Abstract
In nonhuman primates, anxiety levels are typically assessed by observing social hierarchies or behavior in an intruder task. As measures of anxiety might influence performance on a particular cognitive task, it is important to analyze these measures in the same room as used for the cognitive task. As we use a playroom for the spatial maze test, we classified elderly female rhesus macaques (Macaca mulatta) monkeys, as bold or reserved monkeys based on the time spent in specific areas of this room. Based on their exploratory behavior in the playroom, bold monkeys were defined as animals that spent 20% more time in the unprotected areas of the room than in the protected areas, whereas reserved monkeys spent a comparable amount of time in both areas. MRI analyses showed that reserved monkeys had a smaller amygdala compared to bold monkeys but there were no group differences in hippocampal volumes. In addition, the amount of time spent in the corners of the room was negatively correlated with the right and total amygdala size. Finally, reserved monkeys showed a lower phMRI response to the muscarinic receptor antagonist scopolamine compared to the bold monkeys. Thus, in elderly female nonhuman primates measures of anxiety are associated with structural amygdala differences and hippocampal muscarinic receptor function.
Keywords: anxiety, nonhuman primate, amygdala, hippocampus, scopolamine, phMRI
1. Introduction
Anxiety is a general term used to describe a variety of physiological and psychological responses to anxiety-provoking or aversive stimuli. “Anxiety levels” are readily assessed using various types of tasks. In humans, many different neuropsychological tests have been developed to assess anxiety levels, including the Beck Anxiety Scale (Janeway, 2009, Maizels, et al., 2006). These tests usually involve surveys of patients or caregivers, and assess anxiety levels over a period of time, also referred to as “trait anxiety” (Smith and Lay, 1974). A smaller amygdala size was associated with enhanced anxiety-like behaviors (Irle, et al., 2009, Weniger, et al., 2008, Weniger, et al., 2009). Particularly with the elderly, enhanced anxiety has been linked to a decrease in cognitive function as well as increase in morbidity (Beck, 2005, Castaneda, et al., 2008, Jackson, et al., 2009). The neurobiological pathways of anxiety are not fully understood; however, the muscarinic receptor system appears to play a role in regulating measures of anxiety, especially in woman (Furey, et al., 2010).
In nonhuman primates, measures of anxiety are usually assessed with social hierarchies, intruder or predator confrontation tasks, or presentation of a stressful stimulus such as a toy snake (Amaral, 2003, Amaral, et al., 2003, Barros, et al., 2007, Barros, et al., 2008, Cagni, et al., 2009, Lesch, 2001, Sullivan, et al., 2010, Wrase, et al., 2006). In one version of the intruder task, measures of anxiety are assessed in response to 5 min social isolation in a test room followed by a 30 sec exposure to two humans wearing a leather capture glove (Newman and Farley, 1995). In another version, measures of anxiety are compiled when the monkey is alone in the room, a human intruder enters the room sitting in a profile position (not looking at the monkey), and when the human intruder stares at the monkey (Fairbanks, et al., 2004). In addition to these tests, an initially stressful wire-mesh box attached to the home cage has also been used to assess anxiety levels in nonhuman primates. Further, removing of a monkey from the home cage in a familiar multiple animal housing room and placing the monkey in a novel test cage in an unfamiliar room not containing other monkeys, has also been used to assess anxiety levels (Parker, et al., 2004). The novel environment contained polyvinyl chloride perches and a variety of familiar and unfamiliar objects. Monkeys also had access to biscuits, cantaloupe, marshmallows, and water throughout testing. Tests lasted 30 minutes, once a day for 5 consecutive days. Object exploration and food consumption were analyzed. As these tasks are performed in a home cage, it is conceivable that a freely moving animal might perform differently in an environment outside of its home cage, with different reference points and protected places. As measures of anxiety might influence performance on a particular cognitive task, it is important to analyze these measures in the same room in which they are cognitively tested. It is conceivable that such measures are distinct from those assessed using anxiety tests like the intruder test described above.
The anxiety tasks are amygdala- and hippocampus-dependent (Oler, et al., 2010, Parker, et al., 2004). In young Rhesus macaques, increased brain activity, as assessed by high-resolution 18F-labelled deoxyglucose positron emission tomography, in the amygdala and hippocampus could predict anxious temperament assessed as response in a test cage for 30 min in a potentially threatening situation in which a human intruder enter the room and stood 2.5 m from the cage presenting his profile to the monkey ensuring avoiding of eye contact with the animal (Oler, et al., 2010). In this particular study, involving 238 young monkeys from a multigenerational single-family pedigree, anxious temperament-related metabolic activity in the hippocampus, but not amygdala, showed significant heritability (Oler, et al., 2010). Consistent with differential roles of the amygdala and hippocampus in regulation of anxiety, hippocampal and amygdala lesions in rodents and nonhuman primates caused distinct alterations in measures of anxiety (Machado and Bachevalier, 2008, McHugh, et al., 2004). As heritability estimates are influenced by age of the population (Davis, et al., 2009, Visscher, et al., 2008), anxiety-related activity in the amygdala might show heritability in aged monkeys.
