Abstract
Aim
To analyze potential and actual drug-drug interactions reported to the Spontaneous Reporting Database of the Croatian Agency for Medicinal Products and Medical Devices (HALMED) and determine their incidence.
Methods
In this retrospective observational study performed from March 2005 to December 2008, we detected potential and actual drug-drug interactions using interaction programs and analyzed them.
Results
HALMED received 1209 reports involving at least two drugs. There were 468 (38.7%) reports on potential drug-drug interactions, 94 of which (7.8% of total reports) were actual drug-drug interactions. Among actual drug-drug interaction reports, the proportion of serious adverse drug reactions (53 out of 94) and the number of drugs (n = 4) was significantly higher (P < 0.001) than among the remaining reports (580 out of 1982; n = 2, respectively). Actual drug-drug interactions most frequently involved nervous system agents (34.0%), and interactions caused by antiplatelet, anticoagulant, and non-steroidal anti-inflammatory drugs were in most cases serious. In only 12 out of 94 reports, actual drug-drug interactions were recognized by the reporter.
Conclusion
The study confirmed that the Spontaneous Reporting Database was a valuable resource for detecting actual drug-drug interactions. Also, it identified drugs leading to serious adverse drug reactions and deaths, thus indicating the areas which should be in the focus of health care education.
Adverse drug reactions (ADR) are among the leading causes of mortality and morbidity responsible for causing additional complications (1,2) and longer hospital stays. Magnitude of ADRs and the burden they place on health care system are considerable (3-6) yet preventable public health problems (7) if we take into consideration that an important cause of ADRs are drug-drug interactions (8,9). Although there is a substantial body of literature on ADRs caused by drug-drug interactions, it is difficult to accurately estimate their incidence, mainly because of different study designs, populations, frequency measures, and classification systems (10-15).
Many studies including different groups of patients found the percentage of potential drug-drug interactions resulting in ADRs to be from 0%-60% (10,11,16-25). System analysis of ADRs showed that drug-drug interactions represented 3%-5% of all in-hospital medication errors (3). The most endangered groups were elderly and polimedicated patients (22,26-28), and emergency department visits were a frequent result (29). Although the overall incidence of ADRs caused by drug-drug interactions is modest (11-13,15,29,30), they are severe and in most cases lead to hospitalization (31,32).
Potential drug-drug interactions are defined on the basis of on retrospective chart reviews and actual drug-drug interactions are defined on the basis of clinical evidence, ie, they are confirmed by laboratory tests or symptoms (33). The frequency of potential interactions is higher than that of actual interactions, resulting in large discrepancies among study findings (24).
A valuable resource for detecting drug-drug interactions is a spontaneous reporting database (15,34). It currently uses several methods to detect possible drug-drug interactions (15,29,35,36). However, drug-drug interactions in general are rarely reported and information about the ADRs due to drug-drug interactions is usually lacking.
The aim of this study was to estimate the incidence of actual and potential drug-drug interactions in the national Spontaneous Reporting Database of ADRs in Croatia. Additionally, we assessed the clinical significance and seriousness of drug-drug interactions and their probable mechanism of action.
Methods
Data source
A retrospective observational study was performed from March 2005 to December 2008. Data were obtained from the Spontaneous Reporting Database of Pharmacovigilance Department of the Croatian Agency for Medicinal Products and Medical Devices (HALMED). Spontaneous Reporting Database uses VigiFlow, web-based Individual Case Safety Report management system that is specially designed for use by national centers in the World Health Organization Programme for International Drug Monitoring. Data elements for transmission of individual case safety reports are entered according to standardized ICH E2B format (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use) (37).
ADR reporting is a legal obligation of every health care professional in Croatia, and patients are free to report as well (38,39). Reports are made in writing and sent by land mail, fax, or electronic mail using a prescribed form. Reporters are classified into the following groups: primary care physicians, secondary and tertiary care physicians, pharmacists, and patients. Drugs are grouped according to the Anatomical-Therapeutic-Chemical drug classification (ATC) and entered into the spontaneous reporting database. One report can describe one or more ADRs.
Data concerning the suspected ADRs were coded into the related Preferred Term and System Organ Class using the Medical Dictionary for Drug Regulatory Affairs (MedDRA) adverse drug reaction terminology (40).
Data analysis
ADR reports were considered only if pharmacovigilance assessors evaluated the relatedness to the use of the suspected drug as possible, probable, or certain (41). We analyzed all reports involving at least two drugs suspected of causing an ADR. Drug-drug interactions were detected with online version of computerized interaction detection system Drug-Reax (42). To confirm Drug Reax results, we used online version of Stockley drug interaction program (43) and literature search of Medline.
For each report containing a potential drug-drug interaction, we verified if the description of the ADR corresponded to the interaction effect described in drug interaction programs and if it did it was considered actual (eg, first-dose hypotension of ACE inhibitors in patients receiving long-term treatment with ACE inhibitors and diuretics). Evaluation was made by a panel of five experts, including a pharmacist, clinical pharmacist, two physicians, and a clinical pharmacologist. They reviewed all the available information in the reports, and when necessary, the reporter was contacted for follow-up information. ADRs were considered serious if they resulted in one of the following outcomes according to the ICH E 2A (44) guidelines: death, life threatening condition, inpatient hospitalization, or prolongation of existing hospitalization, persistent or significant disability/incapacity, a congenital anomaly/birth defect, or other important medical event according to CIOMS V (Council for International Organizations of Medical Sciences, 2001) (45). Data were analyzed in respect to the total number of reports, demographic characteristics of the patients, classification of suspected drugs, System Organ Class, seriousness, and the source of reports. Medians (ranges) and percentages were used to present the data. ADR reports caused by actual drug-drug interactions were compared with ADR reports not caused by actual drug-drug interactions. The results were analyzed using the Mann-Whitney and χ2 test. Data analysis was done by the Predictive Analytics Software, version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Incidence of drug-drug interactions
From March 2005 to December 2008, HALMED received 2076 reports of ADRs, 1209 of which (58.2%) involved more than one drug. In 94 (7.8%) of these, ADRs were caused by actual drug-drug interactions, with 106 different pairs of concomitantly administered drugs. In 83 reports (88.3%), ADRs were caused by only one drug-drug interaction, in 10 reports (10.6%) by 2 drug-drug interactions, and in 1 report (1.1%) by 3 drug-drug interactions.
