Abstract
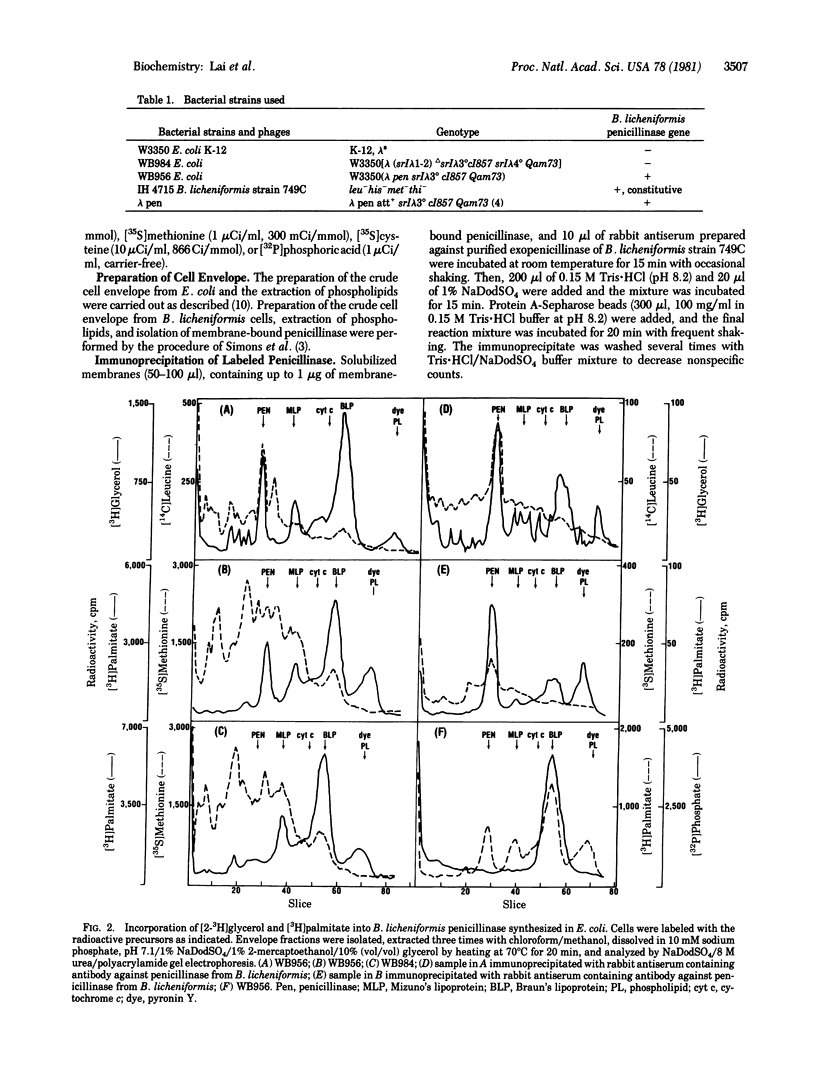
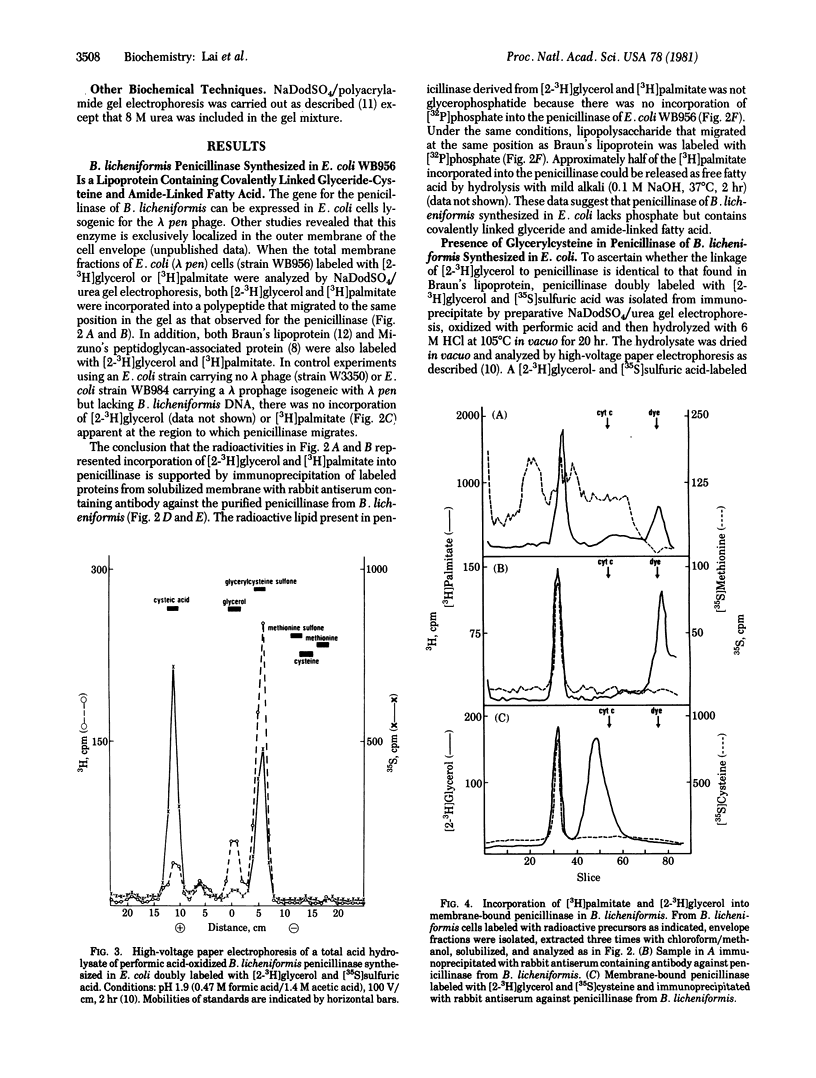
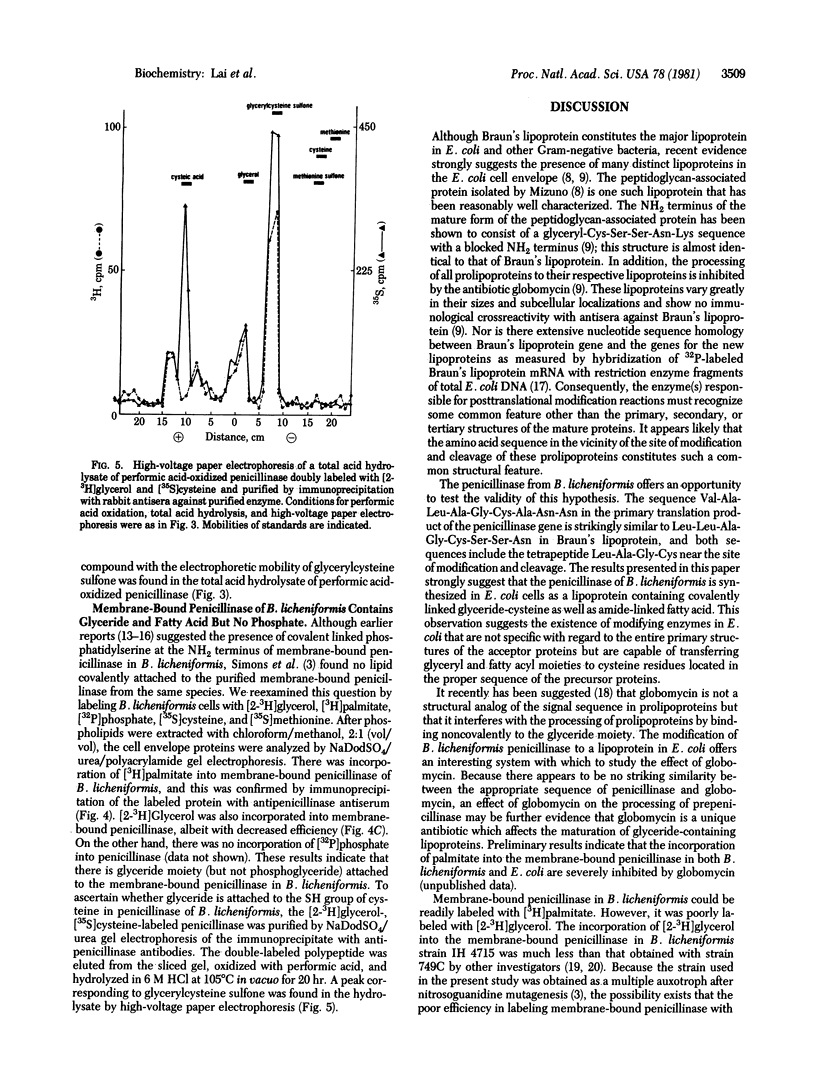
DNA sequence analysis of the structural gene for Bacillus licheniformis penicillinase has revealed a tetrapeptide sequence of Leu-Ala-Gly-Cys within the NH2-terminal part of the precursor form of penicillinase (penicillin amido-beta-lactamhydrolase, EC 3.5.2.6). The same tetrapeptide occurs in the signal sequence of the prolipoprotein of Escherichia coli, and the cysteine residue in the tetrapeptide of prolipoprotein is modified to form glyceride-cysteine which becomes the NH2 terminus of Braun's lipoprotein. On the basis of labeling, with [2-3H]glycerol, [3H]palmitate, [35S]methionine, and [35S]sulfuric acid, of an E. coli strain lysogenic for a lambda vector containing the penicillinase gene from B. licheniformis and of immunoprecipitation with rabbit antisera against purified B. licheniformis penicillinase, we conclude that B. licheniformis penicillinase synthesized in E. coli contains covalently linked glyceride and fatty acid. These results strongly suggest the operation of a modification system in E. coli, and presumably in other Gram-negative bacteria, which results in the formation of a glyceride-cysteine residue if the proper peptide sequence is present in the signal sequence of membrane proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brammar W. J. The thirteenth Colworth Medal Lecture: The construction in vitro and exploitation of transducing derivatives of bacteriophage lambda. Biochem Soc Trans. 