In experiment 1, behavioral measures of middle-aged female rhesus macaques (Macaca mulatta) were compared in a temperament test, similar to the intruder task, and the playroom. In experiment 2, behavioral performance of a second independent cohort of animals was assessed in the playroom. Based on the time spent in specific areas of a playroom, we classified elderly female rhesus monkeys as bold or reserved monkeys. Subsequently, we assessed whether anxiety levels were associated with amygdala or hippocampal volumes using MRI. Finally, as muscarinic receptors play an important role in the regulation of anxiety (Degroot and Nomikos, 2006, Furey, et al., 2010, Laplante, et al., 2005, Smythe, et al., 1998, Wall, et al., 2001), we also assessed whether anxiety levels were associated with the pharmacological MRI response to the muscarinic receptor antagonist scopolamine.
2. METHODS
2.1 Animals
Middle-aged female rhesus macaques (Experiment 1; Macaca mulatta; n = 7; age range 18–19 years; ovarectomized; 7.2 ± 0.5 kg) or old female rhesus macaques (Experiment 2; Macaca mulatta; n = 16; age range 21–27 years; reproductively inactive; 7.9 ± 0.2 kg) were cared for by the Division of Animal Resources at the Oregon National Primate Research Center (ONPRC) in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals. They were pair-housed (when possible) indoors under controlled conditions: 24°C temperature, 12L:12D photoperiod (lights on at 0700 h), regular meals at 0800 and 1500 h (Purina High Protein Chow Diet, Purina Mills, Inc., St. Louis, MO) supplemented with fresh fruit, vegetables and candy treats; fresh drinking water was available ad libitum. All studies were approved by ONPRC IACUC.
2.2 Temperament test in a cage (Experiment 1)
Behavioral responses to anxiety-provoking stimuli were assessed in a modified version of the Human Intruder test (Coleman, et al., 2003, Kalin and Shelton, 1989, Kalin, et al., 2004, Kalin, et al., 1991). Briefly, monkeys were placed in a testing room in a standard monkey cage. Monkeys acclimated to the room and surroundings for 3 minutes (Control 1). Following acclimation, monkeys remained in the room for an additional 2 minutes (Control 2). An unfamiliar human entered the room and stared at the wall, with his gaze away from the monkey and remained in the room for 2 minutes (Human 1, side gaze). The human exited the room and the monkey was alone in the room for another 2 minutes (Control 3). Subsequently, the human re-entered the room and stared at the monkey, following the monkey with his/her eyes for 2 minutes (Human 2, stare). The human exited the room after the stare period, and the monkey remained in the room alone for 2 minutes (Control 4). The human then re-entered the room for a last time, and after situating himself/herself exactly the same way as during the Human 2 period, he/she presented the monkey with a treat. The treat was brought to the cage and held for 5 seconds to determine if the monkey would retrieve the reward from the stranger.
The temperament task was recorded by videotape and manually analyzed. Fifteen behaviors were analyzed, comparing “bold” behaviors to “reserved” behaviors. Bold behaviors included: lip smacking, staring, ear flicking, and gaping. Reserved behaviors included: bending down, pacing, stereotypy (including eye poking), sitting in the corner of the cage, yawning, and shaking. The frequency of each behavior for each monkey was recorded. Data are presented as a ratio of bold and reserved behaviors.
2.3 Playroom Anxiety Measures (Experiments 1 & 2)
In Experiment 1, monkeys were introduced to a playroom (2.44 × 3.45 m) one at a time for a total of 2 minutes per trial. There were 6 food boxes equally spaced around the room and baited with preferred food rewards. Each monkey was repeatedly placed in the room until they were trained to a criterion level. In order to start the cognitive task, each monkey had to enter and exit the room and reliably search all the boxes in the room. The average number of trials for all monkeys was 67 ± 8.0. The average number of trials to criterion for the reserved monkeys was 79.5 ± 8.4 trials, whereas the average number of trials to criterion for the bold was 50 ± 7.6 trials.
In experiment 2, monkeys were introduced to a playroom (2.44 × 3.45 m), one at the time, for a total of 2 minutes per trial. There were a total of 50 trials over 10 days; two days per battery, with batteries approximately 2 months apart. The room consisted of a human entry door, one-way observation window, monkey entry door, and a series of 10 ports (Haley, et al., 2009). Three of the 10 ports were baited with preferred rewards. Movement and location of the animal within the room were recorded with Ethovision video tracking (Noldus Information Technologies, Leesburg, VA). For data analysis, the room was divided into 5 areas (Figure 1), the animal door (A), the ports (B), the middle of the room (C), corners of the room (D), and the perimeter of the room (E). Videos were analyzed using Button Box (Behavior Research Solutions LLC, Hudson, WI) to measure the duration of time spent in each area. The time spent in the corner and perimeter was collapsed (protected areas) as were the time spent in the middle of the room, the jump door, and the ports (unprotected areas). In the corner of the room, the animals are protected by 2 walls and have their back to the walls, while examining the rest of the room (Figure 1, areas D and E). In the unprotected areas of the room, the monkeys are more exposed to the rest of the room (Figure 1, areas A, B, and C). Specifically at the jump door, the monkeys have their back to the rest of the room, a posture that would not or hardly be present in the reserved monkeys. If a monkey did not show preference for one area of the room, the monkey was considered a bold monkey.
Figure 1.