Potential drug-drug interactions were identified in 468 of 1209 reports (38.7%) involving more than one drug, and actual drug-drug interactions in 94 reports (20.8% of potential drug-drug interactions).
Characteristics of actual drug-drug interactions
In 94 reports of actual drug-drug interactions, the age range of patients was 3 days-85 years (median 58 years). In 14 cases, patients were less than thirty years old, and all interacting drugs prescribed to them belonged to nervous system medications. On average, they were taking 4 medications, ranging from 2 to 12. Fifty-three patients were female and 41 were male.
Most ADRs belonged to the following System Organ Classes: gastrointestinal disorders (24.3%), nervous system disorders (14.5%), investigations (11.8%), and psychiatric disorders (11.2%) (Table 1). Most frequently reported MedRA Preferred Terms of drug-drug interactions were gastro-intestinal tract hemorrhage and muscular disorders.
Table 1.
Distribution of adverse drug reactions (ADR) caused and not caused by actual drug-drug interactions (DDI) classified by Medical Dictionary for Drug Regulatory Affairs System Organ Class (MedDRA SOC) (40)
| No. (%) of ADRs |
||
|---|---|---|
| MedDRA SOC | caused by actual DDIs* | not caused by DDIs† |
| Gastrointestinal disorders |
37
(24.3) |
625
(21.3) |
| Nervous system disorders |
22
(14.5) |
325
(11.1) |
| Investigations |
18
(11.8) |
96
(3.3) |
| Psychiatric disorders |
17
(11.2) |
123
(4.2) |
| General disorders and administration site conditions |
16
(10.5) |
324
(11.0) |
| Skin and subcutaneous tissue disorders |
10
(6.6) |
625
(21.3) |
| Blood and lymphatic system disorders |
9
(5.9) |
62
(2.1) |
| Muscle disorders |
9
(5.9) |
111
(3.8) |
| Vascular disorders |
4
(2.6) |
27
(0.9) |
| Respiratory, thoracic, and mediastinal disorders |
2
(1.3) |
187
(6.4) |
| Metabolism and nutrition disorders |
2
(1.3) |
37
(1.3) |
| Cardiac disorders |
2
(1.3) |
50
(1.7) |
| Reproductive system and breast disorders |
1
(0.7) |
43
(1.5) |
| Eye disorders |
1
(0.7) |
103
(3.5) |
| Renal and urinary disorders |
1
(0.7) |
42
(1.4) |
| Infections and infestations |
1
(0.7) |
22
(0.7) |
| Other | / | 132 (4.5) |
*94 reports contained 152 adverse drug reactions.
†1982 reports contained 2934 adverse drug reactions.
Fifty-three out of 94 ADR reports (56.4%) caused by actual drug-drug interactions were serious, 2 of which were fatal (3.8%), 12 were life threatening (22.6%), 23 led to hospitalization (43.4%), and 16 were serious according to the CIOMS V criteria (30.2%). Fatal cases were 69-year old woman who died from stomach and duodenal perforation due to additive gastrointestinal toxicity of amlodipine and indometacin and a 55-year old man who died from pancytopenia and sepsis developed from fluorouracil toxicity caused by concomitantly administered fluorouracil and leucovorin.
Drugs most frequently involved in actual drug-drug interactions
The most frequent drugs involved in actual drug-drug interactions were cyclosporine (n = 15), warfarin (n = 9), and fluvastatin (n = 8) (Table 2). According to the ATC classification, the drugs involved in 34.0% of actual drug-drug interactions were nervous system medications (ATC group N). In most of these interactions, both interacting drugs belonged to nervous system medications (33 out of 39) and the most frequently interacting drug was paroxetine (Table 2). Interaction between lamotrigine and valproic acid caused serious rash with systematic involvement in one young man (23 years), two girls (4 and 14 years, respectively), and one boy (16 years) (Table 2).
Table 2.
