Abstract
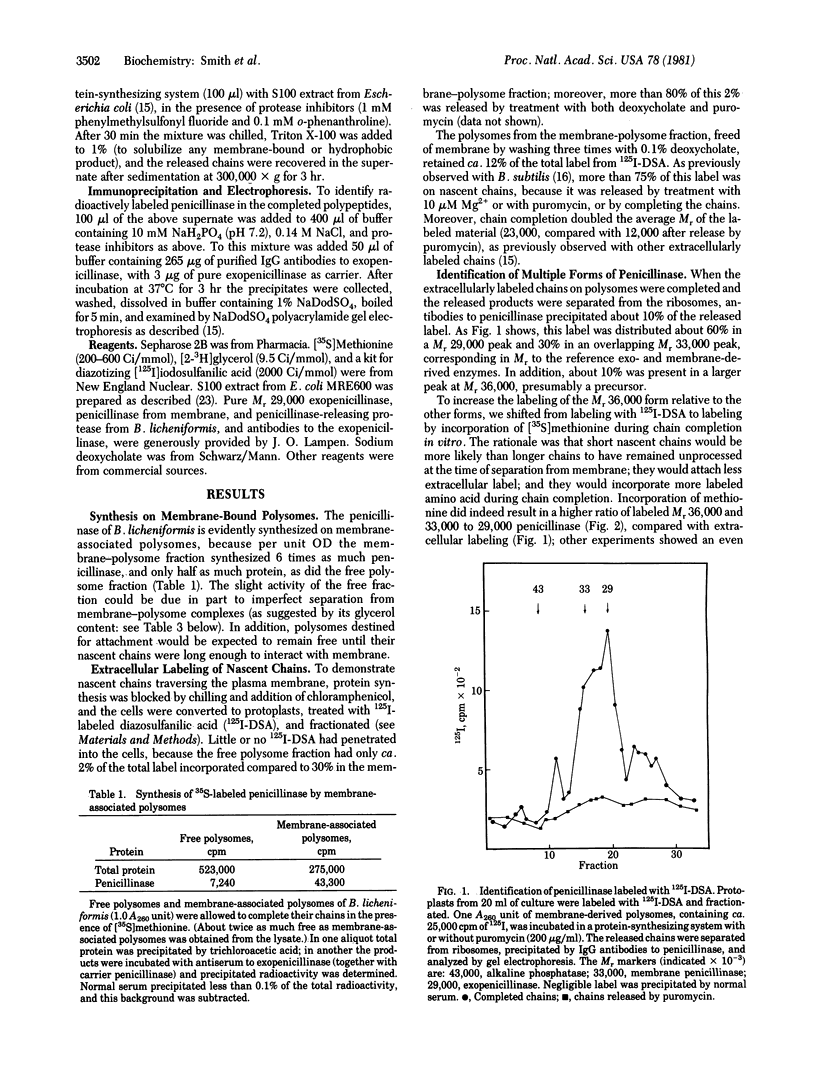
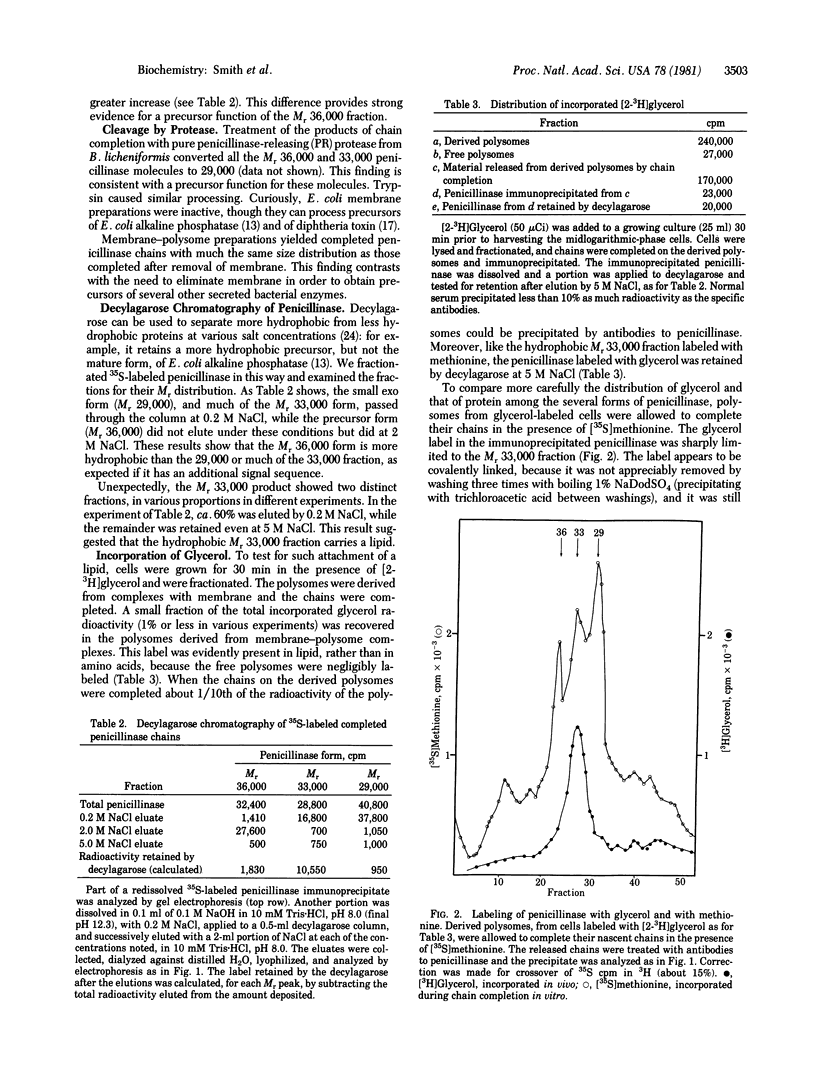
The penicillinase of Bacillus licheniformis is shown to be secreted cotranslationally. In extracts it was formed by membrane-associated but not by free polysomes; and after extracellular labeling of cells, followed by completion of the growing chains on polysomes in vitro, labeled penicillinase could be immunoprecipitated. This product contained electrophoretic peaks of Mr 36,000, 33,000, and 29,000, which correspond to previously reported forms of the enzyme. The Mr 36,000 form exhibits moderate hydrophobicity, as expected of a precursor with an NH2-terminal signal sequence for secretion. In addition, part of the Mr 33,000 fraction evidently contains a lipid: it is even more hydrophobic, and [2-3H]glycerol was found to be incorporated into these molecules but not into the other forms of the enzyme. These findings renew the earlier, discarded suggestion that the Mr 33,000 membrane-bound penicillinase in the cells contains lipid. The incorporation of lipid and two different cleavages can evidently all occur during growth of the penicillinase chain. Moreover, the resulting terminal regions are all accessible to extracellular labeling on growing chains. Several additional, unidentified lipoproteins also incorporate lipid during chain growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyappa P. S., Lampen J. O. Membrane associated phospholipoproteins of Bacillus lichenformis 749. Biochim Biophys Acta. 1976 Oct 19;448(3):401–410. doi: 10.1016/0005-2736(76)90296-0. [DOI] [PubMed] [Google Scholar]
- Aiyappa P. S., Lampen J. O. Penicillinase-releasing protease of Bacillus licheniformis 749 Specificity for hydroxyamino acids. J Biol Chem. 1977 Mar 10;252(5):1745–1747. [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui K., Nielsen J. B., Caulfield M. P., Lampen J. O. Large exopenicillinase, initial extracellular form detected in cultures of Bacillus licheniformis. Biochemistry. 1980 Apr 29;19(9):1882–1886. doi: 10.1021/bi00550a023. [DOI] [PubMed] [Google Scholar]