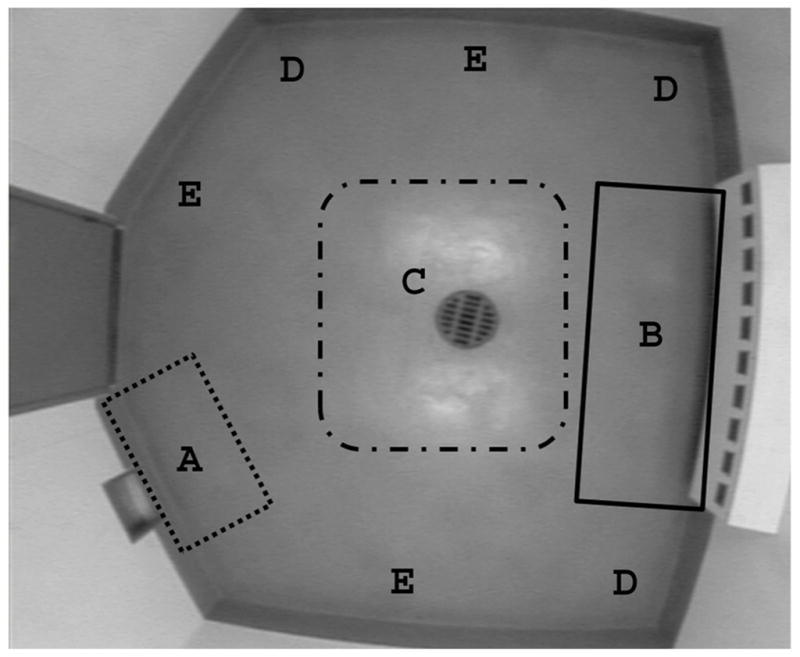
Bird’s eye view of the playroom. The playroom was divided into 5 areas, the jump door (A), the ports (B), the middle of the room (C), the corners (D), and the perimeter of the room (E).
In experiment 1, based on their exploratory behavior in the playroom, 3 of the monkeys were classified as bold monkeys and 4 of the monkeys were classified as reserved monkeys. To compare behavior in the playroom to bold and reserved behavior in the temperament task, the ratio of the unprotected areas of the playroom (boxes and middle)(bold behavior) to the protected areas of the playroom (perimeter and corner) (reserved behavior) was used. The larger the ratio represented the more bold behaviors being observed compared to reserved behaviors. In experiment 2, of the 16 monkeys in the study and based on their behavioral exploration of the play room, 9 were classified as having reserved behaviors and 7 were classified as having bold behaviors.
2.4 MRI analysis
Within 2 weeks of completing the cognitive battery, of which the final component was the assessment of measures of anxiety, an MRI was performed on each animal. Using a voxel based morphology (VBM) method, the size of the amygdala and hippocampus was assessed for each monkey (Bodnar, et al., 2010, Woodward, et al., 2006). Monkeys were fasted the night before and the morning of the MRI experiments for the anesthesia. They were sedated with ketamine (1 mg/kg body weight) and transported from their home cages to a Siemens whole-body 3-T trio MRI system (Erlinger, Germany) located on the ONPRC campus. Animals were immediately intubated and respirated, free-breathing, with 0.9–1.1% isoflurane. Physiological parameters, including blood oxygen levels, carbon dioxide, blood pressure, heart rate, temperature, and respiration rate were monitored continuously throughout the experiment using an MRI-compatible Precess monitor (Invivo, Orlando, FL).
The animal’s head was placed inside a quadrature transmit/8-channel receive human extremity RF coil (Invivo) and fitted with pads to eliminate head movement. After acquisition of a series of “localizer images”, 20 contiguous T2*-weighted multi-shot echo planar images were obtained over the course of 7 minutes. (TR = 1800 ms, TE = 30 ms, 4 segments, 2 mm slices, flip angle 90°, 24 slices, 20 volumes). For BOLD measurements, these T2*-weighted images were designated “baseline”. Immediately following the baseline scans, scopolamine (0.05 mg/kg in 1 ml of isotonic saline i.v., n = 12; or 1 ml of isotonic saline for control animals, n = 5) was infused over 1 minute using an infusion pump (Harvard Apparatus, Holliston, MA). Following the intravenous infusion, a T2-weighted (TR = 5280 ms, TE = 57 ms, 1 mm slice thickness, flip angle 120°, 50 slices) and 2 T1-weighted (TR = 2500 ms, TE = 4.38, 0.6 mm slice thickness, flip angle 12°, 88 slices) anatomical images were acquired. The total time for acquisition of anatomical scans was 46 minutes, sufficient time for scopolamine to cause effects in the brain (Ali-Melkkila, et al., 1993). Immediately following the anatomical scans, a second BOLD series of T2*-weighted images was acquired using the same parameters as the baseline scan (“challenge scan”).
Using the MRI analysis software AFNI [NIH (Gold, et al., 1998)], the change in BOLD signal from baseline to challenge scan (scopolamine or vehicle) was determined. In addition, voxel based morphology (VBM) was also assessed for each amygdala, right and left side, and hippocampus, right and left side, and PFC. Isotonic saline control was used to ensure that the change observed between the baseline and challenge scan was due to a scopolamine effect and not an artifact due to signal drift of the magnet. Slice dependent time shifts were interpolated, odd-even slice intensity differences removed, motion corrected, and T1 and T2 anatomical scans were co-registered. Baseline and challenge phMRI scans were averaged across the 20 volumes and the single image was normalized to the co-registered anatomical scan of each individual animal. For each animal, the Regions of Interest (ROI) was drawn for the hippocampus based on the anatomical scan using key neuroanatomical landmarks from anatomical scans (Small, et al., 2004). The ROI was the same for the baseline and scopolamine scans, both of which were co-registered to the anatomical scans (Haley, et al., 2010).
