Abstract
Incubation of fear has been used to account for the delayed manifestation of symptoms of fear and anxiety including the delayed onset of Post Traumatic Stress Disorder (PTSD). We have shown the utility of classical conditioning-specific modification of the rabbit nictitating membrane response (NMR) as a model of PTSD. This modification includes an exaggeration in the size and a change in the timing of the unconditioned NMR after several days of classical conditioning. To assess the effects of incubation on conditioning-specific modification, we measured changes in responding as a function of the time between classical conditioning and NMR testing. After just one day of classical conditioning resulting in modest levels of learning, increases in response size were an inverted-U shaped function of days of incubation with little if any change occurring one and ten days after training but significant change occurring after six days. The incubation effect persisted for a week. An unpaired control group showed no change in the size of the response confirming the incubation effect was associative. The results bear a striking resemblance to symptoms of PTSD that do not always occur immediately after trauma and become exacerbated over time and then persist. They point to a window when incubation can exacerbate symptoms and speak to the vulnerability of re-experiencing trauma too soon. This could be a serious problem for military or emergency personnel recalled to combat or a disaster site without sufficient time to deal with the effects of their initial experiences.
Keywords: classical conditioning, eyeblink, nictitating membrane response, post traumatic stress disorder, rabbit, stress
Introduction
An increase in fear and anxiety that occurs over time following fear conditioning, termed fear incubation, has been studied for more than sixty years in animals and humans (Pickens et al., 2010) and has been proposed more recently as a model for delayed-onset post traumatic stress disorder (PTSD) (Pickens et al., 2009a). Although there is considerable debate about whether delayed-onset PTSD actually exists (Andrews et al., 2007; Frueh et al., 2009; Smid et al., 2011), there is good evidence that PTSD symptoms do not always occur immediately after trauma and that they can become exacerbated over time (Frueh et al., 2009; Smid et al., 2011). Those who study delayed-onset PTSD attribute it to a number of different causes most notably the presence of stress before, during, or after the traumatic experience (Andrews et al., 2009; Horesh et al., 2010). From a neurobiological point of view, different forms of stress have been shown to generate structural changes in a number of brain areas particularly the amygdala (Roozendaal et al., 2009) – a structure long associated with fear and fear conditioning in both animals and humans (Holland & Gallagher, 1999; Yehuda & LeDoux, 2007; Knight et al., 2010; Linnman et al., 2011) and implicated in PTSD (Shin et al., 2005; Williams et al., 2006; Gianaros et al., 2008). Roozendaal et al. (2009) describe work that has shown there are more immediate synaptic changes in the amygdala following chronic stress than acute stress which leads to a delayed change. Therefore, changes in the amygdala may account for both immediate and delayed onset PTSD depending on the nature of the stress exposure.
We have shown previously that exaggerated responding after classical conditioning of both the rabbit nictitating membrane response (NMR) and heart rate change, called conditioning-specific reflex modification (CRM), may be useful as a model of some features of PTSD (Seager et al., 2003; Schreurs et al., 2005; Burhans et al., 2008; Schreurs et al., 2010). For example, the increased size and complexity of the NMR to weak unconditioned stimuli following classical conditioning may be similar to the heightened responsivity to stressful stimuli exhibited by people who suffer from PTSD (McTeague et al., 2010). CRM is measured as an increase in the size and shape and a change in the timing of unconditioned responses (URs) to an electrical stimulus or air puff unconditioned stimulus (US) when these responses are measured in the absence of a conditioned stimulus (CS) following classical conditioning of the rabbit NMR (Gruart & Yeo, 1995; Schreurs et al., 1995; Schreurs et al., 2000; Buck et al., 2001; Wikgren et al., 2002; Seager et al., 2003; Schreurs et al., 2006; Burhans et al., 2008; Schreurs et al., 2010). CRM is assessed by presenting a series of USs that varies in intensity and duration before (US Pretest) and after (US Post Test) classical conditioning. The largest changes in response frequency, amplitude, timing, and shape occur at intermediate US intensities where the UR has not reached its maximum (Schreurs, 2003; Burhans et al., 2008).
In previous rabbit NMR conditioning experiments, CRM was only detected after several days of classical conditioning and although CRM was shown to be long-lasting (Burhans et al., 2008), there has been no evidence to date that it can be incubated or that it is delayed in onset. In the present experiment, we used a single 80-trial session of CS-US pairings to establish classical conditioning rather than the three to six sessions normally used to consolidate conditioning and then, one, six or ten days later, assessed responding to the US. The purpose of the present experiment was to assess the effects of incubation on conditioning-specific modification of the NMR and whether CRM effects persist. If CRM does incubate and persist, it would to extend the validity of CRM as an animal model of PTSD (Burhans et al., 2008).
