Abstract
Neurophysiological responses related to lessening of pain sensitivity are a suggested mechanism of manual therapy. Prior studies have observed generalized lower pain thresholds associated with carpal tunnel syndrome (CTS) in comparison to healthy controls. The present study sought to determine whether similar findings were present in suprathreshold measures and measures specific to central integration of pain (temporal summation and after sensations). Additionally, we wished to determine whether measures of pain sensitivity were related to clinical outcomes in participants with signs and symptoms of CTS receiving a manual therapy intervention. Individuals with signs and symptoms of CTS reported greater pain sensitivity to suprathreshold measures of mechanical pain, temporal summation, and after sensation in comparison to healthy controls. Immediate lessening of mechanical pain sensitivity and after sensations in response to a manual therapy intervention and 3- week attenuation of temporal summation following a 3- week course of manual therapy were associated with 3- week changes in clinical pain intensity in participants with signs and symptoms of CTS. These findings suggest heightened pain sensitivity across several parameters may be associated with CTS. Furthermore, changes in mechanical pain, after sensation, and temporal summation may be related to improvements in clinical outcomes.
INTRODUCTION
Neurodynamic interventions (NDI) result in force and motion along peripheral nerves (Coppieters and Alshami, 2007) and are often referred to as “neural mobilization” (Haddick, 2007, Sebastian, 2010) suggesting a mechanical mechanism. Carpal tunnel syndrome (CTS) is characterized by compression of the median nerve (Gelberman et al., 1988); adhesions and edema within the carpal tunnel (Oh et al., 2006); and limited mobility of the median nerve (Hough et al., 2007). NDI are frequently included in the conservative management of individuals with CTS with evidence suggesting effectiveness (Medina McKeon and Yancosek, 2008) and improved mobility of the median nerve could represent one mechanism.
In addition to mechanical abnormalities, CTS is characterized by generalized pain sensitivity (de la Llave-Rincon AI et al., 2009, Fernandez-de-Las-Penas et al., 2009, Fernandez-de-Las-Penas et al., 2010b) and altered central nervous system processing (Tecchio et al., 2002). For example, bilateral heightened pain sensitivity is observed in individuals presenting with unilateral CTS (de la Llave-Rincon AI et al., 2009, Fernandez-de-Las-Penas et al., 2010b). Furthermore, alterations in cortical hand somatotopy are associated with CTS (Tecchio et al., 2002). These findings suggest central nervous system alterations in pain processing accompany the peripheral dysfunction associated with CTS. Decreased pain sensitivity occurs in response to manual therapy (Vicenzino et al., 2001, Frey Law et al., 2008) suggesting favorable alteration of neuroplastic changes associated with central sensitization (Boal and Gillette, 2004). Subsequently, one mechanism of NDI could be lessening of pain sensitivity corresponding to central sensitization.
The first purpose of the current study was to compare age and sex matched healthy participants to those with signs and symptoms of CTS to observe whether differences existed in pain sensitivity. Generalized heightened sensitivity to threshold measures of pain accompanies CTS (Fernandez-de-Las-Penas et al., 2009, de la Llave-Rincon AI et al., 2009). The present study sought to determine whether pain sensitivity was also heightened in response to suprathreshold measures. We hypothesized individuals with signs and symptoms of CTS would demonstrate increased pain sensitivity to both threshold and suprathreshold measures of pain sensitivity.
Our second purpose was a planned secondary analysis of previously published data (Bialosky et al., 2009). The original study compared NDI to a sham intervention for outcomes related to clinical pain intensity and thermal and mechanical pain sensitivity in participants presenting with signs and symptoms of CTS. Significant improvements, not differing by group, were observed in measures of mechanical pain sensitivity, clinical pain, and function over 3 weeks. Additionally, immediate reductions in thermal pain sensitivity were observed in response to a three week trial of the studied NDI and not in response to the sham (Bialosky et al., 2009). This secondary analysis sought to study the relationship between changes in thermal and mechanical pain sensitivity and 3- week changes in clinical pain intensity in participants with signs and symptoms of CTS who received NDI. A recent study observed threshold measures of pain sensitivity were not predictive of treatment success in response to a single session of physical therapy including NDI and soft tissue intervention in individuals with CTS (Fernandez-de-Las-Penas et al., 2010a). We wished to determine the relationship between suprathreshold measures of pain sensitivity and clinical outcomes. Suprathreshold measures of pain sensitivity are highly related to clinical pain (Staud et al., 2003b) and are potentially more predictive of clinical pain than threshold measures (Granot et al., 2003, Staud et al., 2003b, Werner et al., 2004). Accordingly, we hypothesized changes in suprathreshold measures of pain sensitivity would correlate to 3- week changes in clinical pain intensity.