1977;5(6):1633–1652. doi: 10.1042/bst0051633. [DOI] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Ichihara S., Hussain M., Mizushima S. Characterization of new membrane lipoproteins and their precursors of Escherichia coli. J Biol Chem. 1981 Mar 25;256(6):3125–3129. [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J. S., Philbrick W. M., Hayashi S., Inukai M., Arai M., Hirota Y., Wu H. C. Globomycin sensitivity of Escherichia coli and Salmonella typhimurium: effects of mutations affecting structures of murein lipoprotein. J Bacteriol. 1981 Jan;145(1):657–660. doi: 10.1128/jb.145.1.657-660.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Wu H. C. Biosynthesis and assembly of envelope lipoprotein in a glycerol-requiring mutant of Salmonella typhimurium. J Bacteriol. 1976 Mar;125(3):892–904. doi: 10.1128/jb.125.3.892-904.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T. A novel peptidoglycan-associated lipoprotein found in the cell envelope of Pseudomonas aeruginosa and Escherichia coli. J Biochem. 1979 Oct;86(4):991–1000. doi: 10.1093/oxfordjournals.jbchem.a132631. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Katz-Wurtzel E. T., Pirtle R. M., Inouye M. Restriction enzyme cleavage sites surrounding the structural gene for the lipoprotein of the Escherichia coli outer membrane. J Bacteriol. 1979 Jun;138(3):715–720. doi: 10.1128/jb.138.3.715-720.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Caulfield M. P., Lampen J. O. Lipoprotein nature of Bacillus licheniformis membrane penicillinase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3511–3515. doi: 10.1073/pnas.78.6.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. The mechanism of liberation of penicillinase from Bacillus subtilis. J Gen Microbiol. 1961 Oct;26:267–276. doi: 10.1099/00221287-26-2-267. [DOI] [PubMed] [Google Scholar]
