Abstract
Nerve root compression is a common cause of radiculopathy and induces persistent pain. Mammalian fibrin is used clinically as a coagulant but presents a variety of risks. Fish fibrin is a potential biomaterial for neural injury treatment because it promotes neurite outgrowth, is non-toxic, and clots readily at lower temperatures. This study administered salmon fibrin and thrombin following nerve root compression and measured behavioral sensitivity and glial activation in a rat pain model. Fibrin and thrombin each significantly reduced mechanical allodynia compared to injury alone (p<0.02). Painful compression with fibrin exhibited allodynia that was not different from sham for any day using stimulation by a 2 g filament; allodynia was only significantly different (p<0.043) from sham using the 4 g filament on days 1 and 3. By day 5, responses for fibrin treatment decreased to sham levels. Allodynia following compression with thrombin treatment were unchanged from sham at any time point. Macrophage infiltration at the nerve root and spinal microglial activation were only mildly modified by salmon treatments. Spinal astrocytic expression decreased significantly with fibrin (p<0.0001) but was unchanged from injury responses for thrombin treatment. Results suggest that salmon fibrin and thrombin may be suitable biomaterials to mitigate pain.
Keywords: Fibrin, thrombin, inflammation, astrocyte, pain, macrophage
Introduction
Chronic neck pain affects between 12–71% of the adult population, and imposes high financial burdens, with annual costs reaching over $29 billion [1–4]. Cervical radiculopathy is a leading cause of such pain, and can result from nerve root compression via injury, spinal stenosis, or disc herniation [5–8]. We have developed a rat model of cervical nerve root compression that produces persistent behavioral hypersensitivity (i.e. pain symptoms), Wallerian degeneration, and sustained inflammation at the injury site [9–12]. Painful nerve root compression also induces local and spinal nociceptive and inflammatory changes including increased expression of neuropeptides, growth factors, and cytokines, as well as glial activation, macrophage infiltration, and edema [11, 13–22]. Despite the clinical prevalence of painful radiculopathy and a clearer picture of the relationship between behavioral and inflammatory responses, treatments for effective management of the associated pain symptoms remain lacking.
Soft biocompatible hydrogels have, in some cases, shown promise as materials that limit glial cell activation and proliferation and support neuronal survival and neurite extension. Fibrin gels are one such material that has been tested in settings of trauma, including injuries to the central and peripheral nervous systems [23, 24]. Fibrin gels formed by purified fibrinogen and thrombin derived from teleost fish blood are distinctly more effective at promoting neuronal growth in vitro, while also limiting glial activation, compared to fibrin derived from mammalian sources [25, 26]. Mammalian fibrin is used clinically as a coagulant and as a substitute for sutures in some circumstances [27, 28]. However, proteins derived from human blood present the risk of disease, infection, or prion transmission, and its autologous use can present problems for those individuals with clotting disorders [23]. In some settings, both human and bovine fibrin degrade too rapidly to allow recovery of damaged cells unless they are supplemented with protease inhibitors, which present additional potential complications, such as abnormal bleeding patterns [29], venous thrombosis [30], and even anaphylaxis [31, 32]. Compared to mammalian fibrin, salmon fibrin promotes more neurite growth, shows a slower degradation profile, and is non-toxic in animal models [26]. Further, salmon fibrin clots readily at temperatures as low as 0°C, while human fibrinogen activated by thrombin clots slowly below 23°C [33]; this difference provides an advantage in situations where hypothermia must be used. A swine aortotomy model demonstrated salmon fibrin dressing to be effective in controlling severe bleeding [34]. Although there is an increased focus on the use of salmon-derived blood products for repair and tissue healing, few studies have investigated these materials for repair of neuronal tissue or for their potential to alleviate pain.
Several studies demonstrate advantages of salmon fibrin and describe thrombin as having a role in mediating many cellular cascades responsible for pain [24–26, 33, 35–37]. Yet, no study has investigated salmon fibrin and/or thrombin in the context of chronic pain. Therefore, the purpose of this study was to investigate the hypothesis that salmon fibrin and thrombin could mitigate behavioral sensitivity (i.e. mechanical allodynia) and inflammatory responses in the nerve root and spinal cord that are induced by painful cervical nerve root compression. As such, salmon fibrin and salmon thrombin were separately applied immediately following a transient painful nerve root compression in a rat model and mechanical allodynia (pain resulting from a non-noxious stimulus) was monitored after treatment to assess behavioral sensitivity. Macrophage infiltration, astrocytic activation, and microglial activity were qualitatively and quantitatively assessed by ED1, GFAP, and Iba1 immunohistochemistry in the nerve root and spinal cord following treatment, as well.
Materials and Methods
Formulation of Salmon-Derived Fibrin & Thrombin
Salmon fibrinogen and thrombin were prepared from ammonium sulfate precipitates prepared from anticoagulated salmon blood as previously described [38]. Salmon thrombin (Sea Run Holdings; Freeport, ME) was eluted from a heparin-Sepharose column and stored at high concentration (>1000 U/mL) in a buffer solution consisting of 1 M NaCl, 1 mM EGTA, 20 mM Tris, pH 7.0 and 0.6 mg/ml sucrose [39]. Immediately before use, the thrombin solution was diluted to an activity concentration of 2 NIH units/ml with neurobasal media (Invitrogen, Carlsbad, CA). Salmon fibrin was prepared by mixing 3 mg/ml purified salmon fibrinogen (Sea Run Holdings; Freeport, ME) with 2 NIH units/ml salmon thrombin in neurobasal media [40]. The fibrin solution was mixed only immediately prior to its application in order to ensure that it gelled within the desired anatomic location in vivo. All treatments were sterile upon administration.
