Abstract
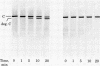
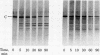
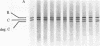
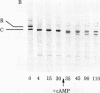
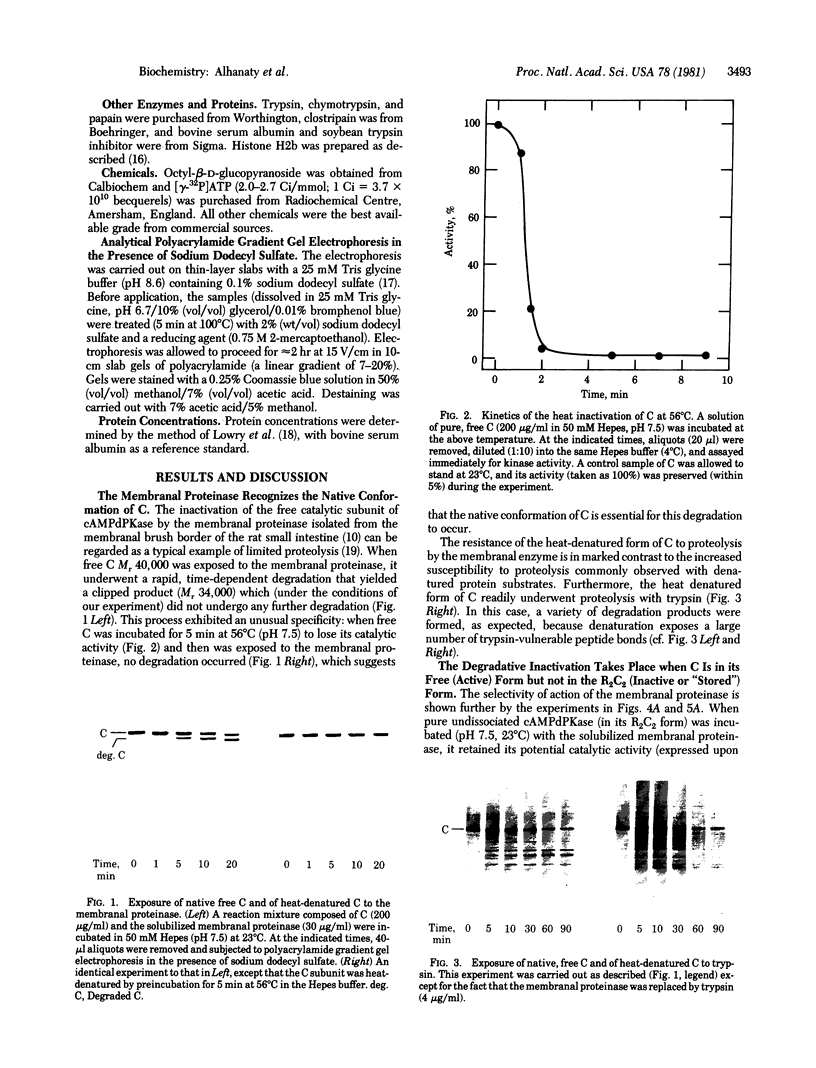
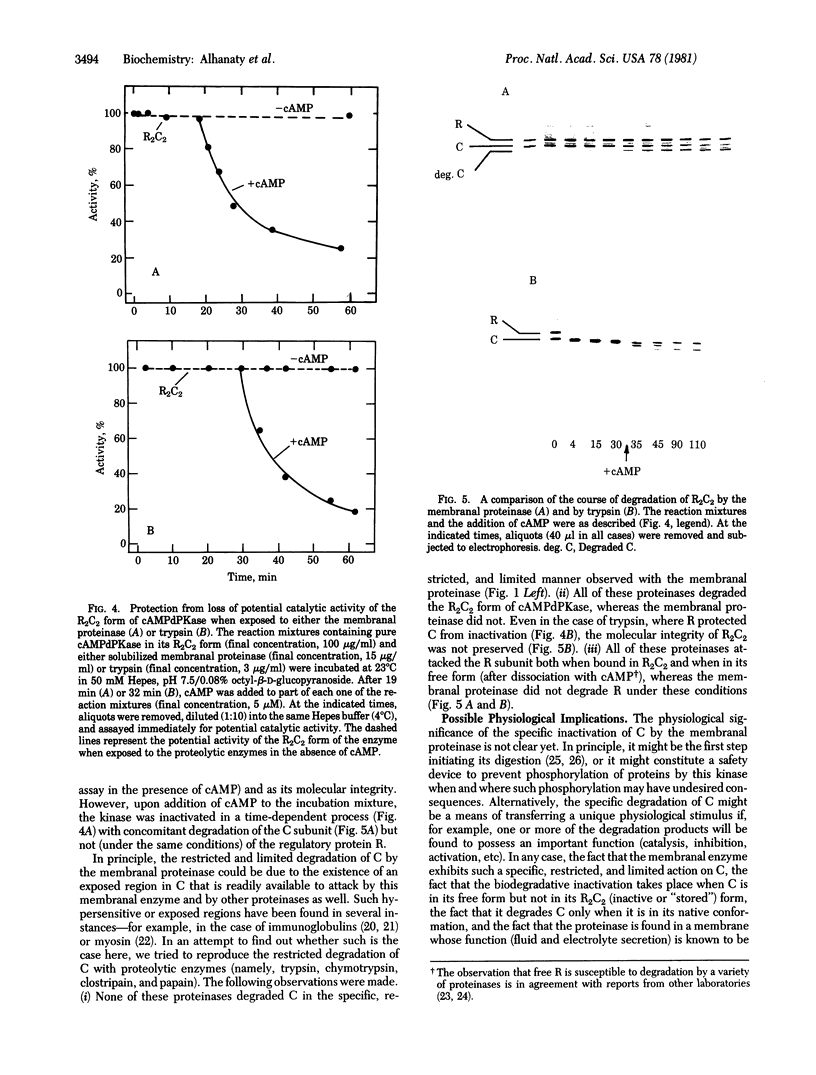
A membranal proteinase from brush-border epithelial cells of the rat small intestine was shown to bring about a restricted and limited degradation of the free catalytic subunit (C) of cyclic AMP-dependent protein kinase (ATP:protein phosphotransferase, EC 2.7.1.37) with concomitant inactivation of the kinase. This membranal proteinase exhibits a remarkable specificity. (i) It degrades C in its native conformation, but not after it has been heat-denatured. (ii) The degradation of C (Mr 40,000) does not proceed further, once a distinct clipped product (Mr 34,000) is formed. (iii) The undissociated ("stored") form of the enzyme (R2C2) is not attacked by the membranal proteinase, preserving both its potential catalytic activity and its molecular integrity. Only upon addition of cyclic AMP to release free C does the proteinase attack it. (iv) The membranal proteinase does not degrade the regulatory subunit (R), released by cyclic AMP from R2C2, although R is quite susceptible to degradation by other proteolytic enzymes. None of these features of the membranal proteinase could be reproduced with trypsin, chymotrypsin, clostripain, or papain. The specific, restricted, and limited action of this membranal enzyme raises the possibility that it may have a distinct physiological assignment associated with the bioregulation of cyclic AMP-dependent protein kinase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhanaty E., Shaltiel S. Limited proteolysis of the catalytic subunit of cAMP-dependent protein kinase--a membranal regulatory device? Biochem Biophys Res Commun. 1979 Jul 27;89(2):323–332. doi: 10.1016/0006-291x(79)90633-8. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- Builder S. E., Beavo J. A., Krebs E. G. Stoichiometry of cAMP and 1,N6-etheno-cAMP binding to protein kinase. J Biol Chem. 1980 Mar 25;255(6):2350–2354. [PubMed] [Google Scholar]
- Böhm E. L., Strickland W. N., Strickland M., Thwaits B. H., van der Westhuizen D. R., von Holt C. Purification of the five main calf thymus histone fractions by gel exclusion chromatography. FEBS Lett. 1973 Aug 15;34(2):217–221. doi: 10.1016/0014-5793(73)80797-5. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., West L., Flockhart D. A., Lincoln T. M., McCarthy D. Studies on the properties and mode of action of the purified regulatory subunit of bovine heart adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1978 Jun 10;253(11):3997–4003. [PubMed] [Google Scholar]