Drugs most frequently involved in actual drug-drug interactions (DDI)*
| Drug (number of DDIs*) | Drug pairs (number of DDI combinations) | Most relevant reported ADRs | Mechanism of interaction |
|---|---|---|---|
| Cyclosporine (15) |
cyclosporine - fluvastatin (6) |
rhabdomyolysis, myopathy |
inhibition of fluvastatin metabolism |
| cyclosporine- amlodipine
(3) |
cyclosporine toxicity |
inhibition of cyclosporine metabolism by amlodipine |
|
| cyclosporine – methylprednisolone
(2) |
cyclosporine toxicity |
decreased metabolism of cyclosporine |
|
| cyclosporine – prednisone (1) |
cyclosporine toxicity |
decreased metabolism of cyclosporine |
|
| cyclosporine- carvedilol
(1) |
cyclosporine toxicity |
inhibition of cyclosporine metabolism |
|
| cyclosporine- simvastatin (1) |
rhabdomyolysis |
inhibition of cytochrome P450-mediated metabolism of simvastatin and transporters |
|
| cyclosporine- ciprofloxacin (1) |
cyclosporine toxicity |
decreased cyclosporine metabolism; pharmacodynamic antagonism |
|
| Warfarin (9) |
warfarin-enoxaparin (2) |
bleeding |
additive anticoagulation |
| warfarin- acetaminophen (1) |
bleeding |
inhibition of warfarin metabolism or interference with clotting factor formation |
|
| warfarin -allopurinol (1) |
bleeding |
inhibition of warfarin metabolism |
|
| warfarin-amoxicillin (1) |
increased INR |
unknown |
|
| warfarin-atenolol (1) |
increased INR |
unknown |
|
| warfarin-fluvastatin (1) |
INR increased and bleeding |
inhibition of warfarin metabolism |
|
| warfarin- methylprednisolone (1) |
anemia |
unknown |
|
| warfarin-simvastatin (1) |
myalgia |
competition for cytochrome P450 3A4-mediated metabolism |
|
| Fluvastatin (8) |
fluvastatin -cyclosporine (6) |
see above |
|
| fluvastatin -diclophenac (1) |
gastrointestinal toxicity |
inhibition of diclophenac metabolism |
|
| fluvastatin -warfarin (1) |
see above |
||
| Paroxetine (8) |
paroxetine - risperidone (4) |
extrapyramidal ADRs, weight increase |
concomitant use of paroxetine (potent CYP2D6 inhibitor) and risperidone (CYP2D6 substrate) has resulted in increased risperidone plasma concentrations and an increased risk of risperidone adverse effects. |
| paroxetine - clozapine (1) |
sedation, hypotension |
decreased clozapine metabolism |
|
| paroxetine- fluphenazine (1) |
extrapyramidal ADRs |
inhibition of cytochrome P450-mediated fluphenazine metabolism by paroxetine |
|
| paroxetine-meloxicam (1) |
hematochezia |
At therapeutic doses SSRIs can block this reuptake of serotonin by platelets, leading to serotonin depletion, impairment of hemostatic function and increase the risk of bleeding |
|
| paroxetine - tramadol (1) |
palpitations, headache, dizziness |
increased concentration of serotonin in the nervous system and periphery; inhibition of the CYP2D6-mediated formation of tramadol active metabolites (-)-M1 and (+)-M1 by paroxetine |
|
| Risperidone (7) |
risperidone- paroxetine (4) |
see above |
see above |
| risperidone- clozapine (1) |
incontinence of urine |
compete for metabolism by the cytochrome P450 isoenzyme CYP2D6 resulting in a reduction in the metabolism of the clozapine |
|
| risperidone - haloperidol (1) |
QT prolongation |
additive effects on QT prolongation |
|
| risperidone - quetiapine (1) |
neuroleptic malignant syndrome |
additive effects |
|
| sertraline (5) |
sertraline - alprazolam (1) |
neonatal respiratory distress syndrome |
inhibition of cytochrome P450 3A-mediated alprazolam metabolism |
| sertraline - amitriptyline (1) |
sedation |
inhibition of amitriptyline metabolism |
|
| sertraline -lithium (1) |
nausea and vomiting |
additive effect |
|
| sertraline - olanzapine (1) |
weight increased |
inhibition of olanzapine mechanism |
|
| sertraline - zolpidem (1) |
hallucinations |
unknown |
|
| Valproic acid (5) |
valproic acid- lamotrigine (4) |
life-threatening rashes |
decreased lamotrigine metabolism |
| valproic acid - amitriptyline (1) |
disorientation, amnesia, hallucinations |
decreased amitriptyline plasma clearance |
|
| Acetylsalicylic acid (5) |
acetylsalicylic acid -clopidogrel (3) |
bleeding |
inhibition of platelet aggregation |
| acetylsalicylic acid -enoxaparin (1) |
bleeding |
decreased platelet function; decreased coagulation |
|
| acetylsalicylic acid - ketoprofen (1) | gastrointestinal toxicity | additive gastrointestinal irritation |
*Abbreviations: INR – International Normalized Ratio; SSRI – Selective Serotonin Reuptake Inhibitors; ADR – adverse drug reaction.
Twenty-seven percent of actual drug-drug interactions involved cardiovascular system medications (ATC group C). In most cases, the other interacting drug did not belong to the ATC group C (21 out of 35). We detected 16 drug-drug interactions involving hydroxymethylglutaryl-CoA reductase inhibitors (statins), 8 of which were caused by fluvastatin, and 4 drug-drug interactions involving simvastatin and atorvastatin each. The most frequent actual interaction was between fluvastatin and cyclosporine (n = 6) (Table 2).
There were 12.3% of actual drug-drug interactions that involved drugs belonging to antineoplastic and immunomodulating medications (ATC group L). Most of them involved cyclosporine (15 out of 21) (Table 2). Two cases of actual interactions involved methotrexate and non-steroidal anti-inflammatory drugs (ibuprofen, tenoxicam) and one involved methotrexate and omeprazole. The mechanism of these interactions involved decreased methotrexate clearance.
There were 10.9% of actual drug-drug interactions that involved medications affecting blood and blood forming organs (ATC group B), and more than half of the cases (9 out of 17) involved warfarin (Table 2). The most frequently reported actual drug-drug interaction in this group was between acetylsalicylic acid and clopidogrel (n = 3) (Table 2).
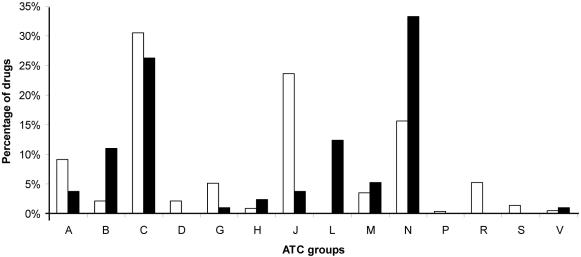
There were 15.8% of actual drug-drug interactions that involved drugs belonging to the musculo-skeletal system (ATC group M), alimentary tract, and metabolism (ATC group A), anti-infectives for systemic use (ATC group J), systemic hormonal preparations, excluding sex hormones and insulins, genito-urinary system and sex hormones (ATC group H), genito-urinary system and sex hormones (ATC group G), and various medicines group (ATC group V) (Figure 1).