Materials and Methods
Subjects
Twenty-four male New Zealand White rabbits (Oryctolagus cuniculus) supplied by Harlan weighed approximately 2.0-2.2 kg at the beginning of the experiment. Animals were housed in individual cages, given free access to food and water, and maintained on a 12-hour light/dark cycle. Rabbits were maintained in accordance with guidelines issued by the National Institutes of Health, and the research was approved by the West Virginia University Animal Care and Use Committee.
Apparatus
The apparatus and recording procedures for the NMR have been detailed by Schreurs and Alkon (1990) who modeled their apparatus after those described by Gormezano (Gormezano, 1966; Coleman & Gormezano, 1971). In brief, each subject was restrained in a Plexiglas box and trained in a sound-attenuating, ventilated chamber (Coulbourn Instruments, Model E10-20). A stimulus panel containing a speaker and house light (1-W, 120-V, 60-lumen LED lamp) was mounted at a 45° angle, 15 cm anterior to and 15 cm above the subject’s head. An ambient noise level of 65 dB in each chamber was provided by an exhaust fan. Periorbital ES was delivered by a programmable two-pole shocker (Coulbourn Instruments, Model E13-35) via stainless steel Autoclip wound clips positioned 10 mm below and 10 mm posterior to the dorsal canthus of the right eye.
Details of transducing NM movements have been reported previously (Gormezano & Gibbs, 1988; Schreurs & Alkon, 1990) In short, a hook connected to an L-shaped lever containing a freely moving ball and socket joint was attached to a 6-0 nylon loop sutured into, but not through, the NM. The other end of the lever was attached to a rotary potentiometer (Novotechnik US Inc., Southborough, MA; Model P2201) that, in turn, was connected to a 12-bit analog-to-digital converter (5-ms sampling rate; 0.05-mm resolution). Individual analog-to-digital outputs were stored on a trial-by-trial basis for subsequent analysis. Data collection, analysis and stimulus delivery were accomplished using a LabVIEW system (National Instruments).
Procedure
Eighteen rabbits were randomly assigned to three paired conditioning groups (n’s=6) that received one session per day starting with adaptation, US testing (Pretest), paired CS-US presentations (Acquisition), US testing (Post Test1) one, six or ten days later, then a second session of US post testing (Post Test2) seven days after Post Test1, and finally, a CS-alone extinction session ( Extinction). An unpaired control group (n=6) received adaptation, Pretest, one session of explicitly unpaired CS and US presentations, a Post Test1 six days later, Post Test2 seven days after Post Test1, and then Extinction. We conducted Post Test1 six days after unpaired stimulus presentations because preliminary testing indicated maximal incubation effects occurred six days after CS-US pairings.
On adaptation day, the rabbits were prepared for ES and recording of NM movement and then adapted to the training chambers for the length of time of subsequent training sessions (80 min). On Pretest and both Post Test sessions the subjects received a total of 80 trials of ES stimulation presented at an average inter-trial interval (ITI) of 60 s (50-70 s range). Each trial involved the presentation of 1 of 20 possible combinations of ES intensity (0.1, 0.25, 0.5, 1.0, or 2.0 mA) and duration (10, 25, 50, or 100 ms). Four separately randomized sequences of the 20 stimulus combinations were presented on each testing day, with the restriction that the same intensity or duration could not occur on more than three consecutive trials. Previous research has shown that one day of CS-US pairings produces only moderate levels of NMR conditioning and is insufficient to elicit CRM when CRM is assessed across all 80 trials on Post Test (Schreurs et al., 1995). To increase our sensitivity of detecting CRM, we continued our recent practice of examining responding to the US during the first 20 trials of Pretest and both Post Test sessions and collapsing the data across the four US durations (Burhans et al., 2008; Schreurs et al., 2010). To provide a similar fine-grained analysis of responding to the CS during paired and unpaired stimulus presentations, we calculated percent responding in blocks of twenty CS presentations.
The paired conditioning session consisted of 80 presentations of a 400-ms, 1-kHz, 82-dB tone CS that coterminated with a 100-ms, 2.0-mA US (i.e., 300-ms inter-stimulus interval [ISI]) delivered, on average, every 60 s (50-70 s range). The session of unpaired stimulus presentations consisted of 80 CS-alone and 80 US-alone presentations that occurred in an explicitly unpaired manner delivered, on average, every 30 s (20-40 s range). The CS-alone extinction session consisted of 80 presentations of the 400-ms, 1-kHz, 82-dB tone CS delivered, on average, every 60 s (50-70 s range). Responding to the CS during the Acquisition and Extinction sessions was assessed across all 80 paired trials as well as for every 20-trial block. Responding to the CS during the explicitly unpaired session was assessed across all 80 CS-alone trials as well as every block of 20 CS-alone trials.