Surgical Procedures
Male Holtzman rats (Harlan Sprague-Dawley, Indianapolis, IN), weighing 250–350 g at the start of the study, were housed under U.S. Department of Agriculture and Association for Assessment and Accreditation of Laboratory Animal Care compliant conditions with a 12-12 hour light-dark cycle and free access to food and water. All experimental procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and were carried out according to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain [41].
A painful transient nerve root compression was applied to the right C7 cervical dorsal nerve root [9–12, 16, 19, 20, 22]. All surgical procedures were performed under isoflurane inhalation anesthesia (4% for induction, 2% for maintenance). Rats were placed in a prone position and an incision was made from the base of the skull to the second thoracic vertebra. Muscle and soft tissue were resected and a hemilaminectomy and partial facetectomy were performed at C6/C7 to expose the spinal cord and C7 dorsal nerve root on the right side. In order to completely expose the nerve root, a small incision was made in the dura at the dorsal root’s insertion into the spinal cord [9]. The right C7 dorsal nerve root was compressed for 15 minutes using a 10 gram-force microvascular clip (World Precision Instruments; Sarasota, FL) [9–12, 16, 19, 20, 22]. Salmon fibrin (20 μl) was administered directly to the C7 nerve root immediately after the microvascular clip was removed and the fibrin gel was allowed to polymerize in vivo (n=6, fibrin). Under those conditions, the gelation time was about 30 minutes. A separate set of rats received salmon thrombin alone (n=4, thrombin) as treatment. Several sets of separate control groups were also included: (1) neurobasal media (n=4, NB media) administered after compression and clip removal, (2) a group of rats (n=6, injury) that underwent compression alone without any treatment, and (3) a group receiving sham procedures (n=4, sham) as surgical controls that involved exposure of the C7 dorsal nerve root only but no compression or treatment. After surgery, incisions were closed with suture and surgical staples. Rats were allowed to recover in room air while they were monitored.
Behavioral Assessment
Behavioral hypersensitivity was evaluated by measuring forepaw mechanical allodynia at postoperative days 1, 3, 5, and 7. Prior to surgery, baseline measurements were collected to define un-operated control responses for each rat. Rats were allowed to acclimate prior to each testing session. Mechanical allodynia was measured by stimulating the plantar surface of the ipsilateral forepaw using two von Frey filament strengths (2 g and 4 g) (Stoelting Co., Wood Dale, IL). Each testing session with each filament consisted of three rounds of 10 stimulations to each forepaw, with at least 10 minutes between each round to allow for an adequate rest period. For each testing session, the total number of positive responses was counted for each rat and averaged for each group. A one-way analysis of variance (ANOVA) with Bonferroni post-hoc correction tested for differences in the number of responses between groups at each time point for each filament. All statistical analyses were performed using SYSTAT software (version 10.2, SYSTAT, Richmond, CA), and significance was taken as p<0.05.
Tissue Harvest & Immunochemistry Procedures
The ipsilateral C7 dorsal nerve roots were harvested after behavioral testing on day 7 to measure macrophage infiltration at the root. The C7 nerve root from age-matched, un-operated, normal rats (n=2, normal) served as negative controls. Rats were deeply anesthetized with sodium pentobarbital (65 mg/kg) and transcardially perfused with 200 mL of phosphate buffered saline (PBS) followed by 4% formalin. The right C7 nerve roots spanning from the proximal region of the dorsal root ganglion (DRG) to their insertion in the spinal cord were removed and post-fixed in 4% formalin prior to dehydration and paraffin embedding for microtome sectioning. Three to five longitudinal nerve root sections (10 μm thick) from each rat were mounted on gelatin-coated slides. Sections were blocked with 5% normal horse serum (Invitrogen; Carlsbad, CA) for 30 minutes followed by incubation overnight at 4°C in a monoclonal antibody against the CD68 receptor (ED1, 1:250; Serotec, Kidlington, UK). The next day, sections were treated with a horse anti-mouse secondary antibody (1:200, Vector Labs, Burlingame, CA) for 60 minutes, exposed to 3,3-diaminobenzidine (Vector Labs, Burlingame, CA) for color development, and cover-slipped using Permount (Fisher, Fair Lawn, NJ) mounting medium. All antibody dilutions were previously optimized and a negative control with no primary antibody was always included to verify specificity. Sections were digitally imaged at 200X magnification and evaluated by two observers blinded to the procedural groups. Each evaluator independently rated the sections for the amount of macrophage infiltration. Tissue sections with little-to-no staining were scored (−), while those with a mild increase in macrophages over normal levels received a grade of (+), and those with intense macrophage infiltration were assigned a score of (++) [10, 14, 42]. Ratings between groups were compared using a Fisher’s Exact test, with significance at p<0.05.
Spinal cord tissue was also collected at day 7 to assess astrocytic and microglial activation. Spinal cord samples at the C6 spinal level were harvested and post-fixed as described above, transferred to 30% sucrose/PBS, and stored for 3 days at 4°C before being freeze-mounted in Histoprep OCT embedding medium (Fisher; Fair Lawn, NJ) for axial cryosectioning. Four serial spinal cord axial sections (20 μm) from each rat were prepared for free-floating immunohistochemical staining using glial fibrillary acidic protein (GFAP) as a marker of astrocytic reactivity and ionized calcium binding adaptor molecule 1 (Iba1) for microglial reactivity. Tissue sections were blocked for 60 minutes in normal goat serum (Vector Labs; Burlingame, CA) followed by incubation in a primary antibody solution containing either rabbit anti-GFAP (1:20,000; Dako, Carpinteria, CA) or rabbit anti-Iba1 (1:1000; Wako, Richmond, VA) overnight at 4°C. The following day, sections were treated with an Alexa594 conjugated goat anti-rabbit secondary antibody (1:1000; Invitrogen, Carlsbad, CA). Images were taken of the ipsilateral dorsal horns from each rat at 200X magnification. Each image was cropped to include the region of the superficial laminae and inverted for quantitative densitometry analysis. Expression levels of spinal GFAP and Iba1 were separately analyzed to quantify the percentage of pixels above a threshold determined for expression in normal un-operated samples [12, 21, 43]. Expression of GFAP and Iba1 were separately compared between groups using an ANOVA with Bonferroni post-hoc test.