2.5 Statistical analysis
All data analyses were performed using SPSS 19.0 (SPSS Inc., Somers, NY). Student’s t tests were used to assess potential age differences. In order to assess the preference for each area of the room, thus categorizing each monkey, one-way ANOVAs were used. To analyze group differences in habituation during the first day of the task, a two-way ANOVA was used with group × trial. To further assess behavioral performance during all 5 sessions, a repeated measures two-way ANOVA was used with groups as between-subjects variable. To analyze VBM amygdala size and right and left side hippocampus in the bold and reserved monkeys, a repeated measures ANOVA was used with the right and left hemisphere as within subject factors. Percentage change in bold between groups was assessed using the Mann-Whitney U test. Pearson correlations were used to assess correlations between amygdala size and time spent in the 5 different areas of the room. P < 0.05 was considered to be statistically significant.
3. Results
3.1 Experiment 1
3.1.1 Animals
Bold monkeys spent 20% more time in unprotected areas of the room (bold behavior) compared to the reserved monkeys. There were group differences in the first session (P < 0.05) and over the course of all the testing sessions (P < 0.05).
3.1.2 Behavioral responses in intruder test compared to playroom
There was a statistical trend of a difference between the ratio of time spent exhibiting bold to reserved behaviors in the playroom between bold monkeys, 2.8 ± 1.5, and reserved monkeys, 1.3 ± 0.3 (P = 0.08). No difference was observed between the ratio of bold to reserved behaviors in either group of monkeys during the temperament task (data not shown). Interestingly, all of the bold monkeys took the treat from the stranger, but only one of the reserved monkeys took the treat.
3.2 Experiment 2
3.2.1. Animals
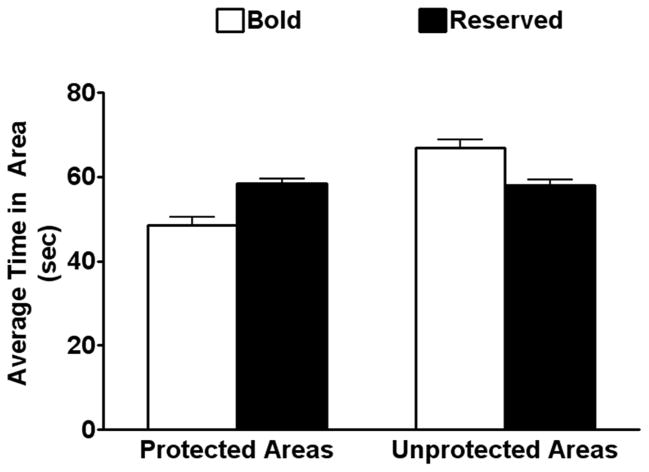
Bold monkeys were defined as animals that spent 20% more time in the unprotected areas of the room than in the unprotected areas, whereas reserved monkeys spent a comparable amount of time in both areas (Figure 2). Compared to bold monkeys, reserved monkeys spent more time in the protected areas of the room, while bold monkeys spent significantly more time in the open areas of the room, i.e. the middle, the ports, and the jump door, compared to the reserved monkeys (Figure 2). There were group differences on the first day of testing (P < 0.01) and over the course of the study (P < 0.05), and there was no effect of day or group × day interaction. The ratio of bold to reserved behaviors in the playroom was significantly different between the groups, where the ratio of the bold monkeys was 2.9 ± 0.8 whereas the ratio of the reserved monkeys was 0.7 ± 0.1 (P < 0.01). The ages of these two anxiety groups were not significantly different (reserved: 26.3 ± 0.7 years; bold group: 25.6 ± 0.7 years).
Figure 2.
Measures of anxiety in the playroom. Average amount of time spent in the unprotected areas (middle and jump door) compared to protected areas (corner and perimeter of the room) collapsed across bold and reserved monkeys. Data are presented as mean time in area in sec ± SEM.