METHODS
Participants
The Institutional Review Board of the University of Florida approved this study. Participants with CTS were recruited from the clinics of orthopedic surgeons at the University of Florida and from the general public through posted flyers and electronic distribution. Participants were included if between the ages of 18 and 70 with signs and symptoms consistent with CTS as defined by pain or paresthesia in the median nerve distribution and at least one of the following clinical signs; 1) a positive Phalen sign, 2) a positive Tinnel sign over the carpal tunnel, or 3) a positive carpal compression test. Participants were required to have CTS symptoms present for greater than twelve weeks and to rate their pain or symptom intensity at least a 4/10 on a numeric rating scale over the past twenty-four hours. Exclusion criteria included non-English speaking; prior surgery for CTS; prior treatment with the studied NDI; pregnancy, other systemic disease known to cause peripheral neuropathy; current or history of chronic pain conditions. Healthy participants were recruited from the general public through posted flyers and electronic distribution and age and sex matched to those with signs and symptoms of CTS. Exclusion criteria for healthy participants included current or history of CTS in either extremity; systemic disease known to cause peripheral neuropathy; current or history of chronic pain condition; and pregnancy.
Measures
identification of CTS signs and symptoms
We based inclusion on a clinical presentation of CTS rather than electrodiagnostic studies. The utility of electrodiagnostic testing in diagnosing CTS is not established (Graham, 2008, Taylor-Gjevre et al., 2010). In fact, recent guidelines necessitate their use only when considering surgical intervention (Keith et al., 2009). We did not base inclusion in this study on electrodiagnostic findings due to 1) our participants were managed conservatively and 2) our belief the clinical diagnosis was consistent with the typical patient presentation commonly treated conservatively with NDI. Inclusion for this study was based upon patient report of pain and paresthesia in the median nerve distribution in conjunction with positive findings on clinical tests for CTS. These tests are commonly included in isolation or in combination (Boland and Kiernan, 2009, Fernandez-de-Las-Penas et al., 2010a) and are common in the clinical diagnosis of CTS. Subsequently, we felt the combination of median nerve symptom distribution along with at least one positive clinical sign was representative of a patient likely to be given a clinical diagnosis of CTS and treated with NDI.
demographic
Participants completed a demographic questionnaire for age, sex, ethnicity, racial group, employment status, marital status, education level, household income.
mechanical pain sensitivity (suprasthreshold)
Mechanical pain sensitivity was obtained with a pressure algometer (Pain Diagnostics & Treatment, Great Neck, NY) through the application of 2.3 kg of force at a rate of 1 kg/second through a 1 cm2 application tip over the carpal tunnel and the brachioradialis. We chose 2.3 kg of force as we desired a suprathreshold measure of mechanical pain sensitivity and have previously observed average mechanical pain thresholds of 2.0 kg in pilot studies from healthy participants (unpublished data). Subsequently, we expected 2.3 kg to produce pain in our healthy participants and those with signs and symptoms of CTS. Sites were chosen as specific to the carpal tunnel and a remote site indicative of general pain sensitivity (brachioradialis). Participants rated associated pain at each region on separate 100 mm mechanical visual analog scales (MVAS) anchored at one end with “no pain at all” and at the other with “worst pain imaginable”.
thermal pain sensitivity
Participants underwent thermal pain assessment using the Medoc Neurosensory Analyzer (TSA-2001, Ramat Yishai, Israel) with a hand-held, peltier-element-based stimulator including the following:
threshold
Thermal pain threshold was assessed at the thenar surface of the involved/more involved palm of participants with CTS and the dominant palm of healthy participants. The stimulator was applied at a baseline temperature of 35°C and increased at a rate of 0.5°C. Participants were instructed to verbally indicate when “the sensation changed from warm to painful”. The temperature at which the participant first indicated pain was recorded. A threshold measure was repeated 2 times and the average calculated.
tolerance (suprathreshold)
Thermal pain threshold was assessed at the thenar surface of the involved/more involved palm of participants with CTS and the dominant palm of healthy participants. The stimulator was applied at a baseline temperature of 35°C and increased at a rate of 0.5°C. Participants were instructed to indicate “when the temperature becomes so painful you can no longer stand it” and this temperature recorded. A thermal tolerance measure was repeated 2 times and the average temperature calculated.
ramp and hold (suprathreshold)
Ramp and hold was assessed on the volar forearm of each participant. The thermode was applied with a baseline temperature of 35°C rising rapidly (10°C/sec) to a peak of 49°C. Participants rated the associated pain using a 100 mm MVAS anchored at one end with “no pain” and at the other with “worst pain imaginable” indicating A-delta fiber mediated pain sensitivity (Price et al., 2002). The protocol was performed two times with the average rating analyzed.
temporal Summation (suprathreshold)
Temporal summation was assessed on the palmar surface of the hand using ten consecutive heat pulses of less than one second duration at an inter- stimulus frequently of .33 Hz. The baseline temperature of each pulse was 35°C and peaked at 51 ° C. Participants rated their delayed (second) pain for each of the ten pulses using a 101 point numeric rating scale (NRS) anchored with “0= no pain” and “100= worst pain imaginable”. Temporal summation was quantified as a measure of the 5th pulse minus the 2nd pulse (Price et al., 2002).
after sensation (suprathreshold)
Participants quantified any pain they continued to feel 15 seconds following the tenth pulse in the temporal summation protocol using the same NRS. After sensation was defined as the rating of any pain persisting at 15 seconds following the last thermal pulse (Staud et al., 2007).
