontitis with sub-gingival debridement and 3-months of daily sub-antimicrobial-dose doxycycline may decrease HbA1c in patients with type 2 diabetes taking normally prescribed hypoglycemic agents.
Keywords: sub-antimicrobial-dose doxycycline, antibiotic dose doxycycline, type 2 diabetes, chronic periodontitis, periodontal therapy, HbA1c
1. INTRODUCTION
Diabetes mellitus and impaired glucose tolerance are disorders characterized by frequent periods of hyperglycemia[1]. Hyperglycemia initiates chemical and molecular pathways that appear to be critical to the initiation of micro- and macro-vascular complications of diabetes. Several distinct mechanisms have been suggested to account for the pathogenicity of complications of hyperglycemiaincluding: protein kinase C isoforms [2], increased hexosamine pathway flux [3,4], increased polyol pathway flux[5], and enhanced formation of advanced glycation end products [6,7]. Elevated intra- and extracellular glucose concentrations lead to the non-enzymatic glycation of protein [6]. An example of an early glycation product is glycosylated hemoglobin (HbA1c). Glycemic control is evaluated by measuring the hemoglobin fraction A1c [8],a reflection of plasma glucose levels during the 120 day half life of the red blood cell. HbA1c is widely used as a surrogate measure for glycemic control [9-15] and treatment decision-making in clinical medicine[16].
Glycemic control is essential to the prevention of diabetes-related morbidity and mortality.Elevated HbA1c has been linked to micro- and macro- vascular diabetes complications [9,15,17] and lowering of HbA1c results in reduced morbidity and mortality [18]. While recommended target HbA1c levelsare < 7%[19], recent evidence indicates that a ‘safe’ lower threshold for HbA1c may not exist [9,20]. New treatment strategies for diabetes are needed in order to cope with this increasing public health problem.
Current medical treatments for hyperglycemia include insulin treatment, and oral medications that target insulin secretion and utilization pathways. These treatments act to increase insulin sensitivity or insulin availability and thereby reduce hyperglycemia indirectly. As a consequence of improved long term glucose utilization, decreased HbA1c levels are observed. Despite best treatment efforts however, HbA1c levels may remain elevated in patients with established diabetes. The Diabetes Control and Complications Trial [15] and the United Kingdom Prospective Diabetes Study [13] demonstrated that intensive medical management improves levels of HbA1c, and reduces risk of micro-vascular complications. These studies also demonstrated the degenerative nature of diabetes. After initial reductions following treatment with oral agents or insulin, mean HbA1c values returned to their prestudy levels within six years. Additionally, aggressive medical control of hyperglycemia may result in increased incidence of hypoglycemia, which may lead to adverse health outcomes. Because of the difficulty in managing diabetes, new treatment modalities would be useful to prevent diabetes-related micro-and macro-vascular complications.
One therapeutic strategy is to directly target protein glycation. Benfotiamine, a lipid-soluble thiamine derivative, disrupts the pathways associated with advanced glycation end product (AGE) formation, and has been used successfully in vitro and in animal models[2] to inhibit nephropathy[21]. Likewise pyridoxamine has been used in animal models of diabetes and has been demonstrated to inhibit diabetic retinopathy[22]. Aminoguanidine inhibits AGE formation in animal models, but has proven to be too toxic [23] for use in humans [24]. Receptors for advanced glycation end products have been identified in human tissues, that when activated by AGEs result in increased activation of inflammatory pathways. Soluble receptor for advanced glycation end product has been used effectively in animal models to prevent hyperglycemia-associated sequelae [25][26-28], but is not approved for use in humans.