3.2.2 VBM using MRI
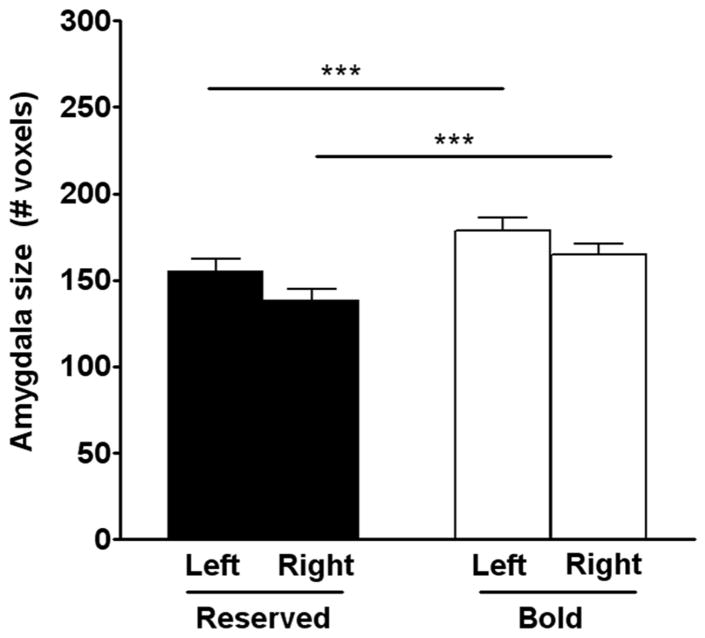
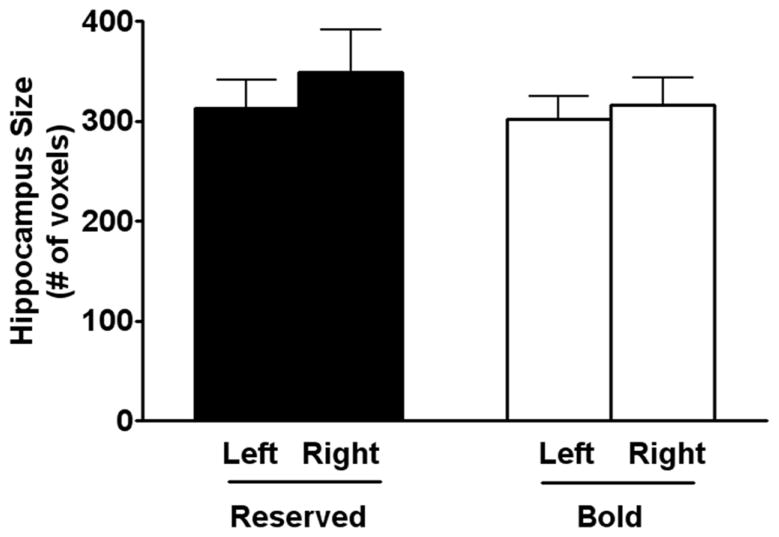
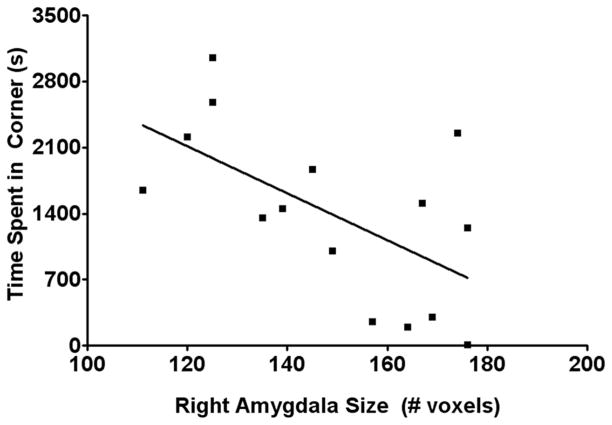
The amygdala size was smaller in reserved monkeys compared to bold animals [F (1, 16) = 50.875, P < 0.0001, Figure 3]. In contrast, no significant differences were observed between the right and the left amygdala sizes or hippocampal size (Figure 4). The amount of time spent in the corner of the room was negatively correlated with the right (r = −0.59, P < 0.05; Figure 5) and total amygdala size (r = −0.54, P < 0.05). No significant correlation was observed in the left amygdala.
Figure 3.
Amygdala size. Reserved monkeys had a smaller amygdala size compared to bold monkeys as measured by voxel based morphology (VBM). Data are presented as mean ± SEM. *P < 0.05 compared to reserved monkeys.
Figure 4.
Hippocampal size. There was no difference in the size of the hippocampus of Reserved and bold monkeys as measured by voxel based morphology (VBM).
Figure 5.
The right amygdala size was inversely correlated with the time spent in the corners of the room. Monkeys with smaller amygdala sizes spent more time in the corners of the room (r = −0.59, P < 0.05).
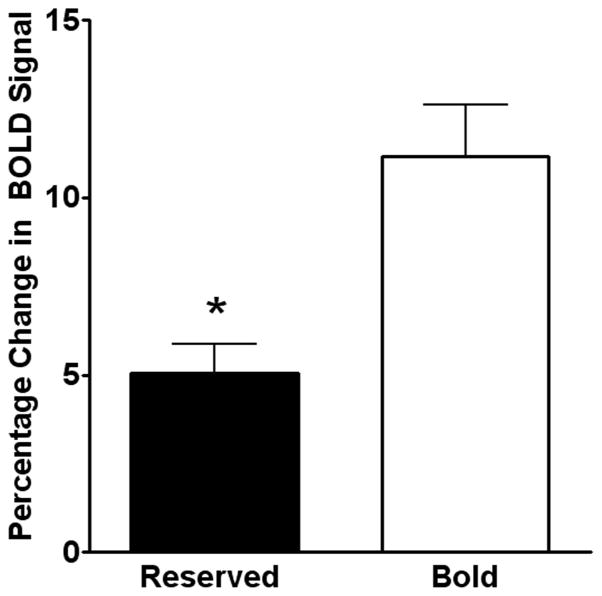
3.2.3 Scopolamine phMRI
Finally, we analyzed the phMRI response in the hippocampus to the muscarinic receptor antagonist scopolamine. Reserved monkeys showed a lower scopolamine response than bold monkeys (Figure 6).
Figure 6.
Reserved monkeys had a lower scopolamine-induced phMRI response than bold monkeys. Following scopolamine challenge, reserved monkeys had a significantly lower percentage change in T2*-weighted signal compared to reserved monkeys (n = 5). *P < 0.05
3.2.4 Cognitive Performance and Measures of Anxiety
These monkeys also performed the Spatial Foodport maze (Haley, et al., 2009). Of the 16 monkeys, 6 were good spatial performers, 6 were poor spatial performers and 4 were unable to complete the task. While all the good spatial performers were bold monkeys (Haley, et al., 2010), 1 of the poor spatial performers was also a bold monkeys. The remaining 4 poor spatial performers and the 4 monkeys who could not complete the task were reserved monkeys.