A CR was defined as any extension of the NM exceeding 0.5 mm that was initiated after CS onset but prior to US onset. A UR was defined as any extension of the NM exceeding 0.5 mm that was initiated within 300 ms of US onset (i.e., the CS-US ISI used to score CRs during pairings). The UR criterion was based on the observation that, following CS-US pairings, post-test URs at lower US intensities had onset latencies that fell into the range of latencies for CRs (Schreurs et al., 2000). Amplitude of a response was scored in millimeters as the maximum extension of the NM. Area of the response was calculated as the total area of the response curve (in arbitrary units) from stimulus onset until the end of trial. For URs during US testing, two additional measures were calculated in order to overcome the statistical limitations of empty data cells produced by subthreshold responses to the US, particularly at the lower intensities and durations. These measures, magnitude of the response and magnitude of the response area, included the amplitudes and areas of all NMRs above baseline regardless of whether the 0.5 mm criterion was met (Garcia et al., 2003; Schreurs et al., 2010).
Results
To determine whether incubation would affect CRM, rabbits were trained for a single session and then returned to their home cages for one, six or ten days before CRM was assessed (Post Test1). The persistence of CRM was assessed a week later by second US test (Post Test2), followed by a CS-alone extinction session to measure retention of conditioned responses (CRs) to the CS.
CR Acquisition and Extinction
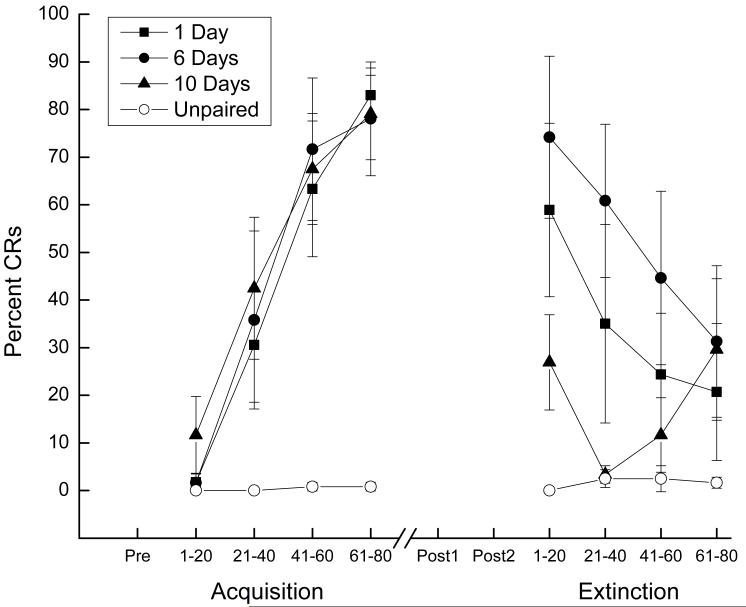
Responding across 80 CS-US pairings for eighteen rabbits in the paired groups reached a mean of level of 46.62 (± 10.62 SEM) percent CRs compared to 0.4 (± 0.29 SEM) percent responding for six rabbits in the unpaired control group. Analysis of variance revealed there was a significant main effect of pairings (F(1, 22) = 40.87, p < .001). Figure 1 shows percent CRs to the CS as a function of 20-trial blocks during CS-US pairings or unpaired stimulus presentations during Acquisition and Extinction that followed one, six or ten days of incubation and the two Post Test sessions. The left side of Figure 1 shows that the level of CRs for rabbits receiving CS-US pairings increased as a function of 20-trial blocks whereas responding for rabbits that received unpaired stimulus presentations did not rise appreciably above zero. Analysis revealed a significant effect of Pairings (F(1,22) = 40.46, p < .001) and 20-trial Blocks (F(3,66) = 17.41, p < .001) and an interaction of Pairings and 20-trial Blocks (F(3,66) = 16.53, p < .001). There were no significant differences in acquisition among the paired groups (F’s <1). The right side of the figure shows the level of responding to the CS during Extinction for rabbits given CS-US pairings decreased across 20-trial blocks after one and six days of incubation but was low and variable after ten days of incubation. Responding to the CS by rabbits previously given unpaired stimulus presentations remained at virtually zero. Analyses revealed a significant main effect of Pairings (F(1,22) = 6.73, p < .05) but no effect or interaction of 20-trial Blocks (F(3,66) = 2.53, p < .07). Analysis of the paired groups (one, six, or ten days incubation) revealed an effect of 20-trial Blocks (F(3,45) = 8.28, p < .001) and an interaction of Days of incubation and 20-trial Blocks (F(3,45) = 2.99, p < .05) that was localized by post hoc comparisons to lower levels of responding after ten days of incubation (p’s < .05).
Figure 1.
Mean (± SEM) percent conditioned responses (CRs) to the conditioned stimulus as a function of 20-trial blocks during conditioned stimulus-unconditioned stimulus pairings or unpaired stimulus presentations during Acquisition and to conditioned stimulus-alone presentations during Extinction. Extinction took place following one, six or ten days of incubation and two Post Test sessions (Post1 and Post2) that were separated by seven days.
Taken together, the CR acquisition data show that rabbits receiving CS-US pairings averaged 46% CRs but reached levels of conditioning over 80% CRs by the final 20-trial block of training compared to unpaired controls that remained at virtually zero. Following incubation and two US Post Tests, the Extinction data show that despite identical levels of CR acquisition, rabbits incubated for ten days exhibited significantly lower overall levels of responding to the CS than rabbits incubated for one and six days who extinguished across the four 20-trial blocks. Unpaired control rabbits continued to show little, if any responding to the CS.