The tetracycline class of drugs may also have an inhibitory effect on protein glycation [29]. In animal models of diabetes[29], repeated doxycycline administration by oral intubation significantly lowered glycated serum albumin without an apparent effect on serum glucose. Also, the results of one human trial [30]demonstrated reduction in HbA1c levels three months following doxycycline usage as an antibiotic adjunct to conventional periodontal debridement procedures in patients with type 2 diabetes. Doxycycline is an inexpensive, well-tolerated, broad-spectrum antibiotic that has the additional benefit of being a potent inhibitor of host-derived matrix metalloproteinases (MMP) even at sub-antimicrobial doses[31].Enzymes of the MMP class, including MMP-9 and MMP-8,have repeatedly been shown to be inhibited in the gingival tissues and periodontal lesions (i.e., “pockets”) at sub-antimicrobial doses of doxycycline[31]. Sub-antimicrobial-dose doxycycline(SDD) (Periostat™, CollaGenex Pharmaceuticals, Inc. Newtown, PA: now Galderma R&D, Fort Worth, TX) was approved as an adjunct to scaling and root planing (mechanical debridement to reduce the bacterial burden in the periodontal pocket) for the treatment of periodontitis [32]. The additional benefit to conventional subgingival debridement derived from sub-antimicrobial doxycycline is due to the potent inhibition of extracellular matrix degradation [33], even in severe cases of periodontitis. The Food and Drug Administration (FDA)-approveddose for sub-antimicrobial doxycycline is 20 mg twice each day for up to 9 months. Antimicrobial action, and antibiotic side –effects (e.g., emergence of antibiotic resistantbacteria)is not achieved at recommended therapeutic doses [34,35].
We hypothesized that periodontal therapy(i.e., mechanical debridement to reduce the bacterial burden in the periodontal pocket or lesion) that includes SDD as an adjunct inhibits diabetes-induced protein glycation as measured by HbA1cin humans. To begin to test this hypothesis, we performed a 3-month, double-masked, randomized, controlled pilot clinical trial comparing three treatments of chronic periodontitis; subgingival debridement(scaling and root planing) plus eithersub-antimicrobial-dose doxycycline 20 mg BID for three months, anti-microbial dose doxycycline 100mg qd for 14 days, or placebo in type 2 diabetes patients with periodontal disease taking stable doses of oral hypoglycemic agents or insulin.
2. METHODS
2.1 Study subjects
Subjects were recruited during their routine maintenance visit with their primary physician at a comprehensive care diabetes center (Naomi Berrie Diabetes Center, Columbia University Medical Center) and were then questioned about their dental care; a subsequent dental screening exam determined subject eligibility. Eligible subjects were then given a comprehensive periodontal examination. Patients were eligible for this study if they had chronic periodontitis defined by loss of clinical attachment (greater than 5 mm in at least one site in each jaw quadrant), had been diagnosed with type 2 diabetes mellitus at least six months previously, and had been taking a stable dose of oral medications or insulin for at least three months.
The following exclusion criteria were applied: present use of coumadin; pregnancy, chronic non-steroidal anti-inflammatory drug use, or antibiotic use within the previous 6 months. Women of childbearing age , on becoming pregnant or not using birth control were excluded. Additional exclusionary criteria: renal impairment, severe liver disease, and grade 3 or 4 retinopathy. All patients gave written informed consent and the Committee on Human Research at Columbia University Medical Center approved the study.
2.2 Treatment protocol and follow-up visits
45 type 2 diabetes patients were randomly assigned by a computer-generated table to receive sub-antimicrobial-dose doxycycline (20mg bid for three months) plus scaling and root planing (n = 15), or antimicrobial-dose doxycycline (100mg each day for 14 days) plus scaling and root planing (n = 15), or placebo for three months plus scaling and root planing (n = 15).
Sub-antimicrobial-dose doxycycline (Periostat™)and matching placebo tablets were kindly provided by Collagenex Pharmaceuticals Inc., Newtown, PA; now Galderma R&D, Inc., Fort Worth, TX). Antimicrobial dose doxycycline tablets were obtained (Columbia University Medical Center Research Pharmacy, New York, NY) which were visually indistinguishable from the SDD or placebo tablets. All study patients were assigned to receive the same total number of pills each day for the duration of the study. Vials containing the study medications were labeled with a unique code. Each subject received two vials at the baseline visit with instructions to take one tablet from one vial in the morning, and one tablet from the other vial each evening for the first two weeks. Hence, subjects in the antimicrobial dose group received one vial containing 14 tablets of 100mg doxycycline and one vial containing 14 identical placebo tablets, while subjects in the sub-antimicrobial-dose doxycycline group received 14 tablets of 20 mg doxycycline in each vial, and placebo subjects received 14 tablets of placebo in each vial. All subjects received a second vial at a two-week follow-up with instructions to take one tablet twice each day until the pills were gone, and to return in two weeks for the one-month visit. Each of these vials contained 28 tablets of placebo, except for the SDD group, which received 28 20 mg doxycycline tablets in each vial. At the one-month visit, each subject received one large vial with instructions to take one tablet twice each day until the end of the study. Each of these large vials contained enough study medication to last for the remainder of the trial, or 120 tablets. The placebo and ADD groups received 120 tablets of placebo while the SDD group received 120 tablets of 20 mg doxycycline. Compliance was estimated by counting the remaining tablets in each returned medication vial.