Results
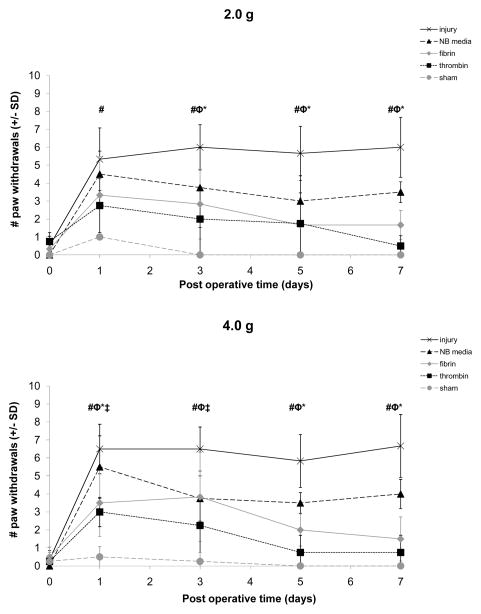
Ipsilateral mechanical allodynia following the painful compression injury alone was significantly elevated (p<0.0001) over baseline for testing with both filaments throughout the postoperative period and was significantly greater (p<0.001) than sham at all postoperative days for testing with both filaments (Figure 1). Sham procedures did not change behavioral hypersensitivity compared to baseline responses. Treatment with the neurobasal media vehicle did not alter allodynia from the injury responses, except on day 7 when it was only slightly reduced; yet, allodynia after NB media treatment remained significantly greater (p<0.01) than sham for both filaments (Figure 1). Allodynia subsequent to treatment with NB media was also greater than responses after treatment with fibrin (p<0.046) and thrombin (p<0.012) by day 7 for the 4 g filament (Figure 1). However, treatment with either salmon fibrin or thrombin significantly (p<0.02 for both treatments) reduced mechanical allodynia compared to injury levels as early as day 1 (Figure 1). This decrease in allodynia was evident for fibrin treatment overall (p<0.02) for both filaments and on most individual days (Figure 1). Although allodynia following treatment with fibrin was not different from responses from sham for testing with the 2 g filament, fibrin responses were elevated over sham using the 4 g filament on days 1 (p<0.043) and 3 (p<0.030) but returned to sham level by day 5. Treatment with thrombin reduced overall sensitivity (p<0.02) and on most individual days (Figure 1). In fact, thrombin abolished sensitivity from injury, and withdrawal responses were not different from sham or baseline responses for either filament on any day.
Figure 1.
Mechanical allodynia in the ipsilateral forepaw for injury, NB media, fibrin, thrombin, and sham for testing with the 2 g and 4 g von Frey filaments. Injury significantly increased the number of paw withdrawals relative to sham (#p<0.001) for testing with both filaments. Responses following treatment with fibrin were significantly reduced relative to injury (*p<0.02) for both filaments. Fibrin responses were not different from sham for any day of testing with the 2 g filament, and were only different on days 1 and 3 (‡p<0.043) using the 4 g filament. Treatment with thrombin significantly reduced allodynia compared to injury (φp<0.02) for testing with both filaments. Thrombin and sham responses were not different at any day for either filament. Data shown as average±standard deviation.
ED1-positive macrophages in the C7 ipsilateral dorsal root at day 7 after compression were increased relative to sham and normal. No ED1 staining was evident in the C7 ipsilateral nerve root of any of the rats undergoing sham procedures (Figure 2, Table 1). In contrast, following untreated injury, ED1 staining was increased compared to sham, with two rats exhibiting intense (++) responses in the nerve root (Figure 2, Table 1). Fibrin treatment with nerve root compression resulted in only mildly (+) increased macrophage infiltration in four rats relative to sham, but that response was reduced compared to the level of macrophage infiltration seen in injury alone (Figure 2, Table 1). Similarly, there was a slight increase in macrophage staining for thrombin treatment after compression, with one root displaying intense infiltration, two displaying mild infiltration, and a fourth showing only baseline infiltration (Figure 2, Table 1). NB media treatment also reduced macrophage infiltration compared to injury, with only one sample displaying mild (+) infiltration (Figure 2, Table 1). Although these semi-quantitative data show that injury alone induced the most ED1 staining and fibrin, thrombin, and NB media all reduced macrophage infiltration to varying degrees, no significant differences were detected between any groups.
Figure 2.
Representative images of ED1 immunostaining at the C7 nerve root at day 7. Intense macrophage infiltration was observed in the nerve root after (A) injury, but roots that had treatment with (B) fibrin or (C) thrombin displayed only mild reactivity. (D) NB media treatment and (E) sham procedures resulted in little-to-no ED1 staining. Scale bar shown is 50 μm and applies to all.
Table 1.