- Gagelmann M., Reed J., Kübler D., Pyerin W., Kinzel V. Evidence for a "mute" catalytic subunit of cyclic AMP-dependent protein kinase from rat muscle and its mode of activation. Proc Natl Acad Sci U S A. 1980 May;77(5):2492–2496. doi: 10.1073/pnas.77.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. S., Brunton L. L., Mayer S. E. Selective activation of particulate cAMP-dependent protein kinase by isoproterenol and prostaglandin E1. J Biol Chem. 1980 Jun 10;255(11):5113–5119. [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Johnson E. M., Hadden J. W., Inoue A., Allfrey V. G. DNA binding by cyclic adenosine 3',5'-monophosphate dependent protein kinase from calf thymus nuclei. Biochemistry. 1975 Aug 26;14(17):3873–3884. doi: 10.1021/bi00688a022. [DOI] [PubMed] [Google Scholar]
- Kimbert D. V. Cyclic nucleotides and their role in gastrointestinal secretion. Gastroenterology. 1974 Nov;67(5):1023–1064. [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Gani V., Jiménez J. S., Shaltiel S. Affinity labeling of the catalytic subunit of cyclic AMP-dependent protein kinase by N alpha-tosyl-L-lysine chloromethyl ketone. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3073–3077. doi: 10.1073/pnas.76.7.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter R. L., Taylor S. S. Relationships between structural domains and function in the regulatory subunit of cAMP-dependent protein kinases I and II from porcine skeletal muscle. J Biol Chem. 1979 Apr 10;254(7):2413–2418. [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Schafer D. E., Lust W. D., Sircar B., Goldberg N. D. Elevated concentration of adenosine 3':5'-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):851–856. doi: 10.1073/pnas.67.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Schmitz J., Preiser H., Maestracci D., Ghosh B. K., Cerda J. J., Crane R. K. Purification of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Weber W., Hilz H. Adenosine-3':5'-monophosphate-binding proteins from bovine kidney. Isolation by affinity chromatography and limited proteolysis of the regulatory subunit of protein kinase II. Eur J Biochem. 1978 Feb 1;83(1):215–225. doi: 10.1111/j.1432-1033.1978.tb12086.x. [DOI] [PubMed] [Google Scholar]
- Weber W., Hilz H. Stoichiometry of cAMP binding and limited proteolysis of protein kinase regulatory subunits R I and R II. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1074–1081. doi: 10.1016/0006-291x(79)91935-1. [DOI] [PubMed] [Google Scholar]
- de Jonge H. R. Cyclic nucleotide-dependent phosphorylation of intestinal epithelium proteins. Nature. 1976 Aug 12;262(5569):591–593. doi: 10.1038/262590a0. [DOI] [PubMed] [Google Scholar]