2.3 Periodontal Clinical Measures
Standard periodontal disease clinical parameters including probing pocket depth, gingival recession, bleeding on probing, and plaque index were assessed at baseline, 1 and 3 months by an examiner calibrated to perform these measurements(the clinical measurements data will be presented elsewhere). Following collection of blood samples and measurement of clinical parameters, subjects received full mouth root planing and scaling with curettes and ultrasonic instruments under local anesthesia for not more than two hours. At one month and three months, blood and clinical parameters were collected as above.Adverse events were monitored and recorded throughout the study.
2.4 Analytic procedures
Measurements of HbA1c and glucoselevels were carried out on freshly drawn ethylenediaminetetraacetic acid-anti-coagulated whole blood using an automated affinity chromatography system (BioRad Micromat II, Hercules, CA). Plasma glucose was determined by the glucose oxidase method. Since subjects were seen at various times throughout the day, these are random glucose samples.
2.5 Statistical analysis
Baseline differences between the groups were tested for significance with chi-square for categorical variables or equality of variances F test for normally distributed continuous variables. Changes in outcomes (HbA1c change from baseline) between groups (HbA1c “units” are expressed as a % of total hemoglobin) were compared to baseline values for each experimental group by analysis of covariance using age and baseline levels of HbA1c or plasma glucose as covariates. Fisher's exact test was used to test categorical outcomes. Intent to treat and per protocol analysis was performed.
3. RESULTS
3.1 Compliance
A total of 45 subjects were recruited into the study. 15 subjects were randomized to SDD plus scaling and root planing, 15 to ADD plus scaling and root planing, and 15 to placebo plus scaling and root planing. Eleven patients either withdrew from the study before the 3-mo visit, or did not return for follow up visits.Compliance, as measured by counting remaining tablets, ranged from 80% to 100%. Of the 10 subjects who were lost to follow up, 3 were from the placebo group, 6 from the SDD group, and 1 from the ADD group.
3.2 Baseline characteristics
Demographic data are shown in Table I. There were no significant differences between the groups with regards to age, duration of diabetes, periodontal disease severity, or baseline HbA1c. The use and dosages of hypoglycemic agents and/or insulin were constant during the 3-month study period as verified with the patients’ treating physician. The prevalence of diabetes-related complications at the baseline appointment was similar between treatment groups. Overall, 18% of study subjects had retinopathy, 55% had neuropathy, and 9% had a nephropathy. 97% of all subjects were taking oral agents for their diabetes and accompanying conditions, including glyburide and/or metformin (n = 44), 26% of subjects were also using insulin. Other drugs taken by subjects for concurrent medical conditions included; 53% statins, 53% anti-hypertensives, and 29% aspirin. The use of these agents was similar in the three groups (not shown).
Table I.
Baseline Characteristics of the Study Subjects in the three treatment groups (mean ± Standard deviation).