Immunohistochemical scoring of ED1 macrophage infiltration in the nerve root at day 7.
| Macrophage (ED1) staining | ||
|---|---|---|
| Condition | Rat ID | Ipsilateral Nerve Root |
| injury | 13 | ++ |
| 17 | ++ | |
| 22 | + | |
| 23 | − | |
| 24 | + | |
| 26 | − | |
| fibrin | 12 | + |
| 18 | − | |
| 19 | − | |
| 347 | + | |
| 348 | + | |
| 349 | + | |
| thrombin | 14 | ++ |
| 20 | + | |
| 351 | + | |
| 352 | − | |
| NB media | 11 | − |
| 15 | − | |
| 16 | + | |
| 25 | − | |
| sham | 9 | − |
| 10 | − | |
| 31 | − | |
| 33 | − | |
Assessments on a 3-point scale with the following gradients: (−) baseline staining, (+) mild response, and (++) intense response.
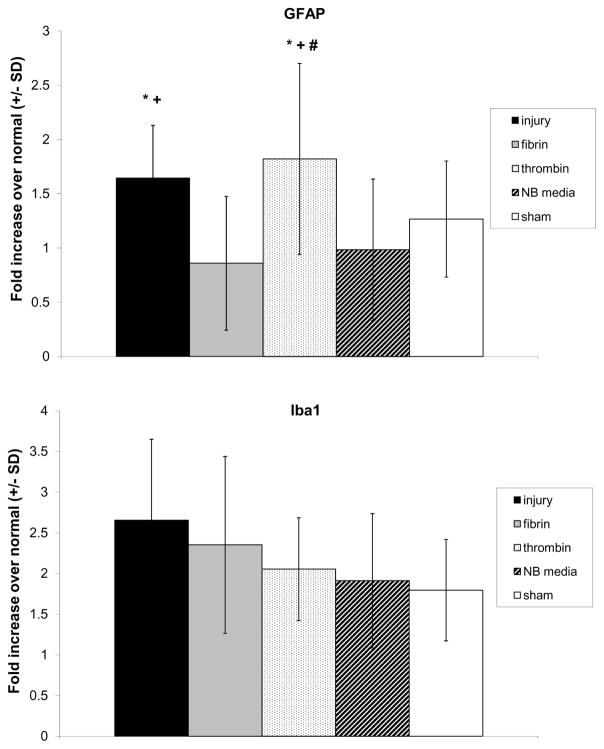
Both spinal astrocytic and microglial activation in the spinal dorsal horn increased at day 7 following injury (Figures 3 & 4). GFAP expression in the spinal cord following injury was significantly elevated over both fibrin (p<0.0001) and NB media (p<0.027). Despite treatment with thrombin producing a decrease in allodynia (Figure 1), thrombin treatment did not reduce spinal astrocytic activation, which remained elevated over fibrin (p<0.0001), NB media (p<0.0001), and sham (p<0.002) (Figures 3 & 4). Spinal Iba1 expression increased after injury and did decrease slightly with treatment by fibrin, thrombin, and NB media, as well as for sham procedures (Figures 3 & 4). However, consistent with macrophage staining in the nerve root, spinal microglial activation was not significantly different for any group (Figure 4).
Figure 3.
Representative C6 spinal cord sections on the side ipsilateral to the nerve root compression showing staining against GFAP (A–E) and Iba1 (F–J) at day 7 for injury, fibrin, thrombin, NB media, and sham. GFAP expression increased following injury and thrombin procedures; Iba1 expression was not significantly different between any group. Scale bar shown is 50 μm and applies to all.
Figure 4.
Automated densitometry quantifying the percentage of positive pixels in the spinal cord reactive for (A) GFAP and (B) Iba1 staining at day 7. GFAP reactivity increased following injury relative to fibrin (p<0.0001) and NB media (p<0.027). Injury with thrombin treatment also exhibited increased GFAP reactivity compared to fibrin (p<0.0001), NB media (p<0.0001) and sham (p<0.002). Iba1 expression was elevated after injury and decreased with treatment of fibrin, thrombin, NB media, and for sham procedures, but was not significant. Asterisk (*) indicates a significant increase over fibrin, plus sign (+) indicates a significant increase over NB media, and pound sign (#) indicates a significant increase over sham. Data shown as average±standard deviation.
Discussion
Effective clinical treatments for radiculopathy are lacking despite its high incidence. To our knowledge, this is the first study to investigate the effects of salmon fibrin and/or thrombin on behavioral outcomes and inflammatory responses in painful neural tissue injury. Both fibrin and thrombin treatments attenuated allodynia produced by nerve root compression for the duration of the study (Figure 1). Furthermore, fibrin significantly decreased spinal astrocytic activation but only slightly decreased macrophage infiltration surrounding the nerve root and spinal microglial activation (Figures 2–4, Table 1). Thrombin did not change spinal astrocytic activation from injury levels but did slightly decrease microglial expression in the nerve root and spinal cord (Figures 2–4, Table 1). These results may be useful to improve treatments for radiculopathy and lead to a better understanding of the mechanisms by which salmon fibrin and thrombin modulate and attenuate pain.
Restoration of normal tissue structure and function after traumatic injury depends on both the chemical and physical features at the wound site. In particular, abnormally rigid structures formed as the result of blood clotting, cell contraction, and extracellular matrix deposition and cross linking can prevent the restoration of normal soft tissues and prolong or exacerbate the inflammatory response [44]. In the context of neural tissue trauma in which damage to neurons and glia is accompanied by bleeding, soft gels formed by coagulation proteins have the potential both to stop bleeding and provide a sufficiently soft provisional matrix to prevent scarring and promote normal healing. Coagulation proteins, however, also exhibit pro-inflammatory effects in part due to sites in mammalian fibrinogen that can activate microglia, and in the multiple effects of mammalian thrombin on inflammatory and other cell receptors [35, 45]. Fibrinogen and thrombin prepared from non-mammalian sources such as salmon blood might potentially lessen the inflammatory effects of fibrin while maintaining its pro-coagulant properties. The salmon fibrin used in the current study significantly decreased spinal GFAP expression but only slightly reduced macrophage infiltration in the nerve root and spinal Iba1 expression (Figures 2–4, Table 1). These results indicate that at day 7, salmon fibrin effectively mediated the astrocytic aspect of the inflammatory response and mildly affected microglial expression.