- Lai J. S., Philbrick W. M., Hayashi S., Inukai M., Arai M., Hirota Y., Wu H. C. Globomycin sensitivity of Escherichia coli and Salmonella typhimurium: effects of mutations affecting structures of murein lipoprotein. J Bacteriol. 1981 Jan;145(1):657–660. doi: 10.1128/jb.145.1.657-660.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J. S., Sarvas M., Brammar W. J., Neugebauer K., Wu H. C. Bacillus licheniformis penicillinase synthesized in Escherichia coli contains covalently linked fatty acid and glyceride. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3506–3510. doi: 10.1073/pnas.78.6.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampen J. O., Nielsen J. B., Izui K., Caulfield M. P. Bacillus licheniformis beta-lactamases: multiple forms and their roles. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):345–348. doi: 10.1098/rstb.1980.0051. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Lai J. S., Wu H. C. Characterization of murein-bound lipoprotein in an Excherichia coli mutant altered in the signal sequence of prolipoprotein. FEBS Lett. 1980 Jan 1;109(1):50–54. doi: 10.1016/0014-5793(80)81309-3. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Modolell J., Davis B. D. Rapid inhibition of polypeptide chain extension by streptomycin. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1279–1286. doi: 10.1073/pnas.61.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Caulfield M. P., Lampen J. O. Lipoprotein nature of Bacillus licheniformis membrane penicillinase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3511–3515. doi: 10.1073/pnas.78.6.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. PURIFICATION AND PROPERTIES OF PENICILLINASES FROM TWO STRAINS OF BACILLUS LICHENIFORMIS: A CHEMICAL, PHYSICOCHEMICAL AND PHYSIOLOGICAL COMPARISON. Biochem J. 1965 Mar;94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. The mechanism of liberation of penicillinase from Bacillus subtilis. J Gen Microbiol. 1961 Oct;26:267–276. doi: 10.1099/00221287-26-2-267. [DOI] [PubMed] [Google Scholar]