Temporal summation and after sensations are considered primarily mediated by C- fiber afferents (Price et al., 1977).
clinical pain intensity
Clinical pain intensity was assessed using a NRS (0= “none” to 100= “worst imaginable”) for “usual pain” over the past week. The NRS is reliable and valid (Jensen et al., 1999) measure of pain intensity.
Procedures
All individuals agreeing to participate signed an informed consent form approved by the University of Florida Institutional Review Board and completed a demographic form. Participants with signs and symptoms of CTS provided a NRS for “usual pain over the previous week”. Next, all participants underwent baseline standardized quantitative sensory assessment of mechanical and thermal pain sensitivity. Healthy participants were then finished with the study.
Participants with signs and symptoms of CTS were randomly assigned to receive either a NDI known to cause specific force and movement across the median nerve (Coppieters and Alshami, 2007) or a sham NDI. Description of the studied and sham NDI and related outcomes have been previously reported (Bialosky et al., 2009). We included only individuals who received the NDI in the current analysis to assess relationships between clinical pain and changes in thermal and mechanical pain sensitivity in response to a common manual therapy intervention. Following randomization, participants underwent the first session of their assigned intervention followed immediately by reassessment of pain sensitivity to observe for an immediate within session treatment effect. Participants with signs and symptoms of CTS were seen 2 times per week over the next 3 weeks for a maximum of 6 treatment sessions. The final treatment session included follow up questionnaires, pre and post intervention pain sensitivity testing, and measurement of clinical pain intensity. Assessment at the 3 week follow up session was performed by an examiner blinded to group assignment.
Data analysis
Twenty participants with signs and symptoms of CTS and 20 age and sex matched healthy participants were included in the analysis for the first purpose of the study. Each participant was assessed unilaterally. The involved side was used for analysis in participants with unilateral CTS. The more involved side was used for analysis in participants with bilateral CTS. The dominant side was used for analysis in participants with bilateral CTS unable to identify a “more involved” side. The dominant arm was used for analysis in the healthy participants. Significance was set at p≤ 0.05 for all outcomes.
differences in pain sensitivity
Baseline group differences in pain sensitivity were assessed through independent t- tests. After sensation was categorized as present or absent based upon whether the participant reported persistent pain 15 seconds following the temporal summation protocol. The incidence of after sensation between healthy participants and those with CTS was assessed using a chi square test.
relationship between pain sensitivity and clinical pain intensity
Measures of pain sensitivity differing between groups were included in these analyses. Bivariate associations were calculated for baseline measures of pain sensitivity and clinical pain intensity in participants with CTS. Next, bivariate associations were calculated for baseline within session changes in pain sensitivity in response to manual therapy and 3 week change in “usual” clinical pain. This analysis indicated the relationship between immediate hypoalgesia in response to manual therapy and clinical outcome. Finally, bivariate associations were calculated for 3 weeks changes in measures of both pain sensitivity and usual clinical pain to examine whether observed change was associated with clinical outcomes.
Cohen’s d was calculated where appropriate as a parallel measure of magnitude.
RESULTS
Group related differences in pain sensitivity
Twenty female participants with CTS were age matched to 20 female healthy participants. Clinical examination findings for the participants with CTS included a positive Tinnel sign in 17/20 (85%), Phalen sign in 15/20 (75%), and carpal compression test in 14/20 (70%). Furthermore, 17/20 (85%) of the participants were positive on 2 of 3 clinical tests and 10/20 (50%) were positive on 3 of 3 clinical tests. Mean age for participants experiencing CTS was 40.75 (sd= 10.38) years old and for healthy participants 38.25 (sd= 12.32). The groups did not differ significantly on age (p= 0.49) and Levene’s test for equality of variance indicated no difference. (F=2.50, p= 0.12). Duration of symptoms for the participants with CTS ranged from 12 weeks to 936 weeks with a median value of 156 weeks. Duration of symptoms had large variability however was not associated with any of the outcome measures (p> 0.05) so we did not include it as a covariate in any of the analyses. Demographic information is reported in Table 1. Education differed significantly between the groups, however was not considered a covariate as a significant association was not observed with any of the studied outcome variables. (p> 0.05)
Table 1.