4. Discussion
In the present study, we present a new way of assessing anxiety-related behaviors in the rhesus macaque in the same environment as they are cognitively tested and show that monkeys defined as having reserved have a smaller amygdala size and a reduced hippocampal phMRI reponse to scopolamine compared to bold monkeys. Compared to previously established paradigms (Coleman, et al., 2003, Fairbanks, et al., 2004, Fairbanks, et al., 2004), our new paradigm demonstrates that conclusions based on animal temperament, including anxiety-related behaviors, may be situational or contextual. Particularly, behavior in an animal cage is not necessarily indicative of behavior in the playroom, suggesting a situational/contextual component to anxiety-like behaviors. Moreover, reserved monkeys required more trials to reach criterion compared to bold monkeys suggesting that behavioral performance in the playroom can affect cognitive performance. Furthermore, not all bold monkeys performed well on cognitive tasks, but some reserved monkeys were not able to complete a spatial task in the same arena (Haley, et al., 2009), suggesting that more reserved behaviors in the playroom, suggesting higher levels of anxiety, might have contributed to their reduced cognitive performance.
Smaller amygdala size was associated with higher anxiety-like measures, or more reserved behaviors, which was not observed in the hippocampus, supporting some anatomical specificity of this effect and consistent with the effects of polymorphism in the serotonin transporter and neuropeptide Y genes on emotion-related amygdala activity (Hariri, et al., 2002, Zhou, et al., 2008). The group difference in anxiety measures based on the first day did not significantly change over time in the study, consistent with an anxiety phenotype (Smith and Lay, 1974). The amount of time spent in the corner of the room was negatively correlated with amygdala size. Interestingly, amygdala size has been shown to be smaller in PTSD subjects than in healthy controls (Irle, et al., 2009, Weniger, et al., 2008, Weniger, et al., 2009). Thus, a smaller amygdala size and higher anxiety levels might increase susceptibility to develop PTSD.
Some of the reserved monkeys were unable to complete a spatial maze administered in the same arena (Haley, et al., 2009). These data are consistent with the relatively worse visiospatial working memory in individuals characterized by high levels of behavioral inhibition and more intense anxiety (Shackman, et al., 2006). However, measures of anxiety can also correlate positively with cognitive performance (Siegel, et al., 2010). The data in the current study confirm that there is no simple relationship between measures of anxiety and cognitive performance as a bold monkey was a poor spatial performer while others were good spatial performers.
Rodent and nonhuman primate models demonstrate that the amygdala has an integral role in the regulation of anxiety (Canteras, et al., 2010, Kalin, et al., 2004, Vinkers, et al., 2010). Although valuable information has been gained from the rodent model, results are not always translational to human applications due to inequalities to the rodent system. Recently, the muscarinic receptor system has been suggested to have an integral role in the regulation of anxiety, particularly in women (Furey, et al., 2010). This is in contrast to the rodent system, where the nicotinic receptor system seems to be involved in the regulation of anxiety (Kenney, et al., 2010, Mineur, et al., 2007, Vicens, et al., 2011). This species difference might not be limited to the cholinergic system. The serotonin system, which has a critical role in anxiety and anxiety disorders (Lesch, et al., 1996), might also be distinct in rodents and humans. Human genetic polymorphisms of the serotonin transporter are implicated in differential neuronal activity and baseline perfusion rate within the amygdala (Munafo, et al., 2008, Rao, et al., 2007). These polymorphisms of the serotonin transporter are observed in the nonhuman primate model (Christian, et al., 2009) but not in the same way in the rodent system (Menard and Treit, 1999). Therefore, the nonhuman primate model may be a useful complementary model for studying mechanisms involved in the regulation of anxiety and for developing potential treatments for humans with anxiety disorders, particularly those associated with altered serotonin receptor-mediated signaling.
In summary, observations based on behavior in a cage are not necessarily indicative of behavior in a playroom. Furthermore, based on classification of anxiety levels in a playroom in nonhuman primates, reserved monkeys have a smaller amygdala and a lower phMRI response to scopolamine compared to bold monkeys. These data support the nonhuman primate as a complementary translational model for studying mechanisms involved in human anxiety disorders particularly for mechanisms involving signaling pathways that are distinct in rodents and humans. As these measures might affect performance in cognitive test, they are valuable to include in the context of cognitive testing of nonhuman primates. Future efforts are warranted to determine the muscarinic receptor subtype(s) involved in these effects.
Highlights.
Nonhuman primates classified as high or low anxiety based on playroom behavior
Anxiety classification associated with amygdala size.
Anxiety clasification associated with hippocampal scopolamine response.