Figure 1.
Distribution of suspected drugs according to Anatomical-Therapeutic-Chemical (ATC) drug classification in the group of adverse drug reaction reports not caused by drug-drug interactions (open bars) and the group of adverse drug reaction reports caused by drug-drug interactions (closed bars). ATC groups: A – Alimentary Tract and Metabolism; B – Blood and Blood Forming Organs; C – Cardiovascular System Drugs; D – Dermatologicals; G – Genito Urinary System and Sex Hormones; H – Systemic Hormonal Preparations, Excluding Sex Hormones and Insulins, Genito Urinary System and Sex Hormones; J – Antiinfectives for Systemic Use; L – Antineoplastic and Immunomodulating Medications; M – Musculo-Skeletal System; N – Nervous System; P – Antiparasitic Products, Insecticides and Repellents; R – Respiratory System; S – Sensory Organs; V – Various.
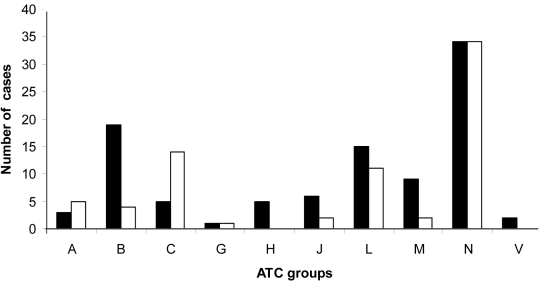
Most actual drug interactions involved pharmacokinetic interactions (44.3%), 32.1% involved pharmacodynamic interactions, and 3.8% involved both pharmacokinetic and pharmacodynamic interactions. In 19.8% of actual drug-drug interactions, the mechanism was unknown. The proportion of serious cases caused by actual drug-drug interactions was highest in the following ATC groups: H and V, B, and J and M (Figure 2), and lowest in H ATC group (5 drugs) and V ATC group (2 drugs).
Figure 2.
Distribution of serious (closed bars) and not serious (open bars) cases of actual drug-drug interactions in different Anatomical-Therapeutic-Chemical (ATC) drug classification groups. ATC groups: A – Alimentary Tract and Metabolism; B – Blood and Blood Forming Organs; C – Cardiovascular System Drugs; G – Genito Urinary System and Sex Hormones; H – Systemic Hormonal Preparations, Excluding Sex Hormones and Insulins, Genito Urinary System and Sex Hormones; J – Anti-infectives for Systemic Use; L – Antineoplastic and Immunomodulating Medications; M – Musculo-Skeletal System; N – Nervous System; V – Various.
In 7 out of 94 ADR reports caused by actual drug-drug interactions, at least one of the interacting drugs was an over-the-counter drug. In 6 cases, it was acetylsalicylic acid and in 2 cases ranitidine. Moreover, 6 out of 7 ADRs involving over-the-counter drugs were serious. Two additional serious ADR cases involved drugs administered in the dose registered as over-the-counter (eg, ibuprofen 400 mg and omeprazole 10 mg).
Reporters of adverse drug reactions involving actual drug-drug interactions
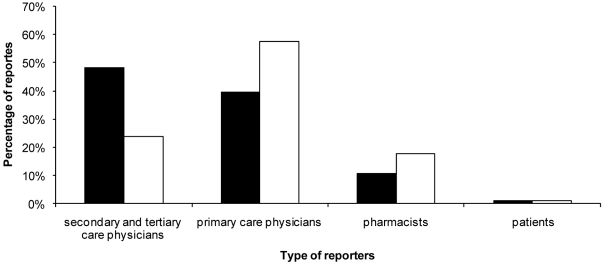
ADRs caused by actual drug-drug interactions were in most cases reported by secondary and tertiary care physicians and ADRs not caused by actual drug-drug interactions by primary care physicians (Figure 3). The most serious ADR reports caused by actual drug-drug interaction were detected by secondary and tertiary care physicians (33 reports).
Figure 3.
Distribution of reporters in the group of adverse drug reaction reports caused by drug-drug interactions (closed bars) and the group of adverse drug reaction reports not caused by drug-drug interactions (open bars).
Actual drug-drug interactions were recognized by the reporter himself or herself in only 12 out of 94 reports. In the remaining reports, the reporter either did not state whether both interacting drugs were the suspected drugs or the box “ADR caused by drug-drug interaction” was not ticked. Reporters most frequently recognized actual drug-drug interactions in which one of the drugs was an anticoagulant/antiplatelet and least frequently drug-drug interactions in which one of the drugs was a psychiatric drug (only one case).
Comparison of reports of adverse drug reactions caused and not caused by actual drug- drug interactions
The number of serious reports (53 out of 94) and the number of drugs (median 4, range 2-12) in therapy was significantly higher in the group of ADR reports caused by actual drug-drug interactions (P < 0.001) than among all other reports (580 out of 1982; median 2, range 1-14, respectively).
These groups did not differ significantly in sex distribution or patient age (Table 3), but did differ in the distribution of the most frequently reported ADRs according to MedDRA System Organ Class (Table 1). The greatest difference between ADR reports caused by actual drug-drug interactions and all other reports was observed in the following System Organ Classes: skin and subcutaneous tissue disorders (6.6% vs 21.3%), investigations (11.8% vs 3.3%), psychiatric disorders (11.2% vs 4.2%), blood and lymphatic system disorders (5.9% vs 2.1%), and respiratory, thoracic and mediastinal disorders (1.3% vs 6.4%) (Table 1).