Demographic Information for Sample
| Signs and Symptoms of CTS | Healthy | p- value | |
|---|---|---|---|
| Age (weeks (sd)) | 40.75 (10.38) | 38.25 (12.32) | 0.49 |
|
| |||
| Ethnicity | |||
| Hispanic/Latino | 3 | 2 | |
| Not Hispanic/Latino | 17 | 18 | 0.63 |
|
| |||
| Race | |||
| Asian | 1 | 2 | |
| African American | 3 | 0 | |
| Caucasian | 16 | 18 | 0.18 |
|
| |||
| Employment | |||
| Full time | 13 | 13 | |
| Part time | 3 | 5 | |
| Unemployed | 3 | 1 | |
| Retired | 1 | 0 | 0.48 |
|
| |||
| Marital Status | |||
| Single | 7 | 10 | |
| Married | 5 | 9 | |
| Living with significant other | 2 | 0 | |
| Divorced | 5 | 0 | |
| Widowed | 1 | 1 | 0.07 |
|
| |||
| Education | |||
| High school | 4 | 1 | |
| Some college | 6 | 2 | |
| Graduated college | 5 | 1 | |
| Some post graduate work | 3 | 5 | |
| Post graduate degree | 2 | 11 | 0.01* |
|
| |||
| Income | |||
| <20,000 | 6 | 9 | |
| 20,000–35,000 | 7 | 2 | |
| 35,001–50,000 | 1 | 4 | |
| 50,001–70,000 | 3 | 0 | |
| >70,000 | 3 | 5 | 0.07 |
Demographic information for individuals with signs and symptoms of carpal tunnel syndrome (CTS) and healthy controls.
p<0.05
Significant (p<0.05) differences in pain sensitivity were observed in measures of mechanical pain sensitivity at the carpal tunnel and brachioradialis, and temporal summation (Table 2). A greater prevalence of after sensation was also observed in participants with CTS (χ2(1) = 3.96, p= 0.05).
Table 2.
Pain Sensitivity Comparison Between Individuals with Signs and Symptoms of Carpal Tunnel Syndrome and Healthy Controls
| s/s CTS | Healthy | Mean Difference (SE) | p- value | Cohen’s d | |
|---|---|---|---|---|---|
| Thermal Threshold | 43.70 (2.40) | 44.88 (2.22) | 1.18 (0.73) | 0.12 | 0.51 |
| Thermal Tolerance | 48.70 (1.39) | 49.06 (1.02) | 0.36 (0.38) | 0.36 | 0.30 |
| Ramp and Hold | 39.05 (24.46) | 27.10 (24.37) | 1.20 (0.77) | 0.13 | 0.49 |
| Temporal Summation | 8.10 (16.86) | −2.35 (8.13) | 10.45 (4.19) | 0.02* | 0.79 |
| After Sensation | 25.70 (23.86) | 10.50 (6.66) | 15.2 (12.4) | 0.24 | 0.87 |
| MP flexor retinaculum | 11.80 (16.31) | 3.60 (6.79) | 0.82 (0.40) | 0.05* | 0.66 |
| MP brachioradialis | 28.80 (31.67) | 10.75 (14.87) | 1.81 (0.78) | 0.03* | 0.73 |
Presented in means (standard deviation). Thermal threshold and tolerance are provided as the temperatures at which the participant first indicated pain perception (threshold) and reported the inability to tolerate any greater temperature (tolerance). SE= standard error of difference. MP= mechanical pain sensitivity to 2.2 kg of force. χ2 was used to determine between group differences in after sensation with a significantly greater proportion of individuals with signs and symptoms of carpal tunnel syndrome (s/s CTS) reporting after sensation (10/20) than healthy participants (4/20). Ratings for after sensation reported in the table are only for the participants who reported persistent pain 15 seconds following the final pulse in the temporal summation protocol (n=12).
significant at p<0.05
Changes in clinical pain and pain sensitivity in response to NDI
Changes in clinical pain and measures of pain sensitivity observed to differ between participants with and without CTS are displayed in Table 3.
Table 3.
Changes in outcome measures associated with neurodynamic intervention
| Baseline Pre NDI | Baseline Post NDI | Change in Baseline | p- value | 3 Week | 3 Week Change | p-value | |
|---|---|---|---|---|---|---|---|
| Clinical Pain | 51.3 (28.7) | NA | NA | NA | 37.9 (29.5) | −13.4 (31.2) | 0.08 |
| MP Brachioradialis | 30.8 (26.7) | 26.5 (29.4) | −4.3 (18.2) | 0.32 | 23.9 (25.5) | −6.9 (16.4) | 0.14 |
| MP Flexor Retinaculum | 14.0 (20.9) | 14.5 (21.2) | 0.05 (13.5) | 0.87 | 8.1(13.8) | −5.9 (17.6) | 0.09 |
| Temporal Summation | 8.1 (19.0) | 1.2 (13.4) | −6.9 (23.9) | 0.21 | 2.5 (16.0) | −5.6 (21.4) | 0.23 |
| After-sensation | 25.4 (21.2) | 22.9 (26.1) | −2.5(16.9) | 0.62 | 30.2 (31.5) | 4.8 (24.8) | 0.43 |
Immediate and 3- week changes in pain sensitivity and clinical pain intensity in response to a neurodynamic intervention (NDI). MP= mechanical pain sensitivity to 2.3 kg of force as indicated along a 100 mm mechanical visual analog scale (mvas). Clinical pain, temporal summation, and after- sensation were all measured by a 0 to 100 numeric rating scale anchored with 0= no pain to 100= the worst pain imaginable.