Acknowledgments
The authors would like to thank Laurie Renner for her assistance with the behavioral testing and Dr Steve Kohama, Dr Chris Kroenke, and Daniel Schwartz for their assistance with the design and analysis of the earlier reported imaging study in the same animals (Haley, et al., 2010). This work was supported by NIH Grants AG-029612 (Dr. Henryk Urbanski) and RR-000163 (Dr. Joseph Robertson), the Medical Research Foundation of Oregon (Dr. Gwendolen Haley), Brookdale Fellowship (Dr. Ilhem Messaoudi), and an OHSU Development Account (Dr. Jacob Raber).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ali-Melkkila T, Kanto J, Iisalo E. Pharmacokinetics and related pharmacodynamics of anticholinergic drugs. Acta Anaesthesiologica Scandinavica. 1993;37:633–642. doi: 10.1111/j.1399-6576.1993.tb03780.x. [DOI] [PubMed] [Google Scholar]
- 2.Amaral DG. The amygdala, social behavior, and danger detection. Ann N Y Acad Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- 3.Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: implications from non-human primate studies. Genes Brain Behav. 2003;2:295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 4.Barros M, Giorgetti M, Souto AA, Vilela G, Santos K, Boas NV, Tomaz C. Persistent anxiety-like behavior in marmosets following a recent predatory stress condition: reversal by diazepam. Pharmacol Biochem Behav. 2007;86:705–711. doi: 10.1016/j.pbb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Barros M, Maior RS, Huston JP, Tomaz C. Predatory stress as an experimental strategy to measure fear and anxiety-related behaviors in non-human primates. Rev Neurosci. 2008;19:157–169. doi: 10.1515/revneuro.2008.19.2-3.157. [DOI] [PubMed] [Google Scholar]
- 6.Beck JG. Cognitive aspects of anxiety and depression in the elderly. Curr Psychiatry Rep. 2005;7:27–31. doi: 10.1007/s11920-005-0020-9. [DOI] [PubMed] [Google Scholar]
- 7.Bodnar M, Malla AK, Czechowska Y, Benoit A, Fathalli F, Joober R, Pruessner M, Pruessner J, Lepage M. Neural markers of remission in first-episode schizophrenia: a volumetric neuroimaging study of the hippocampus and amygdala. Schizophr Res. 2010;122:72–80. doi: 10.1016/j.schres.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Cagni P, Goncalves I, Jr, Ziller F, Emile N, Barros M. Humans and natural predators induce different fear/anxiety reactions and response pattern to diazepam in marmoset monkeys. Pharmacol Biochem Behav. 2009;93:134–140. doi: 10.1016/j.pbb.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Canteras NS, Resstel LB, Bertoglio LJ, Carobrez Ade P, Guimaraes FS. Neuroanatomy of anxiety. Curr Top Behav Neurosci. 2010;2:77–96. doi: 10.1007/7854_2009_7. [DOI] [PubMed] [Google Scholar]
- 10.Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106:1–27. doi: 10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Christian BT, Fox AS, Oler JA, Vandehey NT, Murali D, Rogers J, Oakes TR, Shelton SE, Davidson RJ, Kalin NH. Serotonin transporter binding and genotype in the nonhuman primate brain using [C-11]DASB PET. Neuroimage. 2009;47:1230–1236. doi: 10.1016/j.neuroimage.2009.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman K, Dahl RE, Ryan ND, Cameron JL. Growth hormone response to growth hormone-releasing hormone and clonidine in young monkeys: correlation with behavioral characteristics. J Child Adolesc Psychopharmacol. 2003;13:227–241. doi: 10.1089/104454603322572561. [DOI] [PubMed] [Google Scholar]
- 13.Davis OS, Haworth CM, Plomin R. Dramatic increase in heritability of cognitive development from early to middle childhood: an 8-year longitudinal study of 8,700 pairs of twins. Psychol Sci. 2009;20:1301–1308. doi: 10.1111/j.1467-9280.2009.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degroot A, Nomikos GG. Genetic deletion of muscarinic M4 receptors is anxiolytic in the shock-probe burying model. Eur J Pharmacol. 2006;531:183–186. doi: 10.1016/j.ejphar.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Fairbanks LA, Jorgensen MJ, Huff A, Blau K, Hung YY, Mann JJ. Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. Am J Primatol. 2004;64:1–17. doi: 10.1002/ajp.20057. [DOI] [PubMed] [Google Scholar]
- 16.Fairbanks LA, Newman TK, Bailey JN, Jorgensen MJ, Breidenthal SE, Ophoff RA, Comuzzie AG, Martin LJ, Rogers J. Genetic contributions to social impulsivity and aggressiveness in vervet monkeys. Biol Psychiatry. 2004;55:642–647. doi: 10.1016/j.biopsych.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Furey ML, Khanna A, Hoffman EM, Drevets WC. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35:2479–2488. doi: 10.1038/npp.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold S, Christian B, Arndt S, Zeien G, Cizadlo T, Johnson DL, Flaum M, Andreasen NC. Functional MRI statistical software packages: a comparative analysis. Human Brain Mapping. 1998;6:73–84. doi: 10.1002/(SICI)1097-0193(1998)6:2<73::AID-HBM1>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haley GE, Kroenke C, Schwarz D, Kohama S, Urbanski HF, Raber J. Hippocampal M1 Receptor Function Associated with Spatial Learning and Memory in Aged Female Rhesus Macaques AGE. 2010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haley GE, Landauer N, Renner L, Weiss A, Hooper K, Urbanski HF, Kohama SG, Neuringer M, Raber J. Circadian activity associated with spatial learning and memory in aging rhesus monkeys. Exper Neurol. 2009;217:55–62. doi: 10.1016/j.expneurol.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 22.Irle E, Lange C, Sachsse U, Weniger G. Further evidence that post-traumatic stress disorder but not dissociative disorders are related to amygdala and hippocampal size reduction in trauma-exposed individuals. Acta Psychiatr Scand. 2009;119:330–331. doi: 10.1111/j.1600-0447.2009.01351.x. discussion 331. [DOI] [PubMed] [Google Scholar]