| Treatment group, n | Placebo n=15 | SDD n=15 | ADD n=15 | p |
|---|---|---|---|---|
| Female, n(%) | 9 (60) | 8 (53) | 8 (53) | 0.91 |
| Age(±SD), | 53.8±2 | 53.2±3 | 54.4±2 | 0.93 |
| Probing depth, mm(±SD), | 3.33±0.8 | 3.12±0.7 | 3.66±0.8 | 0.17 |
| Clinical attachment loss, mm(±SD), | 4.10±1.5 | 3.57±1.0 | 4.54±1.2 | 0.11 |
| % Sites bleeding on probing(±SD), | 57±25 | 45±25 | 68±27 | 0.07 |
| % Sites with plaque(±SD), | 82±21 | 67±25 | 82±19 | 0.12 |
| HbA1c(%)(±SD), | 8.2±2.0 | 7.9±1.9 | 7.6±2.0 | 0.75 |
| Duration of diabetes, years(±SD), | 7.6±4.7 | 11.6±13.2 | 6.1±5.1 | 0.33 |
| Plasma glucose, mg/dl(±SD), | 222±121 | 191±83 | 182±97 | 0.13 |
3.3Primary Outcome
The data were analyzed on an intent-to-treat basis. The last recorded HbA1c values of subjects who dropped from the study or who were lost to follow up were carried forward. By intent to treat analysis, subjects in the SDD group (n=15) experienced a mean HbA1c reduction of 0.43% units (1.6) p= 0.15, while subjects in the ADD (n=15) group increased by 0.35%units (0.98) p= 0.62 and placebo (n=15) group increased by0.15%units (1.8) p= 0.20. These differences were not statistically significant.
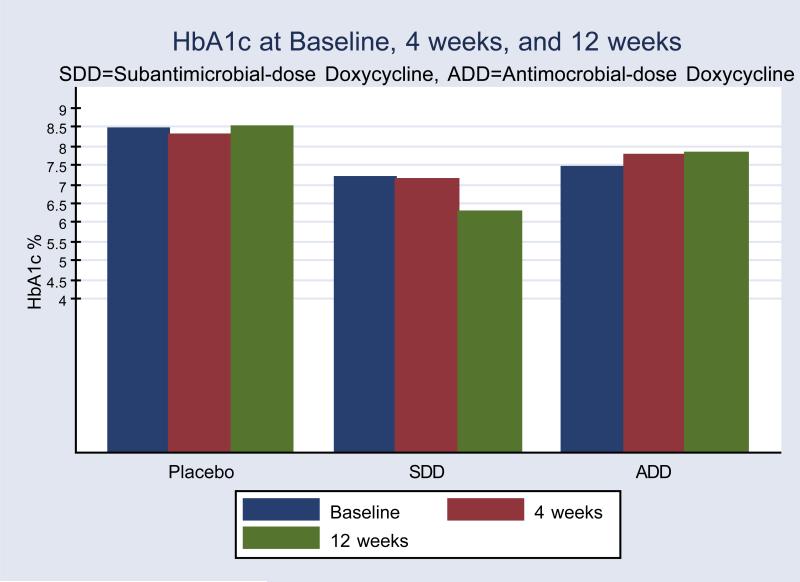
Further exploratory analysis assessed the possible effect of the treatments on ‘compliant’ subjects. In the per protocol analysis (excluding subjects who dropped from the study or did not return for follow up), three month mean HbA1c levels in the SDD group were reduced by 0.9% units from 7.2± 2.2%(±SD), to 6.3 ±1.1%, p=0.07(paired t test), which represented a 12.5% improvement, but not in the ADD (7.5± 1.8% to 7.8± 2.1%) or placebo (8.5± 2.1% to 8.5± 2.6%) groups(Figure 1).Mean HbA1c change (i.e., reduction) from baseline was significantly greater in the SDD group(-0.9± 1.7%, p=0.04)compared with the ADD group (0.4± 1.1%,) but not placebo (0.12± 2.0%, p=0.22). We also conducted an analysis to determine whether a subject had a positive response to treatment as defined by any change in HbA1c that was < 0, calculated at 3 months. According to this definitiona significantly greater proportion of subjects had a positive response to treatment in the SDD group (78%), than in the ADD (28%), or placebo (66%) groups respectively (p=0.049, Fisher's exact test)
Figure 1. Measurements of HbA1c.
In per protocol analysis, after three monthsmean HbA1c levels in the SDD group were reduced by 0.9% units(7.2± 2.2% (±SD), to 6.3 ±1.1%)compared with baseline. Mean HbA1c values in the ADD (7.5± 2.0% to 7.8± 2.1%) or placebo (8.5± 2.0% to 8.5± 2.6%) groups were not lower.