CRM
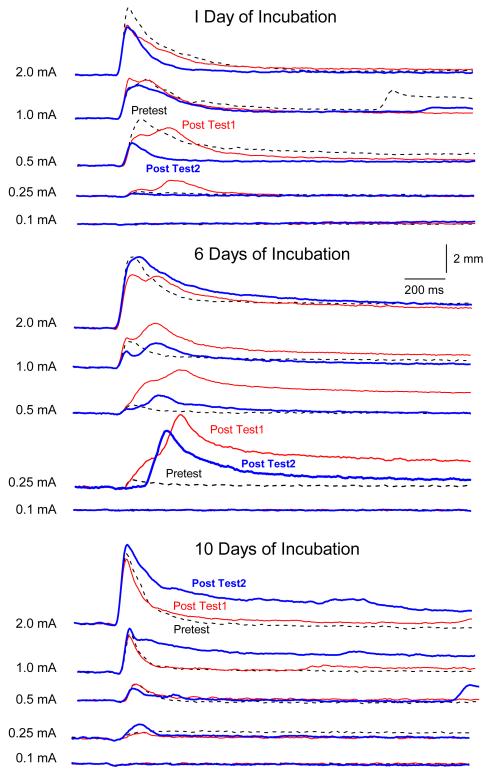
The three panels of Figure 2 show sample UR response topographies for an individual rabbit from each of the paired groups as a function of the first 20-trial block of US test intensities (0.1, 0.25, 0.5, 1.0 and 2.0 mA) presented before CS-US pairings (Pretest), after one, six or ten days of incubation (Post Test-1) and again seven days later (Post Test-2). Focusing on whether CRM occurred as a function of incubation (Pretest versus Post Test-1), the panels show dramatically larger responses that peaked later following six days of incubation but only subtle shifts in peak latency following one day of incubation and no discernible changes ten days after incubation. The middle panel also shows that the changes in size and timing of responding to the US that occurred after six days of incubation weakened slightly but persisted a week later (Post Test1 versus Post Test2).
Figure 2.
The three panels show sample unconditioned response topographies for individual rabbits as a function of the first 20-trial block of unconditioned stimulus test intensities (0.1, 0.25, 0.5, 1.0 and 2.0 mA) presented before conditioned stimulus-unconditioned stimulus pairings (Pretest, dotted line), after one, six or ten days of incubation (Post Test1, red line) and again seven days later (Post Test2, blue line).
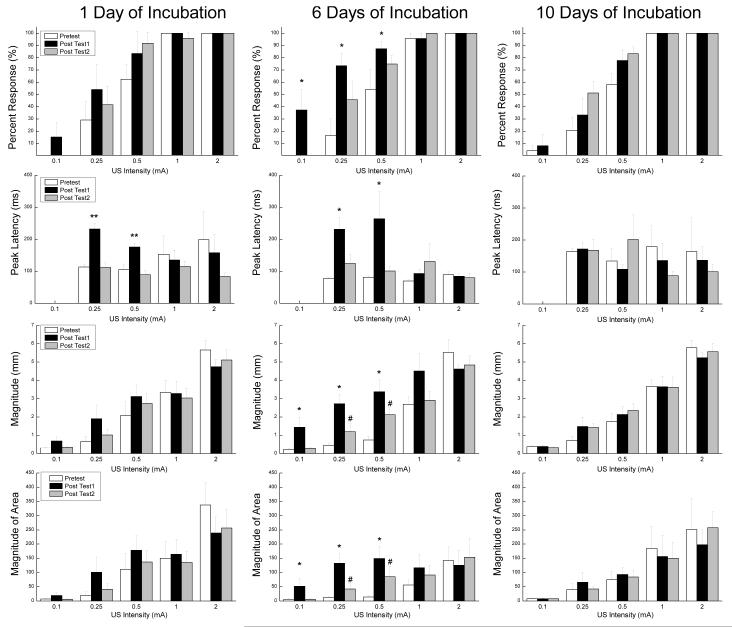
Figure 3 shows the mean and standard error for response frequency (Percent Responding), peak latency, magnitude and area (Magnitude of Area) for paired subjects as a function of the first 20-trial block of US test intensities (0.1, 0.25, 0.5, 1.0 and 2.0 mA) presented before CS-US pairings (Pretest), after one, six or ten days of incubation (Post Test-1), and again seven days later (Post Test-2). The left column of bar graphs shows that there was a significant increase in peak latency on Post Test1 following one day of incubation. The middle columns show significant increases in response frequency, peak latency, magnitude and area on Post Test1 for the group of rabbits tested six days after incubation and an increase in magnitude and area that persisted a week later on Post Test2. The right column of bar graphs shows that there were no reliable changes in responding to the US following ten days of incubation.