Table 3.
Comparison of adverse drug reaction (ADR) reports caused and not caused by actual drug-drug interactions (DDI)
| Characteristic | Median (range) or number (%) of ADR reports* |
P | |
|---|---|---|---|
| not caused by DDIs | caused by DDIs | ||
| Number of drugs prescribed |
2 (1-14) |
4 (2-12) |
<0.001 |
| Patient age |
55 y (1 days-93 years) |
58 y (3 days-85 years) |
0.115 |
| Patients’ sex: |
|||
| female |
1270 (64.1) |
53 (56.4) |
0.535 |
| male |
712 (35.9) |
41 (43.6) |
|
| Seriousness of ADR:* |
|||
| serious |
580 (29.3) |
53 (56.4) |
<0.001 |
| not serious | 1402 (70.7) | 41 (43.6) | |
*Serious adverse drug reactions were those that resulted in the following outcomes according to the ICH E 2A (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use) guideline (44): death, life threatening condition, inpatient hospitalization or prolongation of existing hospitalization, persistent or significant disability/incapacity, a congenital anomaly/birth defect, or other important medical event according to CIOMS V (Council for International Organizations of Medical Sciences 2001).
These two groups also differed according to the distribution of the most frequently suspected drugs according to ATC classification (Figure 1), and a significant difference was found for ATC groups J (P < 0.001), L (P < 0.001), B (P < 0.001), N (P = 0.009), R (P = 0.013), A (P = 0.025), and H (P = 0.029). The percentage of drugs belonging to N, L, B, and H ATC group was significantly higher in the group of actual drug-drug interactions reports (Figure 1).
Discussion
Our study confirmed that the Spontaneous Reporting Database was a valuable resource for detecting ADRs caused by drug-drug interactions and the drugs leading to serious ADRs and deaths, thus emphasizing the areas that should be in the focus of health care education. Most of the studies investigating ADRs associated with drug-drug interactions were set in either hospital (3,10,29,46,47) or general practice setting (14,18,45-49). There was only a single study with the same methodology as ours. Leone et al (15) analyzed Italian spontaneous reporting database and concluded that it can be an important resource for detecting ADRs associated with the concomitant use of interacting drugs. Similar to us, they used all the cases that Drug Reax evaluated as possibly, probably, or certainly related to the use of suspected drugs (15).
The methodology of detecting drug-drug interactions may be a limitation of our study, since at the time of analysis not all drug-drug interactions were known and drug-drug interactions clinically unimportant for the majority of patients can be consequential for the individual with decreased enzyme activity. Further limitations include under-reporting of ADRs, and reports with missing information on concomitant drugs and comorbidities due to retrospective study design. According to some studies, fewer than 6% of all ADRs are reported (50). An additional limitation of our study could be the lack of denominator data such as the user population. Compared to other methodologies, we used more than one source of drug interaction-checking methods (43), which made our drug-drug interaction detection more sensitive, thus minimizing the possibility of unrecognized drug interactions. Also, consensus between experts was reached in all cases.
The percentage of reports involving actual drug-drug interactions was almost double in the Croatian than in Italian spontaneous reporting database (15). Probably because 60.9% of reports in the Italian database involved only one suspected drug without concomitant therapy, while in our study this figure was considerably lower (41.8%). The two studies showed no significant difference in the frequency of ADR reports caused by actual drug-drug interactions involving at least two drugs (P = 0.1446). However, our study had higher percentage of detected reports of actual drug-drug interactions, which could be explained by previously mentioned additional drug interaction-checking methods. Vonbach et al (51) placed Drug-Reax among the top four drug-drug interaction screening software programs, with 0.98 specificity and 0.71 sensitivity when compared with Stockley’s Drug Interactions, which is used as gold standard. Accordingly, our study had significantly higher percentage of reports involving at least one potential drug-drug interaction than the study by Leone et al (15). It had a comparable proportion of actual among potential drug-drug interactions (20.8%) with the study by Leone et al (21.7%) (15), as well as with studies conducted in hospital settings (14.0%) (52) and among geriatric outpatients (25.5%) (53). These findings strongly confirm that drug-drug interactions represent an important and frequent problem in clinical practice.
Variables such as age, polimedication, and the state of disease increase the probability of an interaction (16,22,33,54,55). The percentage of serious ADR reports and the number of drugs in our study was higher in reports on actual drug-drug interactions than among the remaining reports (15,29,56). Lin Chen-Fang et al (54) found that the prevalence of potential drug-drug interactions increased in a linear mode with increasing age and with the number of prescribed drugs (55). Furthermore, these two factors interacted to increase the risk (54). However, we did not find any significant age difference between the patients experiencing ADRs caused by actual drug-drug interactions and those experiencing ADRs not caused by actual drug-drug interactions. This could be explained by the difference in the drugs involved; more than a third of actual drug-drug interactions in our study were caused by nervous system medications (especially paroxetine, risperidon, valproic acid, and lamotrigine), which are more frequently used by younger patients than other ATC groups. Since nervous system medications were most frequently involved in actual drug-drug interactions and hardly ever recognized by the reporter, we identified a special need for alertness in this drug area.
The drug most frequently involved in actual drug-drug interactions in our study was cyclosporine, while in the study by Leone et al it was the combination of digoxin and diuretics (15). Cyclosporine has been particularly hazardous in transplant patients who often require lipid-lowering therapy (57,58). Co-administration of these two types of drugs requires great caution since it can result in serious ADRs including rhabdomyolysis, as has been previously reported by our research group (59).