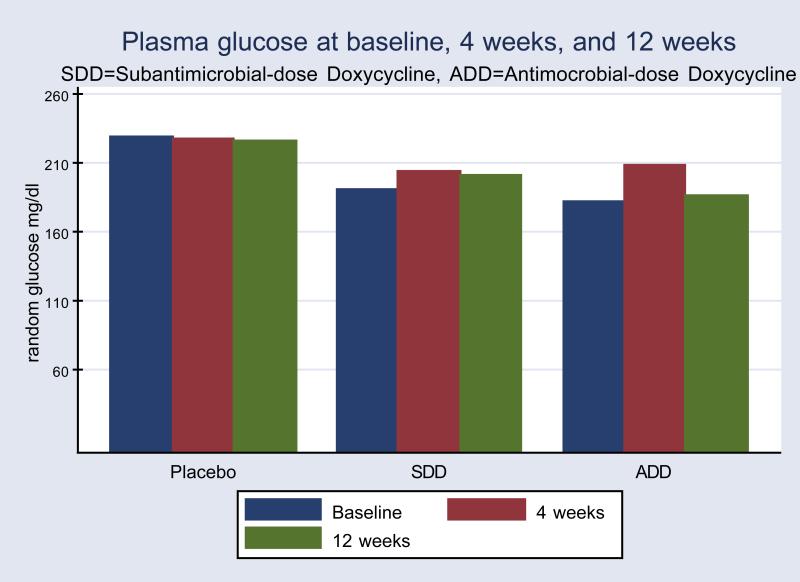
In contrast to the HbA1c changes with SDD treatment, there were no statistically significant changes in random plasma glucose levels for any group based on per-protocol analysis(Figure 2). While not statistically significant, mean random glucose levels were lower at follow up in the SDD group based on intent to treat analysis(data not shown).
Figure 2.
Mean random plasma glucose levels by treatment groups and time point. Significant differences were not seen between or within groups (per protocol analysis).
3.4Safety
There were no serious adverse events reported during the study. Differences in adverse events between groups were not observed, and the treatments appeared to be well tolerated. However,while not statistically significant, there were more subjects who did not return for follow up in the SDD group (6 of 15) than the ADD group (1 of 15) or placebo (3 of 15) group (p=0.11, Fisher's exact test).
4. DISCUSSION
Doxycycline and other tetracycline analogues have been shown to reduce tissue and serum protein glycation in streptozotocin induced diabetic animals without apparent change in serum glucose values[29]. We reasoned therefore, that doxycycline might have utility in the treatment of type 2 diabetes by reducing protein glycation. It was the hypothesis of this study that SDD could play a role in the reduction of protein glycation in humans. In this double-blind placebo-controlled randomized pilot clinical trial, we found that subjects with type 2 diabetes taking SDD twice daily for 3 months experienced reductions in HbA1c. The treatment effect of SDD on HbA1c appeared to be independent of changes in blood glucose levels because doses of hypoglycemic agents and/or insulin remained stable throughout the study. Moreover, all three groups of patients were treated for inflammatory periodontal disease by standard of care, i.e., scaling and root planing to reduce the etiological bacterial burden. Decreased HbA1c was observed in 7 of 9 patients who completed the 3 months of SDD treatment. We also observed that mean random plasma glucose was reduced during the same time periodin the group receiving SDD, but this was not statistically significant. The results of the present study are consistent with previous animal studies that have shown reductions in non-enzymatic glycation following administration of doxycycline without apparent reductions in circulating glucose[29]. Based on these results, larger human studies are warranted to test whether SDD is effective in reducing A1c.
The implications of this pilot trial are potentially far-reaching should the results be confirmed in larger and longer-term studies. First, SDD is an already approved medication for the adjunctive treatment of periodontitis. Since patients with type 2 diabetes are at increased risk for periodontitis, increased usage of this medication in the population will improve periodontal treatment outcomes, and could lead to improvements in diabetes outcomes as well. Second, as in SDD studies in non-diabetic populations[36], we observed no increased incidence of adverse events in patients with type 2 diabetes taking SDD for three months. Third, subjects were taking stable doses of oral hypoglycemic agents and/or insulin, in addition to study medication, and no adverse events were observed, indicating an apparent lack of adverse drug interactions between SDD and these agents.