Correlates of pain sensitivity and clinical pain intensity
We further assessed the variables found to significantly differ by group for a relationship with ratings of clinical pain in participants with CTS. A small to moderate association was observed between baseline measures of both after sensation and temporal summation and baseline clinical pain intensity. (Table 4) Immediate changes following the manual therapy intervention in both mechanical pain sensitivity at the brachioradialis and after sensation were moderately associated with 3- week changes in usual clinical pain. (Table 5) A small to moderate relationship was observed between 3 week changes in temporal summation and 3- week changes in usual clinical pain. (Table 6)
Table 4.
Baseline Relationship Between Clinical Pain and Pain Sensitivity in Participants with Signs and Symptoms of Carpal Tunnel Syndrome (CTS)
| Usual Pain | Temporal Summation | After Sensation | MP flexor retinaculum | MP brachioradialis | |
|---|---|---|---|---|---|
| Usual Pain | 1 | ||||
| Temporal Summation | 0.39 0.09 |
1 | |||
| After Sensation | 0.39 0.21 |
0.37 0.24 |
1 | ||
| MP flexor retinaculum | 0.13 0.58 |
0.15 0.53 |
0.88* <0.01 |
1 | |
| MP brachioradialis | 0.27 0.24 |
0.23 0.34 |
0.08 0.81 |
0.38 0.10 |
1 |
Associations between baseline measures of pain sensitivity and clinical pain intensity. Usual pain obtained with a 101 point numeric rating scale anchored with 0= “no pain at all” to 100= “the worst pain imaginable”. Participants were instructed to indicate their usual level of pain related to carpal tunnel syndrome during the past week. Measures of after sensation are provided only for participants who reported pain at 15 seconds following the final thermal pulse in the temporal summation protocol (n=12). MP= mechanical pain sensitivity to 2.3 kg of force.
= significant at p< 0.05.
Table 5.
Relationship Between Immediate Changes in Pain Sensitivity and 3- Week Changes in Clinical Pain in Participants with Signs and Symptoms of Carpal Tunnel Syndrome (CTS)
| Change in Usual Pain Over 3 Weeks | Temporal Summation | After Sensation | MP flexor retinaculum | MP brachioradialis | |
|---|---|---|---|---|---|
| Change in Usual Pain Over 3 Weeks | 1 | ||||
| Temporal Summation | 0.35 0.14 |
1 | |||
| After Sensation | 0.54 0.07 |
0.24 0.45 |
1 | ||
| MP flexor retinaculum | −0.11 0.68 |
−0.57* 0.05 |
−0.55* 0.01 |
1 | |
| MP brachioradialis | 0.52* 0.03 |
0.34 0.16 |
0.53 0.08 |
−0.16 0.51 |
1 |
Pain sensitivity changes assessed during the initial research session prior to and immediately following a neurodynamic intervention (NDI). Baseline clinical pain was assessed at the first visit when participants with signs and symptoms of CTS were asked to indicate their “usual” pain over the past week using a 101 point numeric rating scale anchored with 0= no pain and 100= the worst pain imaginable. Participants were seen up to 6 sessions over the next 3 weeks to receive the NDI. A measure of “usual” pain was again taken at the final session in 3 weeks. After sensation includes only participants (n=12) reporting pain at 15 seconds following conclusion of the temporal summation protocol. MP= mechanical pain sensitivity to 2.3 kg of force.
significant at p< 0.05.
Table 6.
Relationship Between 3- Week Changes in Pain Sensitivity and 3- Week Changes in Clinical Pain
| Change in Usual Pain Over 3 Weeks | Temporal Summation | After Sensation | MP flexor retinaculum | MP brachioradialis | |
|---|---|---|---|---|---|
| Change in Usual Pain Over 3 Weeks | 1 | ||||
| Temporal Summation | 0.41 0.08 |
1 | |||
| After Sensation | 0.09 0.79 |
0.33 0.32 |
1 | ||
| MP flexor retinaculum | −0.19 0.45 |
0.21 0.40 |
0.49 0.13 |
1 | |
| MP brachioradialis | 0.03 0.91 |
−0.06 0.80 |
−0.39 0.24 |
−0.09 0.73 |
1 |
Pain sensitivity and clinical pain changes occurring over 3 weeks. Clinical pain was assessed with a 101 point numeric rating scale anchored with 0= no pain and 100= the worst pain imaginable for “usual” pain over the past week. Participants were seen up to 6 sessions over the next 3 weeks to receive the NDI. After sensation includes only participants (n=12) reporting pain at 15 seconds following conclusion of the temporal summation protocol during baseline assessment. Measures of pain sensitivity and “usual” pain were again taken at the final session in 3 weeks. MP= mechanical pain sensitivity to 2.3 kg of force.
significant at p< 0.05.