- Sarvas M., Hirth K. P., Fuchs E., Simons K. A precursor form of the penicillinase from Bacillus licheniformis. FEBS Lett. 1978 Nov 1;95(1):76–80. doi: 10.1016/0014-5793(78)80055-6. [DOI] [PubMed] [Google Scholar]
- Shaltiel S., Er-El Z. Hydrophobic chromatography: use for purification of glycogen synthetase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):778–781. doi: 10.1073/pnas.70.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Sarvas M., Garoff H., Helenius A. Membrane-bound and secreted forms of penicillinase from Bacillus licheniformis. J Mol Biol. 1978 Dec 25;126(4):673–690. doi: 10.1016/0022-2836(78)90015-3. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Davis B. D. Extracellular labeling of growing secreted polypeptide chains in Bacillus subtilis with diazoiodosulfanilic acid. Biochemistry. 1979 Jan 9;18(1):198–202. doi: 10.1021/bi00568a030. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Murphy J. R., Davis B. D. Precursor in cotranslational secretion of diphtheria toxin. J Bacteriol. 1980 Jan;141(1):184–189. doi: 10.1128/jb.141.1.184-189.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Thompson R. C., Davis B. D. Extracellular labeling of nascent polypeptides traversing the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2830–2834. doi: 10.1073/pnas.74.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traficante L. J., Lampen J. O. Vesicle penicillinase of Bacillus licheniformis: existence of periplasmic-releasing factor(s). J Bacteriol. 1977 Jan;129(1):184–190. doi: 10.1128/jb.129.1.184-190.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. Membrane penicillinase of Bacillus licheniformis 749/C:sequence and possible repeated tetrapeptide structure of the phospholipopeptide region. Proc Natl Acad Sci U S A. 1976 May;73(5):1457–1461. doi: 10.1073/pnas.73.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. The hydrophobic membrane penicillinase of Bacillus licheniformis 749/C. Characterization of the hydrophilic enzyme and phospholipopeptide produced by trypsin cleavage. J Biol Chem. 1976 Jul 10;251(13):4102–4110. [PubMed] [Google Scholar]