Coagulation and toxicity profiles have been conducted using animal models to ensure the safety and efficacy of salmon fibrin. Intraperitoneal injections of salmon fibrin in both rats and rabbits resulted in no negative effects regarding coagulation or cross-reactivity. After repeated administration of salmon fibrin, animals produced antibodies against the salmon proteins, but did not produce antibodies that cross-reacted with host proteins [33, 46]. Also, there was no effect in coagulation tests, a result that was also found in studies of swine that had been treated with salmon fibrin [47, 48]. These findings are particularly important given the potential for antibody production and subsequent cross-reactivity in response to the use of non-autologous mammalian coagulation proteins which can lead to clotting disorders [49, 50]. In addition to cross-reactivity, mammalian fibrin presents the risk of infectious disease transmission to a host; salmon pathogens are not known to be transmissible to mammals, partly due to the fact that salmon are coldwater creatures and viruses are deactivated at human body temperatures [51]. Collectively, these studies indicate that salmon fibrin is non-toxic and poses minimal risk of disease transmission and cross-reactivity.
Previous studies have shown that salmon fibrinogen and thrombin are nearly indistinguishable from their human analogs in the production of fibrin gels and in preventing bleeding in massive tissue injuries such as an aorta injury [34], but there are subtle differences between human and salmon thrombin in the context of human platelet activation [39]. The similarity in clotting activity of human and salmon proteins is consistent with the establishment of the coagulation system before the evolutionary divergence of mammals and fish. In contrast, the immune systems of mammals and fish are highly divergent, and therefore other functions such as those on human inflammatory cell protease receptors might be expected to differ. Indeed, the site in the gamma chain of human fibrinogen that is predicted to stimulate microglial cells is absent in salmon fibrinogen [24, 26]. In the current study, while not significant, spinal microglial activation at day 7 was slightly decreased for fibrin and thrombin treatments, indicating that these salmon treatments may not have stimulated these cells (Figures 3 & 4). Because microglia initiate pain while astrocytes participate in later stages to maintain pain [52, 53], this difference may be responsible for the differential effects on these two cells evident in this study. Indeed, astrocytic responses are more robustly modulated than either macrophage infiltration or microglial expression at day 7 (Figures 2–4, Table 1).
Although thrombin administration did abolish mechanical allodynia, GFAP expression after this treatment was unchanged from injury at day 7 (Figures 1, 3 & 4). Thrombin stimulates astrocytic outgrowth and proliferation as a mechanism of protection against cell death, regulates neuronal death and neurite outgrowth, and affects pain transmission by mediating the protease-activated receptors, PAR1 and PAR4 [35, 37]. This activity may explain why spinal astrocytic activation on day 7 after thrombin treatment did not differ from injury in the current study. An in vitro study modeling metabolic insults in rat astrocytes and hippocampal neurons showed that thrombin protected against damage and cell death as early as 1 hour after insult by stimulating astrocytic proliferation and outgrowth. Further, while high concentrations of thrombin led to neuronal and astrocytic cell death under normal conditions, elevated thrombin levels actually protected those cells under stressed conditions [37]. In the current study, salmon thrombin was administered directly to the injured nerve root immediately after its compression, which may have stimulated astrocytes as a means of neuronal survival, particularly in response to early cytokine signaling that is known to occur within the first hour after compression in this model [20]. Taken together, these studies suggest that salmon thrombin serves a critical, protective role in the early stages of CNS injury by encouraging astrocytic growth while acting on PAR1 and PAR4 to prevent hypersensitivity.
Although this study evaluated tissue responses at day 7, changes in early microglial and cytokine signaling in response to fibrin and thrombin are still unknown for this model. While the current findings at day 7 do not show significant changes (Figures 3 & 4), it appears that spinal microglial activation may be affected by fibrin and thrombin and it is possible that such changes may be more robust at an earlier time point, such as day 1. In fact, a previous study using this injury model showed that spinal astrocytic and microglial activation exhibit different temporal responses [12]; so, measuring both glial cell responses at a time early after injury and treatment may provide additional insight into the mechanisms by which salmon fibrin and thrombin attenuate pain. Likewise, because macrophage infiltration into the nerve root at day 7 did slightly decrease following each of the salmon treatments (Figure 2, Table 1), it is possible that such responses may actually be diminished at earlier time points. Additional studies defining the temporal cellular and inflammatory responses will provide a clearer understanding of the potential mechanisms mediating the pain responses. Additional studies with this model have also shown that cytokine responses increase in the spinal cord and dorsal root ganglion (DRG) as early as 1 hour after compression [20, 43], providing another potential mechanism by which pain may be modulated following salmon treatments. While the exact mechanism of action by which salmon fibrin and thrombin reduce pain is still unknown, the current findings do support previous work with varied therapeutic approaches for eliminating pain by the administration of pharmacologic formulations at the site of injury. For example, a hydrogel with controlled release of glial cell line-derived neurotrophic factor placed at the root or early intervention with local administration of the soluble TNF-α receptor to the injured nerve root attenuated both allodynia and the associated inflammatory responses in the DRG [16, 20]. Taken together with the results of this current study, it is likely that biomaterials that provide sustained delivery of anti-inflammatory and neural cell promoting factors provide the best promise for treating pain from neural trauma. In prior studies, the effects of treatment on the responses in the DRG were also evaluated. While small and medium diameter neurons in the DRG are important in pain due to their classification as nociceptors [54], they were not evaluated in the current study. Because thrombin has also been reported to regulate neuronal death [35, 37], it would be interesting to assess the survival and function of DRG neurons in the context of antinociceptive salmon-derived treatments. In particular, future work evaluating the effects of salmon fibrin and thrombin on cultured neurons, as well as relevant immune cells, is needed to elucidate the specific cellular and molecular pathways by which inflammation and neuronal plasticity may be modulated.