Figure 3.
Mean (± SEM) response frequency (Percent Responding), peak latency in milliseconds, magnitude in millimeters and area in arbitrary units (Magnitude of Area) for paired subjects as a function of the first 20-trial block of unconditioned stimulus test intensities (0.1, 0.25, 0.5, 1.0 and 2.0 mA) presented before conditioned stimulus-unconditioned stimulus pairings (Pretest), after one, six or ten days of incubation (Post Test1) and again seven days later (Post Test2).
These observations were confirmed by analyses of response frequency, peak latency, magnitude and area. An analysis of response frequency yielded a significant main effect of CRM testing (F(2, 30) = 7.54, p <.01) and US intensities (F(4, 60) = 213.59, p < .001) and an interaction of CRM testing and US intensities (F(8, 120) = 5.36, p <.001). Post hoc comparisons isolated the significant effects of CRM testing on response frequency to lower US intensities (0.1, 0.25, and 0.5 mA) following six days of incubation (p’s < .05). An analysis of peak latency for US intensities at which responses occurred (0.25, 0.5, 1.0, 2.0 mA), yielded a significant main effect of CRM testing (F(2, 30) = 4.37, p <.05), an interaction of CRM testing and Days of incubation F(2, 30) = 2.76, p < .05), and an interaction of CRM testing and US intensities (F(8, 120) = 2.30, p <.05). Post hoc comparisons isolated the significant interaction of CRM testing and Days of incubation on peak latencies to two US intensities (0.25 and 0.5 mA) following one day (p’s < .01) and six days of incubation (p’s < .05). An analysis of magnitude yielded a significant main effect of CRM testing (F(2, 30) = 6.50, p <.01), US intensities (F(4, 60) = 132.58, p < .001), an interaction of CRM testing and Days of incubation (F(2, 30) = 3.00, p <.05) and an interaction of CRM testing and US intensities (F(8, 120) = 6.08, p <.001). Post hoc comparisons isolated the significant interaction of CRM testing and Days of incubation on response magnitude to increases from Pretest at three US intensities (0.1, 0.25 and 0.5 mA) following six days of incubation for Post Test1 and at two intensities (0.25, 0.5 mA) for Post Test2 (p’s < .05) with Post Test2 being significantly lower than Post Test1 at those two intensities (p <.05). Finally, an analysis of area yielded a significant main effect US intensities (F(4, 60) = 25.75, p < .001) and an interaction of CRM testing and US intensities (F(8, 120) = 3.55, p <.01). Post hoc comparisons isolated the significant interaction effects of CRM testing on response area to three US intensities (0.1, 0.25 and 0.5 mA) following six days of incubation for Post Test1 and to two intensities (0.25, 0.5 mA) for Post Test2 (p’s < .05) with Post Test2 being significantly lower than Post Test1 at those two intensities (p <.05).
Taken together, the US testing data suggest that CRM was marginally present following one day of incubation evidenced by a shift in peak latencies and became fully formed following six days of incubation not only as a shift in peak latency but as an increase in the frequency, magnitude and area of responses. Assessment of CRM a week later indicated that responses for rabbits receiving six days of incubation were still significantly larger than on Pretest suggesting a persistence of the effect. Testing of CRM following ten days of incubation showed no significant changes from Pretest values in response frequency, peak latency, magnitude or area suggesting that incubation effects following NMR conditioning do not have a delayed onset but do have a relatively short duration.
Unpaired controls
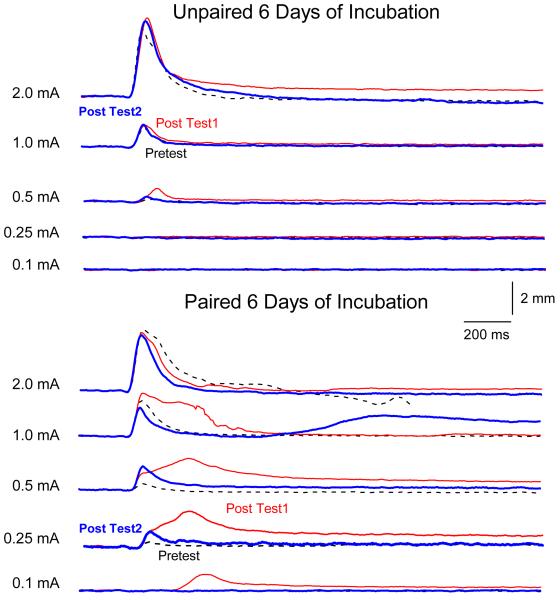
The two panels of Figure 4 show sample UR response topographies for an individual rabbit from the unpaired (top) group and a rabbit from the paired group (different from the one in Figure 2) as a function of the first 20-trial block of US test intensities (0.1, 0.25, 0.5, 1.0 and 2.0 mA) presented before CS-US pairings (Pretest) after six days of incubation (Post Test-1) and again seven days later (Post Test-2). The panels show no evidence of change in the size or timing of responses as a function of unpaired stimulus presentations compared to the changes shown by the rabbit that had received CS-US pairings. Analyses of response frequency, peak latency, and magnitude confirmed these observations by yielding significant main effects of pairings (F’s (2, 20) >11.78, p’s <.01) or, in the case of area, a significant interaction of pairings with CRM testing (F(2, 20) = 10.40, p < .001).