Other interventional studies have examined the effects of periodontal treatment on diabetes outcomes and have included the short-term use of systemic anti-microbial doses of doxycycline. Two clinical trials to date included conventional administration ofsystemic antimicrobial dose doxycycline for individuals with diabetes and periodontitis in conjunction with scaling and root planing. Grossi in 1997showed reduction of HbA1c at three month follow up [30], whileJones et al[37]did not find significant differences between groups at 4 months. Note that in these studies, higher anti-microbial doses of doxycycline were only administered for 2 weeks. Interestingly, in the Jones study, both treatment groups achieved improved glycemic control after four months, but differences between groups were not statistically significant.
The results of the present study differ in several important aspects from those of Grossi et al.[30]. In our study, patients taking twice daily low dose doxycycline for three months experienced a reduction in HbA1c from baseline to three months while subjects taking a standard 2-week antibiotic regimen of doxycycline experienced no such reduction in HbA1c at the three month time period. Additionally, all of our study subjects were on stable doses of hypoglycemic agents and/or insulin, while medication status was not reported in the Grossi trial. The Jones study was able to take concurrent medications into account, which may in part account for improvements that were observed in both treatment groups in that study.
Differences in response to periodontal treatment outcomes in the present study did not appear to influence A1c reductions since clinical parameters were improved among all groups (data not shown). Scaling and root planing removes adherent biofilm from tooth surfaces thereby reducing the microbial and endotoxin challenge in the infected host. In larger (and longer duration, e.g., 9 months) studies of periodontitis, sub-antimicrobial-dose doxycycline given in the doses reported in the present study have been shown to be a safe and useful adjunct to scaling and root planing[33,36,38,39] and resulted in FDA approval of this regimen. As expected, scaling and root planing was effective in reducing mean probing depth and gingival bleeding measures in the present study of subjects with type 2 diabetes (not shown), and will be the subject of a future report.
While these datasupporta possible reduction of A1c by the long-term administration of doxycycline, there are several important limitations to the present study. First, the small sample size is a major limitation, and larger studies are needed in order to determine whether these results are generalizable to other patient populations. Second, the attrition rate in this study was high, at 22% with a high number of those occurring in the SDD group, resulting in only 9 completed subjects in this group. Third, the glucose values reported are random glucose values, and hence are not as reliable as fasting glucose values at indicating metabolic control. Clearly, glucose levels determine A1c levels, and we cannot rule out that variability in glucose values were the major determinant in A1c values observed in this study.Additionally, the relatively short (three month) duration of this study is a limitation. SDD is approved for long-term use, and is typically prescribed for periods of at least nine months for the treatment of periodontitis in humans. Therefore, It will be necessary to determine in future studies whether the short-term reductions in HbA1c that we observed in the present study, are maintained over longer periods. Studies of at least 6-9 months duration are needed to test the safety and efficacy in subjects with type 2 diabetes.
Nevertheless, novel approaches to treatments for diabetes and its complications are necessary in light of the increasing worldwide incidence and prevalence of type 2 diabetes.
As described in a number of larger studies of SDD in non-diabetic populations, in this pilot study serious adverse events were not observed and SDD appeared to be well tolerated. Therefore SDD use appears to be safe and effective for the treatment of periodontitis in subjects with type 2 diabetes. These data however need to be interpreted with caution given the small sample size. Clearly, larger studies of subjects with type 2 diabetes are needed to confirm whether this treatment is safe and effective for the treatment of periodontitis in patients with type 2 diabetes, and to test whether SDD is an effective adjunctive medication for the treatment of diabetes. It also remains to be determined whether long-term administration of SDD is safe and effective in reducing diabetes complications. However, based on these pilot data, such longitudinal studies appear to be warranted.
5. Conclusions
In conclusion, subjects with type 2 diabetestaking stable doses of normally prescribed hypoglycemic agents who received twice daily SDD for 3 months in addition to non-surgical periodontal therapy experienced reductions in HbA1c, while shorter-term antimicrobial doses of doxycycline and scaling and root planing alone were not effective in this regard. SDD therefore, appears to have potential as anA1c lowering agent. Larger and longer-term studies are warranted to test the safety and effectiveness of this potential new use for doxycycline for patients with type 2 diabetes.