The current study supports the potential utility of salmon fibrin as a biomaterial to improve wound healing of soft tissues, such as that surrounding the nerve root. The low elastic modulus of 3 mg/ml fibrin and previous results showing selective growth of neurons and lack of astrocyte activation in these gels in vitro [25] are consistent with the immediate decrease in allodynia and the sustained decrease in spinal GFAP staining observed after salmon fibrin treatment in the current study (Figures 1, 3 & 4). An unexpected result was that salmon thrombin alone was effective in mediating behavioral outcomes in addition to fibrin treatment (Figure 1). In part, this effect might be due to the rapid formation of soft gels formed by salmon thrombin and endogenous rat fibrinogen around the injured nerve root; but this result suggests that there might be a more direct and active role of salmon thrombin either to activate a pain-reducing pathway, or else inhibit a pain-producing pathway. Thrombin has previously been investigated for its role in nociception. Two in vivo studies show that intraplantar injection of either thrombin or a PAR1 agonist reduced inflammatory carrageenan-induced mechanical hyperalgesia in rats and mice [36, 55]. Our study extends these findings, demonstrating that, in addition to mammalian thrombin, salmon thrombin is effective at mediating behavioral hypersensitivity. Although neurobasal media is used commonly to promote neuronal growth and survival [56–58], neuronal responses were not evaluated in this study. Therefore, it is possible that the neurobasal media itself may have worked synergistically with the fibrin and thrombin to alleviate allodynia (Figure 1). However, the fact that treatment with this vehicle only slightly reduced allodynia relative to injury responses (Figure 1), suggests that the effects of the neurobasal media alone may not be sufficient to mediate the pain outcomes. Regardless, future studies should focus on defining the specific mechanisms by which the neuronal and inflammatory cascades are modulated and which aspects are specific to fibrin, thrombin, and/or other materials.
Conclusions
In summary, application of salmon fibrin and salmon thrombin following painful cervical nerve root compression attenuated behavioral hypersensitivity in a rat model of cervical radiculopathy. At day 7, spinal astrocytic expression was reduced after fibrin treatment, but remained elevated after thrombin treatment. At this same time point, both treatments only slightly decreased macrophage infiltration in the nerve root and microglial responses in the spinal cord relative to injury alone. These changes suggest that salmon fibrin and thrombin may play neuroprotective roles by affecting glial responses and hypersensitivity. The application of salmon coagulants in this radiculopathy model supports previous studies in our lab showing the efficacy of simple, direct administration to the injury site. These data suggest that salmon fibrin and thrombin, in addition to posing promising material advantages over their mammalian counterparts, may have potential to alleviate pain.
Acknowledgments
The authors gratefully acknowledge Dr. Evelyn Sawyer (Sea Run Holdings, Inc., Freeport, ME) for supplying the salmon fibrin and thrombin used in this study and Dr. La’Toya Latney for her assistance with statistical analysis. This work was funded by the NIH (R44NS048732-02) and the Catharine D. Sharpe Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Côté P, Cassidy JD, Carroll L. The Saskatchewan health and back pain survey. The prevalence of neck pain and related disability in Saskatchewan adults. Spine. 1998;23:1689–1698. doi: 10.1097/00007632-199808010-00015. [DOI] [PubMed] [Google Scholar]
- 2.Côté P, Cassidy JD, Carroll LJ, Kristman V. The annual incidence and course of neck pain in the general population: a population-based cohort study. Pain. 2004;112:267–273. doi: 10.1016/j.pain.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Haldeman S, Carroll L, Cassidy JD, Schubert J, Nygren A. The bone and joint decade 2000–2010 task force on neck pain and its associated disorders. Spine. 2008;33:S5–S7. doi: 10.1097/BRS.0b013e3181643f40. [DOI] [PubMed] [Google Scholar]
- 4.Freeman MD, Croft AC, Rossignol AM, Weaver DS, Reiser M. A review and methodologic critique of the literature refuting whiplash syndrome. Spine. 1999;24:86–96. doi: 10.1097/00007632-199901010-00022. [DOI] [PubMed] [Google Scholar]
- 5.Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the Maine lumbar spine study. Spine. 2005;30:927–935. doi: 10.1097/01.brs.0000158954.68522.2a. [DOI] [PubMed] [Google Scholar]
- 6.Frymoyer JW. Back pain and sciatica. N Engl J Med. 1988;318:291–300. doi: 10.1056/NEJM198802043180506. [DOI] [PubMed] [Google Scholar]
- 7.Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine. 1995;20:11–19. doi: 10.1097/00007632-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Ohnmeiss DD, Vanharanta H, Ekholm J. Degree of disc disruption and lower extremity pain. Spine. 1997;22:1600–1605. doi: 10.1097/00007632-199707150-00015. [DOI] [PubMed] [Google Scholar]
- 9.Hubbard RD, Winkelstein BA. Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: mechanical factors in painful neck injuries. Spine. 2005;30:1924–1932. doi: 10.1097/01.brs.0000176239.72928.00. [DOI] [PubMed] [Google Scholar]