DISCUSSION
We observed increased pain sensitivity to suprathreshold measures in individuals with signs and symptoms of CTS. Furthermore, we observed a relationship between both immediate and 3- week changes in measures of pain sensitivity and clinical outcomes. These findings provide preliminary evidence for a prognostic value for immediate changes in pain sensitivity and a potential mechanism through which NDI may alter pain associated with CTS.
Group related differences in pain sensitivity
Prior studies suggest heightened pain sensitivity is associated with CTS (de la Llave-Rincon AI et al., 2009, Fernandez-de-Las-Penas et al., 2009, Fernandez-de-Las-Penas et al., 2010b). We have extended this finding to suprathreshold stimuli including temporal summation and after sensation. Temporal summation and after sensation differentiate individuals with other chronic pain conditions from healthy controls (Staud et al., 2003a). Similarly, we observed temporal summation and after sensation to differentiate participants with signs and symptoms of CTS from healthy individuals. Prior studies of CTS suggest a central pain mechanism. (Zanette et al., 2006, Fernandez-de-Las-Penas et al., 2009) Specifically, CTS is associated with generalized pain sensitivity, (Fernandez-de-Las-Penas et al., 2009) complaints beyond the median nerve distribution, (Zanette et al., 2006) and cortical changes (Tecchio et al., 2002). Collectively these studies suggest a central pain mechanism in addition to peripheral dysfunction at the carpal tunnel. Temporal summation and after sensation reflect afferent pain processing at the dorsal horn of the spinal cord (Price et al., 2002) and increased sensitivity to these measures likely represents a change in central nervous system response or processing of afferent pain inputs. Our findings converge with previous studies suggesting a centrally mediated pain mechanism of CTS and identify temporal summation and after sensation as additional indicators of this process.
Interventions for CTS frequently target peripheral dysfunction such as braces to minimize pressure and surgery. Our findings suggest, in addition to a peripheral process, pain associated with CTS is reflective of a central process with a potential role in both the progression of acute pain to chronic pain and the maintenance of chronic pain (Rygh et al., 2005). NDI provides a peripheral stimulus (Coppieters and Alshami, 2007) and may inhibit musculoskeletal pain centrally through a reduction of central sensitization of pain (Boal and Gillette, 2004). Furthermore, a peripheral process may drive or maintain central sensitization (Fernandez-de-Las-Penas et al., 2009, Staud et al., 2009) and NDI may interrupt this process through a peripheral effect. The current study suggests a potential central pain process beyond peripheral impairments through which NDI may inhibit pain in individuals with CTS.
Correlates of changes in pain sensitivity and changes in clinical pain intensity
We did not observe mean changes in measures of pain sensitivity; however, changes were observed at the individual level. (Figures 1 and 2) Treatment effects of conservative interventions for pain conditions are often small leading to the suggestion subgroups of individuals may respond to specific interventions (Stanton et al., 2011). Similar findings are reported in CTS in response to NDI leading to similar suggestions (Medina McKeon and Yancosek, 2008).. The results of the current study suggest changes in pain sensitivity may be a prognostic and mechanistic consideration for a subgroup of individuals with CTS.
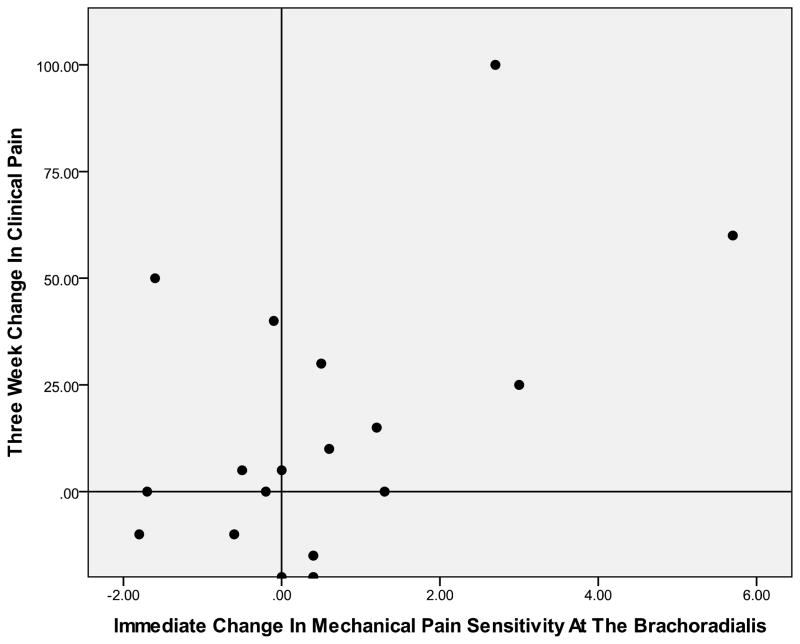
Figure 1.