Serious drug-drug interactions frequently involved antiplatelet (60), anticoagulant (56), and non-steroidal anti-inflammatory drugs (27,53,61,62) that caused gastrointestinal disorders. It is essential that health care providers recognize potentially interacting drug pairs in order to reduce the risk of drug-drug interactions and associated drug-related morbidity and mortality. In our study, reporters less frequently recognized actual drug-drug interactions, which could be a consequence of restricted access to available drug interaction databases or alerting drug-interaction systems and their limited availability. Reporters most frequently recognized drug interactions including anticoagulant and antiplatelet drugs and least frequently those including psychiatric drugs. This finding was also confirmed by Leone et al (15). Our results supported the findings of previous studies on the importance of prescribing-support systems and the need for additional education of health care providers in this area (63).
The main reporters of ADRs caused by actual drug-drug interactions were secondary and tertiary care physicians, while the main reporters of all ADRs were primary care physicians. This shows that most drug-drug interactions occur in hospital settings or that ADRs caused by actual drug-drug interactions are serious and more frequently require hospitalization.
The number of over-the-counter drugs involved in ADRs caused by actual drug-drug interactions was relatively high considering the fact that over-the-counter drugs should have a good safety profile. Therefore, pharmacists, as the main dispensers, should be specially alerted about potential clinically relevant interactions.
In conclusion, the Spontaneous Reporting Database was a valuable resource for detecting ADRs associated with drug-drug interactions. We obtained clinical evidence about ADRs associated with actual drug-drug interactions relevant for clinical practice, which involved nervous system medications (ATC group N), antiplatelet, anticoagulant, and non-steroidal anti-inflammatory drugs (in most serious cases of ADRs). Our findings confirm that ADR reports caused by actual drug-drug interactions are more serious and frequently require hospitalization. Therefore, it is essential to introduce prescribing-support systems and additional education of health care professionals.
Acknowledgments
Funding None.
Ethical approval Not required.
Declaration of authorship NMS performed the assessment of cases, analysis of results, literature search, and writing of the manuscript. VMŠ participated in the presentation of the results, drafting and critical review of the manuscript. IM co-analyzed the data and wrote the manuscript; discussed the results and implications and commented on the manuscript in all stages of development. DK participated in the presentation and interpretation of the results and critical revision of the manuscript. NB performed the literature search and wrote parts of the manuscript. ST participated in the revision of the manuscript.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References:
- 1.Mannesse CK, Derkx FH, de Ridder MA, Man in 't Veld AJ, van der Cammen TJ. Adverse drug reactions in elderly patients as contributing factor for hospital admission: cross sectional study. BMJ. 1997;315:1057–8. doi: 10.1136/bmj.315.7115.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mannesse CK, Derkx FH, de Ridder MA, Man in 't Veld AJ, van der Cammen TJ. Contribution of adverse drug reactions to hospital admission of older patients. Age Ageing. 2000;29:35–9. doi: 10.1093/ageing/29.1.35. [DOI] [PubMed] [Google Scholar]
- 3.Leape LL, Bates DW, Cullen DJ, Cooper J, Demonaco HJ, Gallivan T, et al. Systems analysis of adverse drug events. ADE Prevention Study Group. JAMA. 1995;274:35–43. doi: 10.1001/jama.274.1.35. [DOI] [PubMed] [Google Scholar]
- 4.Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274:29–34. doi: 10.1001/jama.274.1.29. [DOI] [PubMed] [Google Scholar]
- 5.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 6.Committee on Quality of Health Care in America. Institute of Medicine. To error is human: building a safer health system. Washington (DC): National Academy Press; 2000. [Google Scholar]
- 7.Bates DW, Spell N, Cullen DJ, Burdick E, Laird N, Petersen LA, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997;277:307–11. doi: 10.1001/jama.277.4.307. [DOI] [PubMed] [Google Scholar]
- 8.Quinn DI, Day RO. Clinically important drug interactions. In: Speight TM, Holford HG, editors. Avery’s Drug Treatment. Auckland (New Zealand): Adis International; 1997. p. 301-38. [Google Scholar]
- 9.May FE, Stewart RB, Cluff LE. Drug interactions and multiple drug administration. Clin Pharmacol Ther. 1977;22:322–8. doi: 10.1002/cpt1977223322. [DOI] [PubMed] [Google Scholar]
- 10.Jankel CA, Fitterman LK. Epidemiology of drug-drug interactions as a cause of hospital admissions. Drug Saf. 1993;9:51–9. doi: 10.2165/00002018-199309010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Egger SS, Drewe J, Schlienger RG. Potential drug-drug interactions in the medication of medical patients at hospital discharge. Eur J Clin Pharmacol. 2003;58:773–8. doi: 10.1007/s00228-002-0557-z. [DOI] [PubMed] [Google Scholar]
- 12.Pirmohamed M, Orme MLE. Drug interactions of clinical importance. In: Davies D, Ferner R, de Glanville H, editors. Davies’s textbook of adverse drug reactions, 5th ed. London: Chapman & Hall Medical; 1998. p. 888-912. [Google Scholar]
- 13.Hauben M, Zhou X. Quantitative methods in pharmacovigilance: focus on signal detection. Drug Saf. 2003;26:159–86. doi: 10.2165/00002018-200326030-00003. [DOI] [PubMed] [Google Scholar]