- 10.Hubbard RD, Winkelstein BA. Dorsal root compression produces myelinated axonal degeneration near the biomechanical thresholds for mechanical behavioral hypersensitivity. Exp Neurol. 2008;212:482–489. doi: 10.1016/j.expneurol.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothman SM, Kreider RA, Winkelstein BA. Spinal neuropeptide responses in persistent and transient pain following cervical nerve root injury. Spine. 2005;30:2491–2496. doi: 10.1097/01.brs.0000186316.38111.4b. [DOI] [PubMed] [Google Scholar]
- 12.Rothman SM, Winkelstein BA. Chemical and mechanical nerve root insults induce differential behavioral sensitivity and glial activation that are enhanced in combination. Brain Res. 2007;1181:30–43. doi: 10.1016/j.brainres.2007.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–175. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 14.Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- 15.Hashizume H, DeLeo JA, Colburn RW, Weinstein JN. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine. 2000;25:1206–1217. doi: 10.1097/00007632-200005150-00003. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard RD, Martínez JJ, Burdick JA, Winkelstein BA. Controlled release of GDNF reduces nerve root-mediated behavioral hypersensitivity. J Orthop Res. 2009;27:120–127. doi: 10.1002/jor.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi S, Sasaki S, Shimada S, Kaneyasu M, Mizukami Y, Kitade I, et al. Changes of calcitonin gene-related peptide in primary sensory neurons and their central branch after nerve root compression of the dog. Arch Phys Med Rehabil. 2005;86:527–533. doi: 10.1016/j.apmr.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 18.Olmarker K, Rydevik B, Holm S. Edema formation in spinal nerve roots induced by experimental, graded compression. An experimental study on the pig cauda equina with special reference to differences in effects between rapid and slow onset of compression. Spine. 1989;14:569–573. [PubMed] [Google Scholar]
- 19.Rothman SM, Guarino BB, Winkelstein BA. Spinal microglial proliferation is evident in a rat model of painful disc herniation both in the presence of behavioral hypersensitivity and following minocycline treatment sufficient to attenuate allodynia. J Neurosci Res. 2009;87:2709–2717. doi: 10.1002/jnr.22090. [DOI] [PubMed] [Google Scholar]
- 20.Rothman SM, Huang Z, Lee KE, Weisshaar CL, Winkelstein BA. Cytokine mRNA expression in painful radiculopathy. J Pain. 2009;10:90–99. doi: 10.1016/j.jpain.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothman SM, Nicholson KJ, Winkelstein BA. Time-dependent mechanics and measures of glial activation and behavioral sensitivity in a rodent model of radiculopathy. J Neurotrauma. 2010;27:803–814. doi: 10.1089/neu.2009.1045. [DOI] [PubMed] [Google Scholar]
- 22.Thorek DL, Weisshaar CL, Czupryna JC, Winkelstein BA, Tsourkas A. Superparamagnetic iron oxide-enhanced magnetic resonance imaging of neuroinflammation in a rat model of radicular pain. Mol Imaging. 2011;10:206–214. [PubMed] [Google Scholar]
- 23.Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface. 2009;6:1–10. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uibo R, Laidmäe I, Sawyer ES, Flanagan LA, Georges PC, Winer JP, et al. Soft materials to treat central nervous system injuries: Evaluation of the suitability of non-mammalian fibrin gels. Biochim Biophys Acta. 2009;1793:924–930. doi: 10.1016/j.bbamcr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju YE, Janmey PA, McCormick ME, Sawyer ES, Flanagan LA. Enhanced neurite growth from mammalian neurons in three-dimensional salmon fibrin gels. Biomaterials. 2007;28:2097–2108. doi: 10.1016/j.biomaterials.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albala DM, Lawson JH. Recent clinical and investigational applications of fibrin sealant in selected surgical specialties. J Am Coll Surg. 2006;202:685–697. doi: 10.1016/j.jamcollsurg.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Weisel J, Cederholm-Williams S. Fibrinogen and Fibrin: characterization, processing and medical applications. In: Domb A, Kost J, Wiseman D, editors. Handbook of biodegradable polymers. Amsterdam: Harwood; 1997. pp. 347–365. [Google Scholar]
- 29.Stanworth SJ, Bolton MJ, Hay CR, Shiach CR. Increased bleeding in HIV-positive haemophiliacs treated with antiretroviral protease inhibitors. Haemophilia. 1998;4:109–114. doi: 10.1046/j.1365-2516.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- 30.Majluf-Cruz A, Silva-Estrada M, Sánchez-Barboza R, Montiel-Manzano G, Treviño-Pérez S, Santoscoy-Gómez M, et al. Venous thrombosis among patients with AIDS. Clin Appl Thromb Hemost. 2004;10:19–25. doi: 10.1177/107602960401000104. [DOI] [PubMed] [Google Scholar]
- 31.Beierlein W, Scheule AM, Antoniadis G, Braun C, Schosser R. An immediate, allergic skin reaction to aprotinin after reexposure to fibrin sealant. Transfusion. 2000;40:302–305. doi: 10.1046/j.1537-2995.2000.40030302.x. [DOI] [PubMed] [Google Scholar]
- 32.Shirai T, Shimota H, Chida K, Sano S, Takeuchi Y, Yasueda H. Anaphylaxis to aprotinin in fibrin sealant. Intern Med. 2005;44:1088–1089. doi: 10.2169/internalmedicine.44.1088. [DOI] [PubMed] [Google Scholar]