ACKNOWLEDGMENTS
This work was supported by a Columbia University Office of Clinical Trials Pilot Award (SE), and a K23 award,DE 00449(SE), from the National Institute of Dental and Craniofacial Research (NIDCR/NIH).
Abbreviations
- SDD
sub-antimicrobial-dose doxycycline
- ADD
antimicrobial-dose doxycycline
- HbA1c
hemoglobin A1c
- MMP
matrix metalloproteinase
- FDA
Food and Drug Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors disclosed no conflicts of interest
REFERENCES
- 1.Luzio SD, Owens DR, Vora J, Dolben J, Smith H. Intravenous insulin simulates early insulin peak and reduces post-prandial hyperglycaemia/hyperinsulinaemia in type 2 (non-insulin-dependent) diabetes mellitus. Diabetes Res. 1991;16:63–67. [PubMed] [Google Scholar]
- 2.Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 3.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M. Inhibition of gapdh activity by poly(adp-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor rage as a progression factor amplifying immune and inflammatory responses. Diabetes. 2001;50:2792–2808. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelgau MM, Thompson TJ, Herman WH, Boyle JP, Aubert RE, Kenny SJ, Badran A, Sous ES, Ali MA. Comparison of fasting and 2-hour glucose and hba1c levels for diagnosing diabetes. Diagnostic criteria and performance revisited. Diabetes Care. 1997;20:785–791. doi: 10.2337/diacare.20.5.785. [DOI] [PubMed] [Google Scholar]
- 9.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: Glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 10.Yale JF, Valiquett TR, Ghazzi MN, Owens-Grillo JK, Whitcomb RW, Foyt HL. The effect of a thiazolidinedione drug, troglitazone, on glycemia in patients with type 2 diabetes mellitus poorly controlled with sulfonylurea and metformin. A multicenter, randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;134:737–745. doi: 10.7326/0003-4819-134-9_part_1-200105010-00010. [DOI] [PubMed] [Google Scholar]
- 11.Maggs DG, Buchanan TA, Burant CF, Cline G, Gumbiner B, Hsueh WA, Inzucchi S, Kelley D, Nolan J, Olefsky JM, Polonsky KS, Silver D, Valiquett TR, Shulman GI. Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:176–185. doi: 10.7326/0003-4819-128-3-199802010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: Scientific review. Jama. 2002;287:360–372. doi: 10.1001/jama.287.3.360. [DOI] [PubMed] [Google Scholar]
- 13.Uk prospective diabetes study (ukpds) group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (ukpds 33). Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 14.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loe H. Periodontal disease. The sixth complication of diabetes. DiabetesCare. 1993;16:329–334. [PubMed] [Google Scholar]
- 16.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 17.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin a1c with cardiovascular disease and mortality in adults: The european prospective investigation into cancer in norfolk. Ann Intern Med. 2004;141:413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 18.Wagner EH, Sandhu N, Newton KM, McCulloch DK, Ramsey SD, Grothaus LC. Effect of improved glycemic control on health care costs and utilization. Jama. 2001;285:182–189. doi: 10.1001/jama.285.2.182. [DOI] [PubMed] [Google Scholar]
- 19.Executive summary: Standards of medical care in diabetes--2010. Diabetes Care. 2010;33(Suppl 1):S4–10. doi: 10.2337/dc10-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan KC, Chow WS, Tam S, Bucala R, Betteridge J. Association between acute-phase reactants and advanced glycation end products in type 2 diabetes. Diabetes Care. 2004;27:223–228. doi: 10.2337/diacare.27.1.223. [DOI] [PubMed] [Google Scholar]
- 21.Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes. 2003;52:2110–2120. doi: 10.2337/diabetes.52.8.2110. [DOI] [PubMed] [Google Scholar]
- 22.Stitt A, Gardiner TA, Alderson NL, Canning P, Frizzell N, Duffy N, Boyle C, Januszewski AS, Chachich M, Baynes JW, Thorpe SR. The age inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002;51:2826–2832. doi: 10.2337/diabetes.51.9.2826. [DOI] [PubMed] [Google Scholar]