- 14.Krahenbuhl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krahenbuhl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007;30:379–407. doi: 10.2165/00002018-200730050-00003. [DOI] [PubMed] [Google Scholar]
- 15.Leone R, Magro L, Moretti U, Cutroneo P, Moschini M, Motola D, et al. Identifying adverse drug reactions associated with drug-drug interactions: data mining of a spontaneous reporting database in Italy. Drug Saf. 2010;33:667–75. doi: 10.2165/11534400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Astrand B, Astrand E, Antonov K, Petersson G. Detection of potential drug interactions - a model for a national pharmacy register. Eur J Clin Pharmacol. 2006;62:749–56. doi: 10.1007/s00228-006-0143-x. [DOI] [PubMed] [Google Scholar]
- 17.Bjorkman IK, Fastbom J, Schmidt IK, Bernsten CB, Pharmaceutical Care of the Elderly in Europe Research (PEER) Group Drug-drug interactions in the elderly. Ann Pharmacother. 2002;36:1675–81. doi: 10.1345/aph.1A484. [DOI] [PubMed] [Google Scholar]
- 18.Chen YF, Avery AJ, Neil KE, Johnson C, Dewey ME, Stockley IH. Incidence and possible causes of prescribing potentially hazardous/contraindicated drug combinations in general practice. Drug Saf. 2005;28:67–80. doi: 10.2165/00002018-200528010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Gosney M, Tallis R. Prescription of contraindicated and interacting drugs in elderly patients admitted to hospital. Lancet. 1984;2:564–7. doi: 10.1016/S0140-6736(84)90775-X. [DOI] [PubMed] [Google Scholar]
- 20.Kelly WN. Potential risk and prevention, part 1: fatal adverse drug events. Am J Health Syst Pharm. 2001;58:1317–24. doi: 10.1093/ajhp/58.14.1317. [DOI] [PubMed] [Google Scholar]
- 21.Kelly WN. Potential risk and prevention, part 2: drug induced permanent disabilities. Am J Health Syst Pharm. 2001;58:1325–9. doi: 10.1093/ajhp/58.14.1325. [DOI] [PubMed] [Google Scholar]
- 22.Kohler GI, Bode-Boger SM, Busse R, Hoopmann M, Welte T, Boger RH. Drug-drug interactions in medical patients: effects of in-hospital treatment and relation to multiple drug use. Int J Clin Pharmacol Ther. 2000;38:504–13. doi: 10.5414/cpp38504. [DOI] [PubMed] [Google Scholar]
- 23.Leemans L, Veroeveren L, Bulens J, Hendrickx C, Keyenberg W, Niesten F, et al. Frequency and trends of interventions of prescriptions in Flemish community pharmacies. Pharm World Sci. 2003;25:65–9. doi: 10.1023/A:1023253132487. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell GW, Stanaszek WF, Nichols NB. Documenting drug-drug interactions in ambulatory patients. Am J Hosp Pharm. 1979;36:653–7. [PubMed] [Google Scholar]
- 25.Paulino EI, Bouvy ML, Gastelurrutia MA, Guerreiro M, Buurma H, ESCP-SIR Rejkjavik Community Pharmacy Research Group Drug related problems identified by European community pharmacists in patients discharged from hospital. Pharm World Sci. 2004;26:353–60. [PubMed] [Google Scholar]
- 26.Doucet J, Chassagne P, Trivalle C, Landrin I, Pauty MD, Kadri N, et al. Drug-drug interactions related to hospital admissions in older adults: a prospective study of 1000 patients. J Am Geriatr Soc. 1996;44:944–8. doi: 10.1111/j.1532-5415.1996.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 27.Huic M, Mucolic V, Vrhovac B, Francetic I, Bakran I, Giljanovic S. Adverse drug reactions resulting in hospital admission. Int J Clin Pharmacol Ther. 1994;32:675–82. [PubMed] [Google Scholar]
- 28.McDonnell PJ, Jacobs MR. Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother. 2002;36:1331–6. doi: 10.1345/aph.1A333. [DOI] [PubMed] [Google Scholar]
- 29.Raschetti R, Morgutti M, Menniti-Ippolito F, Belisari A, Rossignoli A, Longhini P, et al. Suspected adverse drug events requiring emergency department visits or hospital admissions. Eur J Clin Pharmacol. 1999;54:959–63. doi: 10.1007/s002280050582. [DOI] [PubMed] [Google Scholar]
- 30.Becker ML, Kallewaard M, Caspers PW, Visser LE, Leufkens HG, Stricker BH. Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16:641–51. doi: 10.1002/pds.1351. [DOI] [PubMed] [Google Scholar]
- 31.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–9. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS ONE. 2009;4:e4439. doi: 10.1371/journal.pone.0004439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaeta TJ, Fiorini M, Ender K, Bove J, Diaz J. Potential drug-drug interactions in elderly patients presenting with syncope. J Emerg Med. 2002;22:159–62. doi: 10.1016/S0736-4679(01)00471-1. [DOI] [PubMed] [Google Scholar]
- 34.Thakrar BT, Grundschober SB, Doessegger L. Detecting signals of drug-drug interactions in a spontaneous reports database. Br J Clin Pharmacol. 2007;64:489–95. doi: 10.1111/j.1365-2125.2007.02900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian Y, Ye X, Du W, Ren J, Sun Y, Wang H, et al. A computerized system for detecting signals due to drug-drug interactions in spontaneous reporting systems. Br J Clin Pharmacol. 2010;69:67–73. doi: 10.1111/j.1365-2125.2009.03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tavassoli N, Sommet A, Lapeyre-Mestre M, Bagheri H, Montrastruc JL. Drug interactions with cholinesterase inhibitors: an analysis of the French pharmacovigilance database and a comparison of two national drug formularies (Vidal, British National Formulary). Drug Saf. 2007;30:1063–71. doi: 10.2165/00002018-200730110-00005. [DOI] [PubMed] [Google Scholar]
- 37.ICH E2B guideline: Clinical safety data management: data elements for transmission of individual case safety reports. Available from: http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html Accessed: September 22, 2011.
- 38.Pharmacovigilance regulations [in Croatian]. Narodne novine. 125/09.