- 33.Laidmäe I, McCormick ME, Herod JL, Pastore JJ, Salum T, Sawyer ES, et al. Stability, sterility, coagulation, and immunologic studies of salmon coagulation proteins with potential use for mammalian wound healing and cell engineering. Biomaterials. 2006;27:5771–5779. doi: 10.1016/j.biomaterials.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 34.Rothwell SW, Reid TJ, Dorsey J, Flournoy WS, Bodo M, Janmey PA, et al. A salmon thrombin-fibrin bandage controls arterial bleeding in a swine aortotomy model. J Trauma. 2005;59:143–149. doi: 10.1097/01.ta.0000171528.43746.53. [DOI] [PubMed] [Google Scholar]
- 35.García PS, Gulati A, Levy JH. The role of thrombin and protease-activated receptors in pain mechanisms. Thromb Haemost. 2010;103:1145–1151. doi: 10.1160/TH09-12-0848. [DOI] [PubMed] [Google Scholar]
- 36.Martin L, Augé C, Boué J, Buresi MC, Chapman K, Asfaha S, et al. Thrombin receptor: An endogenous inhibitor of inflammatory pain, activating opioid pathways. Pain. 2009;146:121–129. doi: 10.1016/j.pain.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Vaughan PJ, Pike CJ, Cotman CW, Cunningham DD. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. J Neurosci. 1995;15:5389–5401. doi: 10.1523/JNEUROSCI.15-07-05389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winer JP, Oake S, Janmey PA. Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation. PLoS One. 2009;4:e6382. doi: 10.1371/journal.pone.0006382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michaud SE, Wang LZ, Korde N, Bucki R, Randhawa PK, Pastore JJ, et al. Purification of salmon thrombin and its potential as an alternative to mammalian thrombins in fibrin sealants. Thromb Res. 2002;107:245–254. doi: 10.1016/s0049-3848(02)00333-x. [DOI] [PubMed] [Google Scholar]
- 40.Wang LZ, Gorlin J, Michaud SE, Janmey PA, Goddeau RP, Kuuse R, et al. Purification of salmon clotting factors and their use as tissue sealants. Thromb Res. 2000;100:537–548. doi: 10.1016/s0049-3848(00)00362-5. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 42.Rutkowski MD, Winkelstein BA, Hickey WF, Pahl JL, DeLeo JA. Lumbar nerve root injury induces central nervous system neuroimmune activation and neuroinflammation in the rat: relationship to painful radiculopathy. Spine. 2002;27:1604–1613. doi: 10.1097/00007632-200208010-00003. [DOI] [PubMed] [Google Scholar]
- 43.Rothman SM, Winkelstein BA. Cytokine antagonism reduces pain and modulates spinal astrocytic reactivity after cervical nerve root compression. Ann Biomed Eng. 2010;38:2563–2576. doi: 10.1007/s10439-010-0012-8. [DOI] [PubMed] [Google Scholar]
- 44.Ryu JK, McLarnon JG. A leaky blood–brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med. 2009;13:2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryu JK, Davalos D, Akassoglou K. Fibrinogen signal transduction in the nervous system. J Thromb Haemost. 2009;7:151–154. doi: 10.1111/j.1538-7836.2009.03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laidmäe I, Salum T, Sawyer ES, Janmey PA, Uibo R. Characterization of the biological effect of fish fibrin glue in experiments on rats: immunological and coagulation studies. J Biomed Mater Res A. 2010;93:29–36. doi: 10.1002/jbm.a.32505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothwell SW, Sawyer E, Dorsey J, Flournoy WS, Settle T, Simpson D, et al. Wound healing and the immune response in swine treated with a hemostatic bandage composed of salmon thrombin and fibrinogen. J Mater Sci Mater Med. 2009;20:2155–2166. doi: 10.1007/s10856-009-3769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothwell SW, Settle T, Wallace S, Dorsey J, Simpson D, Bowman JR, et al. The long term immunological response of swine after two exposures to a salmon thrombin and fibrinogen hemostatic bandage. Biologicals. 2010;38:619–628. doi: 10.1016/j.biologicals.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ortel TL, Mercer MC, Thames EH, Moore KD, Lawson JH. Immunologic impact and clinical outcomes after surgical exposure to bovine thrombin. Ann Surg. 2001;233:88–96. doi: 10.1097/00000658-200101000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schoenecker JG, Johnson RK, Lesher AP, Day JD, Love SD, Hoffman MR, et al. Exposure of mice to topical bovine thrombin induces systemic autoimmunity. Am J Pathol. 2001;159:1957–1969. doi: 10.1016/S0002-9440(10)63043-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolf K. Fish viruses and fish viral diseases. Ithaca, NY: Cornell University Press; 1988. [Google Scholar]
- 52.Romero-Sandoval A, Chai N, Nutile-McMenemy N, Deleo JA. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. 2008;1219:116–126. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. Philadelphia: Elsevier; 2008. pp. 3–34. [Google Scholar]
- 55.Asfaha S, Brussee V, Chapman K, Zochodne DW, Vergnolle N. Proteinase-activated receptor-1 agonists attenuate nociception in response to noxious stimuli. Br J Pharmacol. 2002;135:1101–1106. doi: 10.1038/sj.bjp.0704568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 57.Brewer GJ, Boehler MD, Jones TT, Wheeler BC. NbActiv4 medium improvement to Neurobasal/B27 increases neuron synapse densities and network spike rates on multielectrode arrays. J Neurosci Methods. 2008;170:181–187. doi: 10.1016/j.jneumeth.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kivell BM, McDonald FJ, Miller JH. Serum-free culture of rat post-natal and fetal brainstem neurons. Brain Res Dev Brain Res. 2000;120:199–210. doi: 10.1016/s0165-3806(00)00010-9. [DOI] [PubMed] [Google Scholar]