- 23.Thornalley PJ. Use of aminoguanidine (pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:31–40. doi: 10.1016/j.abb.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, Foiles PG, Freedman BI, Raskin P, Ratner RE, Spinowitz BS, Whittier FC, Wuerth JP. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24:32–40. doi: 10.1159/000075627. [DOI] [PubMed] [Google Scholar]
- 25.Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D, Schmidt AM. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996;97:238–243. doi: 10.1172/JCI118397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flyvbjerg A, Denner L, Schrijvers BF, Tilton RG, Mogensen TH, Paludan SR, Rasch R. Long-term renal effects of a neutralizing rage antibody in obese type 2 diabetic mice. Diabetes. 2004;53:166–172. doi: 10.2337/diabetes.53.1.166. [DOI] [PubMed] [Google Scholar]
- 27.Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM. Rage blockade stabilizes established atherosclerosis in diabetic apolipoprotein e-null mice. Circulation. 2002;106:2827–2835. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 28.Lalla E, Lamster IB, Feit M, Huang L, Spessot A, Qu W, Kislinger T, Lu Y, Stern DM, Schmidt AM. Blockade of rage suppresses periodontitis-associated bone loss in diabetic mice. J Clin Invest. 2000;105:1117–1124. doi: 10.1172/JCI8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan ME, Ramamurthy NS, Golub LM. Tetracyclines inhibit protein glycation in experimental diabetes. Adv Dent Res. 1998;12:152–158. doi: 10.1177/08959374980120011201. [DOI] [PubMed] [Google Scholar]
- 30.Grossi SG, Skrepcinski FB, DeCaro T, Robertson DC, Ho AW, Dunford RG, Genco RJ. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol. 1997;68:713–719. doi: 10.1902/jop.1997.68.8.713. [DOI] [PubMed] [Google Scholar]
- 31.Golub LM, Sorsa T, Lee HM, Ciancio S, Sorbi D, Ramamurthy NS, Gruber B, Salo T, Konttinen YT. Doxycycline inhibits neutrophil (pmn)-type matrix metalloproteinases in human adult periodontitis gingiva. Journal of Clinical Periodontology. 1995;22:100–109. doi: 10.1111/j.1600-051x.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 32.Caton JG, Ciancio SG, Blieden TM, Bradshaw M, Crout RJ, Hefti AF, Massaro JM, Polson AM, Thomas J, Walker C. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71:521–532. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- 33.Novak MJ, Johns LP, Miller RC, Bradshaw MH. Adjunctive benefits of subantimicrobial dose doxycycline in the management of severe, generalized, chronic periodontitis. J Periodontol. 2002;73:762–769. doi: 10.1902/jop.2002.73.7.762. [DOI] [PubMed] [Google Scholar]
- 34.Walker C, Thomas J, Nango S, Lennon J, Wetzel J, Powala C. Long-term treatment with subantimicrobial dose doxycycline exerts no antibacterial effect on the subgingival microflora associated with adult periodontitis. J Periodontol. 2000;71:1465–1471. doi: 10.1902/jop.2000.71.9.1465. [DOI] [PubMed] [Google Scholar]
- 35.Thomas J, Walker C, Bradshaw M. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472–1483. doi: 10.1902/jop.2000.71.9.1472. [DOI] [PubMed] [Google Scholar]
- 36.American diabetes association: Clinical practice recommendations 1997. Diabetes Care. 1997;20(Suppl 1):S1–70. [PubMed] [Google Scholar]
- 37.Jones JA, Miller DR, Wehler CJ, Rich SE, Krall-Kaye EA, McCoy LC, Christiansen CL, Rothendler JA, Garcia RI. Does periodontal care improve glycemic control? The department of veterans affairs dental diabetes study. Journal of clinical periodontology. 2007;34:46–52. doi: 10.1111/j.1600-051X.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 38.American diabetes association clinical practice recommendations 2001. Diabetes Care. 2001;24(Suppl 1):S1–133. [PubMed] [Google Scholar]
- 39.Supplement 1. American diabetes association: Clinical practice recommendations 2000. Diabetes Care. 2000;23(Suppl 1):S1–116. [PubMed] [Google Scholar]