Association Between Immediate Change In Mechanical Pain Sensitivity At The Brachioradialis And 3- Week Change In Clinical Pain (r= 0.52)
Positive numbers on the y- axis indicate greater improvement in clinical pain related to carpal tunnel syndrome over a 3- week period. Positive numbers on the x- axis indicate lessening of mechanical pain sensitivity at the brachioradialis over a 3- week period. Individuals in the top right quadrant and lower left quadrant demonstrated a positive association between immediate changes in mechanical pain sensitivity at the brachioradialis and 3- week changes in clinical pain.
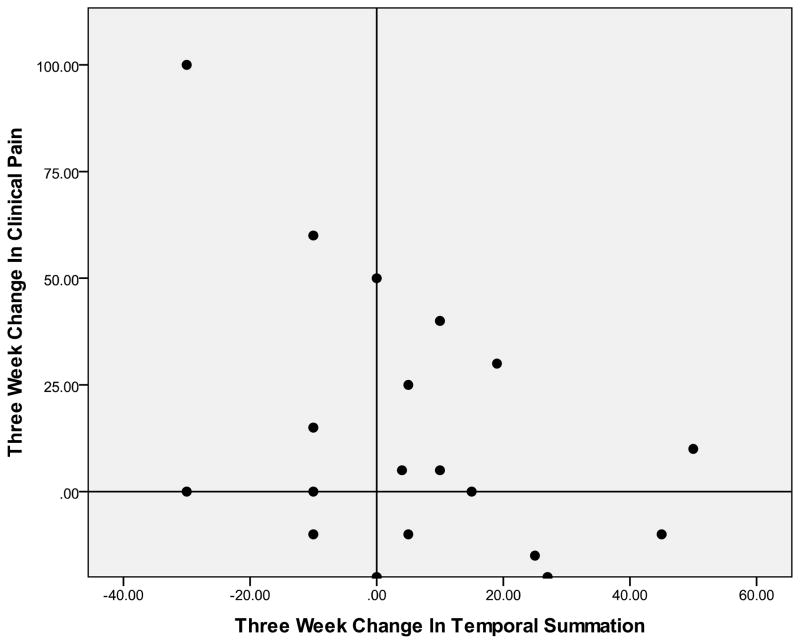
Figure 2.
Association Between 3- Week Change in Temporal Summation and 3- Week Change in Clinical Pain (r= 0.41)
Positive numbers on the y- axis indicate greater improvement in clinical pain related to carpal tunnel syndrome over a 3- week period. Positive numbers on the x- axis indicate lessening of temporal summation of thermal pain over a 3- week period. Individuals in the top right quadrant and lower left quadrant demonstrated a positive association between 3- week changes in temporal summation and clinical pain.
Prior studies of manual therapy have reported within session changes in clinical pain intensity predict between session changes (Tuttle, 2005). Our findings suggest immediate changes in mechanical pain sensitivity and after sensation may be prognostic for clinical pain relief following a 3- week course of NDI. Temporal summation is a behavioral measure of wind up at the dorsal horn of the spinal cord (Price et al., 2002) and immediate hypoalgesia of temporal summation corresponds to the studied NDI (Beneciuk et al., 2009, Bialosky et al., 2009). The findings from the current study also provide preliminary support for attenuation of temporal summation as a potential mechanism through which NDI inhibits pain related to CTS.
Limitations of the current study are we included only women and our findings may not translate to men. Additionally, the individuals in our study were followed for only 3 weeks. We cannot say with certainty the relationships observed between changes in pain sensitivity and clinical pain would persist over a longer time period. Our sample size was a further limitation. A larger sample size may have resulted in additional findings or allowed for multivariate analyses.
CONCLUSION
Participants with signs and symptoms of CTS differed from healthy age and sex matched controls in suprathreshold measures of pain sensitivity suggesting a central mechanism of pain. Immediate change in mechanical pain sensitivity and after sensation and 3-week change in temporal summation were associated with improvements in clinical pain intensity suggesting prognostic factors and a potential mechanism for improvement respectively.
Acknowledgments
This study was supported by a grant (R21AT002796) awarded to SZG from The National Institutes of Health National Center for Complementary and Alternative Medicine (NCCAM), the same grant also provided supported for JEB and MDB.