- Sarvas M., Hirth K. P., Fuchs E., Simons K. A precursor form of the penicillinase from Bacillus licheniformis. FEBS Lett. 1978 Nov 1;95(1):76–80. doi: 10.1016/0014-5793(78)80055-6. [DOI] [PubMed] [Google Scholar]
- Simons K., Sarvas M., Garoff H., Helenius A. Membrane-bound and secreted forms of penicillinase from Bacillus licheniformis. J Mol Biol. 1978 Dec 25;126(4):673–690. doi: 10.1016/0022-2836(78)90015-3. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Davis B. D. Bacillus licheniformis penicillinase: cleavages and attachment of lipid during cotranslational secretion. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3501–3505. doi: 10.1073/pnas.78.6.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. Membrane penicillinase of Bacillus licheniformis 749/C, a phospholipoprotein. J Biol Chem. 1975 Apr 25;250(8):3212–3213. [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. Membrane penicillinase of Bacillus licheniformis 749/C:sequence and possible repeated tetrapeptide structure of the phospholipopeptide region. Proc Natl Acad Sci U S A. 1976 May;73(5):1457–1461. doi: 10.1073/pnas.73.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. Purification of plasma membrane penicillinase from Bacillus licheniformis 749/C and comparison with exoenzyme. J Biol Chem. 1976 Jul 10;251(13):4095–4101. [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. The hydrophobic membrane penicillinase of Bacillus licheniformis 749/C. Characterization of the hydrophilic enzyme and phospholipopeptide produced by trypsin cleavage. J Biol Chem. 1976 Jul 10;251(13):4102–4110. [PubMed] [Google Scholar]