Figure 4.
The two panels show sample unconditioned response topographies for an individual rabbit from the unpaired (top) group and a rabbit from the paired group (different from the one in Figure 2) as a function of the first 20-trial block of unconditioned stimulus test intensities (0.1, 0.25, 0.5, 1.0 and 2.0 mA) presented before conditioned stimulus-unconditioned stimulus pairings (Pretest, dotted line), after one, six or ten days of incubation (Post Test1, red line) and again seven days later (Post Test2, blue line).
Discussion
The principal findings of the current experiment were: (1) after one day of classical conditioning resulting in modest overall levels of conditioning, changes of the NMR were an inverted-U shaped function of the number of days of incubation; (2) these changes were indexed by a shift in the peak latency and an increase in the size of the NMR following six days of incubation; (3) the incubation effect persisted for a week but does not have a delayed onset; and (4) unpaired controls showed no change in the size of the NMR suggesting the incubation effect was associative – a suggestion supported by the inverted “U-shaped” incubation function.
The symptoms of PTSD do not always occur immediately after trauma and they can become more pronounced over time (Frueh et al., 2009; Smid et al., 2011). In fact, a delay in the onset of symptoms by as much as six months has been incorporated into previous diagnostic criteria of PTSD (Smid et al., 2011). However, there is now considerable debate about whether delayed-onset PTSD actually exists in either veterans or civilians with evidence for both points of view (Andrews et al., 2007; Frueh et al., 2009; Andrews et al., 2009; Horesh et al., 2010; Smid et al., 2011). The present data suggest that in an animal model of PTSD symptoms, rabbits do not show a delay in onset of CRM but there is a window during which incubation exacerbates CRM and CRM persists. The results are consistent with clinical data in which exacerbation or reactivation of prior symptoms accounted for 38.3% of military cases of PTSD and 15.3% of civilian cases (Andrews et al., 2007).
The development and exacerbation of NMR CRM to the US in the current experiment overlapped with elevated conditioned response levels suggesting a potential connection between the two types of response (Buck et al., 2001; Schreurs, 2003; Burhans et al., 2008). We have shown previously that CRM has a number of characteristics in common with CRs including timing and topography (Schreurs, 2003; Burhans et al., 2008). The present data show that after both one and six days of incubation there was a shift in the timing of the UR because the response peaked closer to a point where the US would have occurred on paired trials (Schreurs et al., 2000). The fact that the level of both CRs and CRM were significantly lower following ten days of incubation provides additional support for a connection between the two types of response. However, as noted in the past, there is not a simple one-to-one correspondence between CRs and CRM (Buck et al., 2001). In the present experiment, it was not just the level of CRs that determined the level of CRM because the level of conditioning was identical among the three paired groups at the end of CS-US pairings. Moreover, the level of responding early in extinction was not significantly different between one and six days following CS-US pairings but the level of CRM was much higher in the latter. Finally, extinguishing CRs does not extinguish CRM, and it is only when both CS-alone and US-alone trials are presented in an unpaired fashion that both CRs and CRM can be extinguished (Schreurs et al., 2000; Schreurs et al., 2010). The clinical implication is that therapy designed to reduce fear of the sights, sounds, and smells associated with trauma may not be very effective because the fear of the trauma itself must also be extinguished (Schreurs et al., 2010). However, re-exposure to the trauma in order to extinguish it raises serious ethical as well as practical concerns. We have shown elsewhere that using very weak versions of the US in an unpaired procedure is sufficient to extinguish both CRs and CRM (Schreurs et al., 2010). This could be implemented with cognitive behavioral therapy in a virtual reality environment (Gerardi et al., 2010) and timed to occur with the appearance of symptoms be they immediate or delayed (Andrews et al., 2009).
The search for the neurobiology of incubation has met with limited success (Pickens et al., 2009b) but a number of studies have implicated the amygdala (Roozendaal et al., 2009) and cingulate cortex (Freeman, Jr. & Gabriel, 1999). Roozendaal and colleagues showed that ten days after acute stress, structural changes occurred in the amygdala that did not occur one day after stress (Roozendaal et al., 2009). Freeman and Gabriel (1999) found significant increases in neural activity in the posterior cingulate cortex and anterior thalamus following a 48-hour incubation period after limited initial fear-motivated avoidance learning in rabbits. These changes did not occur immediately after the limited avoidance learning. Previous work from our laboratory has shown a critical role for the amygdala in the expression of CRM, suggesting it may be one of the neural substrates for the incubation effects on CRM (Burhans & Schreurs, 2008).