- 39.Medicines law [in Croatian]. Narodne novine. 71/07.
- 40.MedDRA terminology. Available from: www.meddramsso.com. Accessed: September 22, 2011.
- 41.Karch FE, Lasagna L. Adverse drug reactions. A critical review. JAMA. 1975;234:1236–41. doi: 10.1001/jama.234.12.1236. [DOI] [PubMed] [Google Scholar]
- 42.DRUG-REAX System [internet database]. Greenwood Village (CO): Thomson Reuters (Healthcare); 2010.
- 43.Baxter K. (9ed). Stockley’s drug interactions. Available from: http://www.medicinescomplete.com/mc/stockley/current/. Accessed: September 22, 2011.
- 44.ICH E2A guideline: note for guidance on clinical safety data management: definitions and standards for expedited reporting. Available from: http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html. Accessed: September 22, 2011.
- 45.Current challenges in pharmacovigilance: pragmatic approaches, reports of CIOMS (Council for International Organizations for Medicinal Sciences) Working group V. Geneva (Switzerland): CIOMS; 2001. [Google Scholar]
- 46.Tirkkonen T, Ryynänen A, Vahlberg T, Irjala K, Klaukka T, Huupponen R, et al. Frequency and clinical relevance of drug interactions with lovastatin and simvastatin: an observational database study. Drug Saf. 2008;31:231–40. doi: 10.2165/00002018-200831030-00004. [DOI] [PubMed] [Google Scholar]
- 47.Straubhaar B, Krahenbuhl S, Schlienger RG. The prevalence of potential drug-drug interactions in patients with heart failure at hospital discharge. Drug Saf. 2006;29:79–90. doi: 10.2165/00002018-200629010-00006. [DOI] [PubMed] [Google Scholar]
- 48.Magro L, Conforti A, Del Zotti F, Leone R, Iorio ML, Meneghelli I, et al. Identification of severe potential drug-drug interactions using an Italian general-practitioner database. Eur J Clin Pharmacol. 2008;64:303–9. doi: 10.1007/s00228-007-0394-1. [DOI] [PubMed] [Google Scholar]
- 49.Ibanez A, Alcala M, Garcia J, Puche E. Drug-drug interactions in patients from an internal medicine service. Farm Hosp. 2008;32:293–7. [in Spanish] [PubMed] [Google Scholar]
- 50.Francetić I, Huić M. Side effects and interactions of medicines [in Croatian]. In Francetić I, Vitezić D, editors. Basics of clinical pharmacology. Zagreb: Medicinska naklada; 2007. p. 147-64. [Google Scholar]
- 51.Vonbach P, Dubied A, Krahenbuhl S, Beer JH. Evaluation of frequently used drug interaction screening programs. Pharm World Sci. 2008;30:367–74. doi: 10.1007/s11096-008-9191-x. [DOI] [PubMed] [Google Scholar]
- 52.Tulner LR, Frankfort SV, Gijsen GJ, van Campen JP, Koks CH, Beijnen JH. Drug-drug interactions in a geriatric outpatient cohort: prevalence and relevance. Drugs Aging. 2008;25:343–55. doi: 10.2165/00002512-200825040-00007. [DOI] [PubMed] [Google Scholar]
- 53.Carter BL, Lund BC, Hayase N, Chrischilles E. The extent of potential antihypertensive drug interactions in a Medicaid population. Am J Hypertens. 2002;15:953–7. doi: 10.1016/S0895-7061(02)03026-1. [DOI] [PubMed] [Google Scholar]
- 54.Lee A, Stockley IH. Drug interactions. In: Walker R, Edwards C. Clinical pharmacy and therapeutics, 3rd ed. Philadelphia (PA): Churchill Livingstone; 2003. p. 21-32. [Google Scholar]
- 55.Lin CF, Wang CY, Bai CH. Polypharmacy, aging and potential drug-drug interactions in outpatients in Taiwan: a retrospective computerized screening study. Drugs Aging. 2011;28:219–25. doi: 10.2165/11586870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 56.Bergman U, Wiholm BE. Drug-related problems causing admission to a medical clinic. Eur J Clin Pharmacol. 1981;20:193–200. doi: 10.1007/BF00544597. [DOI] [PubMed] [Google Scholar]
- 57.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–81. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther. 2006;112:71–105. doi: 10.1016/j.pharmthera.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Skvrce NM, Bozina N, Sarinic VM, Tomic S. Adverse drug reactions of hydroxymethylglutaryl-CoA reductase inhibitors reported to agency for medicinal products and medical devices. Lijec Vjesn. 2010;132:277–82. [in Croatian] [PubMed] [Google Scholar]
- 60.Hallas J, Harvald B, Gram LF, Grodum E, Brřsen K, Haghfelt T, et al. Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med. 1990;228:83–90. doi: 10.1111/j.1365-2796.1990.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 61.Lin SH, Lin MS. A survey on drug-related hospitalization in a community teaching hospital. Int J Clin Pharmacol Ther Toxicol. 1993;31:66–9. [PubMed] [Google Scholar]
- 62.Chan TY, Chan JC, Tomlinson B, Critchley JA. Adverse reactions to drugs as a cause of admissions to a general teaching hospital in Hong Kong. Drug Saf. 1992;7:235–40. doi: 10.2165/00002018-199207030-00008. [DOI] [PubMed] [Google Scholar]
- 63.Ko Y, Malone DC, Skrepnek GH, Armstrong EP, Murphy JE, Abarca J, et al. Prescribers' knowledge of and sources of information for potential drug-drug interactions: a postal survey of US prescribers. Drug Saf. 2008;31:525–36. doi: 10.2165/00002018-200831060-00007. [DOI] [PubMed] [Google Scholar]