- 23.Jackson JC, Mitchell N, Hopkins RO. Cognitive functioning, mental health, and quality of life in ICU survivors: an overview. Crit Care Clin. 2009;25:615–628. x. doi: 10.1016/j.ccc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Janeway D. An integrated approach to the diagnosis and treatment of anxiety within the practice of cardiology. Cardiol Rev. 2009;17:36–43. doi: 10.1097/CRD.0b013e3181867fe3. [DOI] [PubMed] [Google Scholar]
- 25.Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- 26.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: ontogeny and context-dependent selective expression. Child Dev. 1991;62:1175–1183. [PubMed] [Google Scholar]
- 28.Kenney JW, Wilkinson DS, Gould TJ. The enhancement of contextual fear conditioning by ABT-418. Behav Pharmacol. 2010;21:246–249. doi: 10.1097/FBP.0b013e32833a5b9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laplante F, Nakagawasai O, Srivastava LK, Quirion R. Alterations in behavioral responses to a cholinergic agonist in post-pubertal rats with neonatal ventral hippocampal lesions: relationship to changes in muscarinic receptor levels. Neuropsychopharmacology. 2005;30:1076–1087. doi: 10.1038/sj.npp.1300640. [DOI] [PubMed] [Google Scholar]
- 30.Lesch KP. Molecular foundation of anxiety disorders. J Neural Transm. 2001;108:717–746. doi: 10.1007/s007020170048. [DOI] [PubMed] [Google Scholar]
- 31.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 32.Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology. 2008;33:926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maizels M, Smitherman TA, Penzien DB. A review of screening tools for psychiatric comorbidity in headache patients. Headache. 2006;46(Suppl 3):S98–109. doi: 10.1111/j.1526-4610.2006.00561.x. [DOI] [PubMed] [Google Scholar]
- 34.McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- 35.Menard J, Treit D. Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci Biobehav Rev. 1999;23:591–613. doi: 10.1016/s0149-7634(98)00056-6. [DOI] [PubMed] [Google Scholar]
- 36.Mineur YS, Somenzi O, Picciotto MR. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology. 2007;52:1256–1262. doi: 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman JD, Farley MJ. An ethologically based, stimulus and gender-sensitive nonhuman primate model for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:677–685. doi: 10.1016/0278-5846(95)00111-8. [DOI] [PubMed] [Google Scholar]
- 39.Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, Kalin NH. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Arch Gen Psychiatry. 2004;61:933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- 41.Rao H, Gillihan SJ, Wang J, Korczykowski M, Sankoorikal GM, Kaercher KA, Brodkin ES, Detre JA, Farah MJ. Genetic variation in serotonin transporter alters resting brain function in healthy individuals. Biol Psychiatry. 2007;62:600–606. doi: 10.1016/j.biopsych.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 42.Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6:40–61. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- 43.Siegel JA, Haley GE, Raber J. Apolipoprotein E isoform-dependent effects on anxiety and cognition in female TR mice. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.03.002. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith RC, Lay CD. State and trait anxiety: an annotated bibliography. Psychol Rep. 1974;34:519–594. [PubMed] [Google Scholar]
- 46.Smythe JW, Bhatnagar S, Murphy D, Timothy C, Costall B. The effects of intrahippocampal scopolamine infusions on anxiety in rats as measured by the black-white box test. Brain Res Bull. 1998;45:89–93. doi: 10.1016/s0361-9230(97)00311-0. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, Smith MS, Coleman K, Grove KL. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 2010;30:3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vicens P, Ribes D, Torrente M, Domingo JL. Behavioral effects of PNU-282987, an alpha7 nicotinic receptor agonist, in mice. Behav Brain Res. 2011;216:341–348. doi: 10.1016/j.bbr.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Vinkers CH, Bijlsma EY, Houtepen LC, Westphal KG, Veening JG, Groenink L, Olivier B. Medial amygdala lesions differentially influence stress responsivity and sensorimotor gating in rats. Physiol Behav. 2010;99:395–401. doi: 10.1016/j.physbeh.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Visscher PM, Hill WG, Wray NR. Heritability in the genomics era-- concepts and misconceptions. Nat Rev Genet. 2008;9:255–266. doi: 10.1038/nrg2322. [DOI] [PubMed] [Google Scholar]
- 51.Wall PM, Flinn J, Messier C. Infralimbic muscarinic M1 receptors modulate anxiety-like behaviour and spontaneous working memory in mice. Psychopharmacology (Berl) 2001;155:58–68. doi: 10.1007/s002130000671. [DOI] [PubMed] [Google Scholar]
- 52.Weniger G, Lange C, Sachsse U, Irle E. Amygdala and hippocampal volumes and cognition in adult survivors of childhood abuse with dissociative disorders. Acta Psychiatr Scand. 2008;118:281–290. doi: 10.1111/j.1600-0447.2008.01246.x. [DOI] [PubMed] [Google Scholar]
- 53.Weniger G, Lange C, Sachsse U, Irle E. Reduced amygdala and hippocampus size in trauma-exposed women with borderline personality disorder and without posttraumatic stress disorder. J Psychiatry Neurosci. 2009;34:383–388. [PMC free article] [PubMed] [Google Scholar]
- 54.Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 2006;59:582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 55.Wrase J, Reimold M, Puls I, Kienast T, Heinz A. Serotonergic dysfunction: brain imaging and behavioral correlates. Cogn Affect Behav Neurosci. 2006;6:53–61. doi: 10.3758/cabn.6.1.53. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]