A case has been made that exaggerated responding after classical conditioning of the rabbit NMR may be useful as a model of some symptoms of PTSD (Seager et al., 2003; Schreurs et al., 2005; Burhans et al., 2008; Schreurs et al., 2010). Increases in size and complexity of the NMR to weak USs following classical conditioning bear some similarity to the heightened responsiveness to stressful stimuli characteristic of PTSD (McTeague et al., 2010). An important aspect of these previous studies is that CRM only emerged as a result of an extensive number CS-US pairings across several days of classical conditioning (Schreurs et al., 1995). Although there are many cases of PTSD that emerge after chronic trauma such as a protracted period of exposure to stress (Kaysen et al., 2003), the most common perception is that PTSD results from a single traumatic experience. In our previous experiments, rabbits were given CS-US pairings for at least three days but in many cases six days of pairings before we observed profound changes in the nature of the UR (Schreurs et al., 2000; Buck et al., 2001). With just a single day of CS-US pairings, the present experiment shows that comparatively short-term stress can also induce profound changes in the nature of the UR if the effects of CS-US pairings are permitted to incubate. Clinically, having a time window when incubation can exacerbate symptoms speaks to the vulnerability of re-experiencing trauma too soon after a traumatic event. The literature suggests that it is the presence of stressors before, during or after the trauma that may be more predictive of PTSD than a specific period of vulnerability. Andrews et al. (2009) suggest that delayed onset PTSD is an accumulation of symptoms over time and is more likely in those reporting a recent severe stressor. Horesh et al. (2010) say that more stressful life experiences (and greater perceived severity of the combat trauma) are associated with a shorter PTSD onset. Both groups would make the point that PTSD could be a serious problem for military or emergency personnel who are recalled to a combat zone or disaster site without sufficient time to deal with and overcome the severe stress of their initial experiences.
Acknowledgements
Preparation of this manuscript and experiments described herein were supported by NIMH grant MH081159. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIMH.
Role of Funding Source:
Funding for this study was provided by NIMH Grant MH081159; the NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. American Journal of Psychiatry. 2007;164:1319–1326. doi: 10.1176/appi.ajp.2007.06091491. [DOI] [PubMed] [Google Scholar]
- Andrews B, Brewin CR, Stewart L, Philpott R, Hejdenberg J. Comparision of immediate-onset and delayed-onset posttraumatic stress disorder in military veterans. Journal of Abnormal Psychology. 2009;118:767–777. doi: 10.1037/a0017203. [DOI] [PubMed] [Google Scholar]
- Buck DL, Seager MA, Schreurs BG. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: generality and nature of the phenomenon. Behavioral Neuroscience. 2001;115:1039–1047. [PubMed] [Google Scholar]
- Burhans LB, Schreurs BG. Inactivation of the central nucleus of the amygdala abolishes conditioning-specific reflex modification of the rabbit nictitating membrane response and delays classical conditioning. Behavioral Neuroscience. 2008;122:75–88. doi: 10.1037/0735-7044.122.1.75. [DOI] [PubMed] [Google Scholar]
- Burhans LB, Smith-Bell CA, Schreurs BG. Conditioning-specific reflex modification of the rabbit’s nictitating membrane response and heart rate: behavioral rules, neural substrates, and potential applications to posttraumatic stress disorder. Behavioral Neuroscience. 2008;122:1191–1206. doi: 10.1037/a0013599. [DOI] [PubMed] [Google Scholar]
- Coleman SR, Gormezano I. Classical conditioning of the rabbit’s (Oryctolagus cuniculus) nictitating membrane response under symmetrical CS-US interval shifts. Journal of Comparative and Physiological Psychology. 1971;77:447–455. doi: 10.1037/h0031879. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Jr., Gabriel M. Changes of cingulothalamic topographic excitation patterns and avoidance response incubation over time following initial discriminative conditioning in rabbits. Neurobiology of Learning and Memory. 1999;72:259–272. doi: 10.1006/nlme.1998.3896. [DOI] [PubMed] [Google Scholar]
- Frueh BC, Grubaugh AL, Yeager DE, Magruder KM. Delayed-onset post-traumatic stress disorder among war veterans in primary care clinics. British Journal of Psychiatry. 2009;194:515–520. doi: 10.1192/bjp.bp.108.054700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD, Weidemann G, Kehoe EJ. Covariation of alternative measures of responding in rabbit (Oryctolagus cuniculus) eyeblink conditioning during acquisition training and tone generalization. Behavioral Neuroscience. 2003;117:292–303. doi: 10.1037/0735-7044.117.2.292. [DOI] [PubMed] [Google Scholar]
- Gerardi M, Cukor J, Difede J, Rizzo A, Rothbaum BO. Virtual reality exposure therapy for posttraumatic stress disorder and other anxiety disorders. Current Psychiatry Reports. 2010;12:298–305. doi: 10.1007/s11920-010-0128-4. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. Journal of Neuroscience. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormezano I. Classical conditioning. In: Sidowski JB, editor. Experimental methods and instrumentation in psychology. McGraw-Hill; New York: 1966. pp. 385–420. [Google Scholar]
- Gormezano I, Gibbs CM. Transduction of the rabbit’s nictitating membrane response. Behavior Research Methods, Instruments, & Computers. 1988;20:18–21. [Google Scholar]
- Gruart A, Yeo CH. Cerebellar cortex and eyeblink conditioning: bilateral regulation of conditioned responses. Experimental Brain Research. 1995;104:431–448. doi: 10.1007/BF00231978. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends in Cognitive Sciences. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Horesh D, Solomon Z, Zerach G, Ein-Dor T. Delayed-onset PTSD among war veterans: the role of life events throughout the life cycle. Social Psychiatry and Psychiatric Epidemiology. 2010:xxx. doi: 10.1007/s00127-010-0255-6. In press. [DOI] [PubMed] [Google Scholar]
- Kaysen D, Resick PA, Wise D. Living in danger: the impact of chronic traumatization and the traumatic context on posttraumatic stress disorder. Trauma, Violence, & Abuse. 2003;4:247–264. doi: 10.1177/1524838003004003004. [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters NS, King MG, Bandettini PA. Learning-related diminution of unconditioned SCR and fMRI signal responses. Neuroimage. 2010;49:843–848. doi: 10.1016/j.neuroimage.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Rougemont-Bucking A, Beucke JC, Zeffiro TA, Milad MR. Unconditioned responses and functional fear networks in human classical conditioning. Behavioural Brain Research. 2011;2221:237–245. doi: 10.1016/j.bbr.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante M-C, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biological Psychiatry. 2010;67:346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, Shaham Y. Long-lasting incubation of conditioned fear in rats. Biological Psychiatry. 2009a;65:881–886. doi: 10.1016/j.biopsych.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, ms-Deutsch T, Nair SG, Navarre BM, Heilig M, Shaham Y. Effect of pharmacological manipulations of neuropeptide Y and corticotropin-releasing factor neurotransmission on incubation of conditioned fear. Neuroscience. 2009b;164:1398–1406. doi: 10.1016/j.neuroscience.2009.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Navarre BM, Nair SG. Incubation of conditioned fear in the conditioned suppression model in rats: role of food-restriction conditions, length of conditioned stimulus, and generality to conditioned freezing. Neuroscience. 2010;169:1501–1510. doi: 10.1016/j.neuroscience.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Schreurs BG. Classical conditioning and modification of the rabbit’s (Oryctolagus cuniculus) unconditioned nictitating membrane response. Behavioral and Cognitive Neuroscience Reviews. 2003;2:83–96. doi: 10.1177/1534582303255014. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Alkon DL. US-US conditioning of the rabbit’s nictitating membrane response: emergence of a conditioned response without alpha conditioning. Psychobiology. 1990;18:312–320. [Google Scholar]
- Schreurs BG, Crum JM, Wang D, Smith-Bell CA. Conditioning-specific reflex modification of rabbit (Oryctolagus cuniculus) heart rate. Behavioral Neuroscience. 2005;119:1484–1495. doi: 10.1037/0735-7044.119.6.1484. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Gonzales-Joekes J, Smith-Bell CA. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response is sensitive to context. Learning & Behavior. 2006;34:315–324. doi: 10.3758/bf03192886. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Oh MM, Hirashima C, Alkon DL. Conditioning-specific modification of the rabbit’s unconditioned nictitating membrane response. Behavioral Neuroscience. 1995;109:24–33. doi: 10.1037//0735-7044.109.1.24. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Shi T, Pineda SI, Buck DL. Conditioning the unconditioned response: modification of the rabbit’s (Oryctolagus cuniculus) unconditioned nictitating membrane response. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:144–156. doi: 10.1037//0097-7403.26.2.144. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA, Burhans LB. Unpaired extinction: implications for treating posttraumatic stress disorder. Journal of Psychiatric Research. 2010:xxx. doi: 10.1016/j.jpsychires.2010.10.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seager MA, Smith-Bell CA, Schreurs BG. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: US intensity effects. Learning & Behavior. 2003;31:292–298. doi: 10.3758/bf03195990. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Smid GE, Mooren TT, van der Mast RC, Gersons BP, Kleber RJ. Delayed posttraumatic stress disorder: systematic review, meta-analysis, and meta-regression analysis of prospective studies. Journal of Clinical Psychiatry. 2011;70:1572–1580. doi: 10.4088/JCP.08r04484. [DOI] [PubMed] [Google Scholar]
- Wikgren J, Ruusuvirta T, Korhonen T. Reflex facilitation during eyeblink conditioning and subsequent interpositus nucleus inactivation in the rabbit (Oryctolagus cuniculus) Behavioral Neuroscience. 2002;116:1052–1058. doi: 10.1037//0735-7044.116.6.1052. [DOI] [PubMed] [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Barton M, Oliveri G, Peduto A, Gordon E, Bryant RA. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]