This manuscript was written while JEB received support from Rehabilitation Research Career Development Program (5K12HD055929-02) and MDB received support from the National Institute of Arthritis and Musculoskeletal and Skin Disorders (K01AR054331)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Beneciuk J, Bishop M, George S. Effects of Upper Extremity Neural Mobilization on Thermal Pain Sensitivity: A Sham Controlled Study in Asymptomatic Participants. Journal of Orthopedic and Sports Physical Therapy. 2009;39(6):428–438. doi: 10.2519/jospt.2009.2954. [DOI] [PubMed] [Google Scholar]
- Bialosky JE, Bishop MD, Price DD, Robinson ME, Vincent KR, George SZ. A Randomized Sham- Controlled Trial of a Neurodynamic Technique in the Treatment of Carpal Tunnel Syndrome. Journal of Orthopaedic & Sports Physical Therapy. 2009;39(10):709–723. doi: 10.2519/jospt.2009.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boal RW, Gillette RG. Central neuronal plasticity, low back pain and spinal manipulative therapy. J Manipulative Physiol Ther. 2004;27(5):314–326. doi: 10.1016/j.jmpt.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Boland RA, Kiernan MC. Assessing the accuracy of a combination of clinical tests for identifying carpal tunnel syndrome. J Clin Neurosci. 2009;16(7):929–933. doi: 10.1016/j.jocn.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Coppieters MW, Alshami AM. Longitudinal excursion and strain in the median nerve during novel nerve gliding exercises for carpal tunnel syndrome. J Orthop Res. 2007;25(7):972–980. doi: 10.1002/jor.20310. [DOI] [PubMed] [Google Scholar]
- de la Llave-Rincon AI, Fernandez-de-Las-Penas C, Fernandez-Carnero J, Padua L, Arendt-Nielsen L, Pareja JA. Bilateral hand/wrist heat and cold hyperalgesia, but not hypoesthesia, in unilateral carpal tunnel syndrome. Exp Brain Res. 2009;198(4):455–463. doi: 10.1007/s00221-009-1941-z. [DOI] [PubMed] [Google Scholar]
- Fernandez-de-Las-Penas C, Cleland JA, Ortega-Santiago R, de-la-Llave-Rincon AI, Martinez-Perez A, Pareja JA. Central sensitization does not identify patients with carpal tunnel syndrome who are likely to achieve short-term success with physical therapy. Exp Brain Res. 2010a;207(1–2):85–94. doi: 10.1007/s00221-010-2436-7. [DOI] [PubMed] [Google Scholar]
- Fernandez-de-Las-Penas C, de la Llave-Rincon AI, Fernandez-Carnero J, Cuadrado ML, rendt-Nielsen L, Pareja JA. Bilateral widespread mechanical pain sensitivity in carpal tunnel syndrome: evidence of central processing in unilateral neuropathy. Brain. 2009;132:1472–1479. doi: 10.1093/brain/awp050. [DOI] [PubMed] [Google Scholar]
- Fernandez-de-Las-Penas C, Madeleine P, Martinez-Perez A, Arendt-Nielsen L, Jimenez-Garcia R, Pareja JA. Pressure pain sensitivity topographical maps reveal bilateral hyperalgesia of the hands in patients with unilateral carpal tunnel syndrome. Arthritis Care Res (Hoboken) 2010b;62(8):1055–1064. doi: 10.1002/acr.20189. [DOI] [PubMed] [Google Scholar]
- Frey Law LA, Evans S, Knudtson J, Nus S, Scholl K, Sluka KA. Massage reduces pain perception and hyperalgesia in experimental muscle pain: a randomized, controlled trial. J Pain. 2008;9(8):714–721. doi: 10.1016/j.jpain.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Gelberman RH, Rydevik BL, Pess GM, Szabo RM, Lundborg G. Carpal tunnel syndrome. A scientific basis for clinical care. Orthop Clin North Am. 1988;19(1):115–124. [PubMed] [Google Scholar]
- Graham B. The value added by electrodiagnostic testing in the diagnosis of carpal tunnel syndrome. J Bone Joint Surg Am. 2008;90(12):2587–2593. doi: 10.2106/JBJS.G.01362. [DOI] [PubMed] [Google Scholar]
- Granot M, Lowenstein L, Yarnitsky D, Tamir A, Zimmer EZ. Postcesarean section pain prediction by preoperative experimental pain assessment. Anesthesiology. 2003;98(6):1422–1426. doi: 10.1097/00000542-200306000-00018. [DOI] [PubMed] [Google Scholar]
- Haddick E. Management of a patient with shoulder pain and disability: a manual physical therapy approach addressing impairments of the cervical spine and upper limb neural tissue. J Orthop Sports Phys Ther. 2007;37(6):342–350. doi: 10.2519/jospt.2007.2458. [DOI] [PubMed] [Google Scholar]
- Hough AD, Moore AP, Jones MP. Reduced longitudinal excursion of the median nerve in carpal tunnel syndrome. Arch Phys Med Rehabil. 2007;88(5):569–576. doi: 10.1016/j.apmr.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83(2):157–162. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Keith MW, Masear V, Chung K, Maupin K, Andary M, Amadio PC, Barth RW, Watters WC, III, Goldberg MJ, Haralson RH, III, Turkelson CM, Wies JL. Diagnosis of carpal tunnel syndrome. J Am Acad Orthop Surg. 2009;17(6):389–396. doi: 10.5435/00124635-200906000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina McKeon JM, Yancosek KE. Neural gliding techniques for the treatment of carpal tunnel syndrome: a systematic review. J Sport Rehabil. 2008;17(3):324–341. doi: 10.1123/jsr.17.3.324. [DOI] [PubMed] [Google Scholar]
- Oh J, Zhao C, Zobitz ME, Wold LE, An KN, Amadio PC. Morphological changes of collagen fibrils in the subsynovial connective tissue in carpal tunnel syndrome. J Bone Joint Surg Am. 2006;88(4):824–831. doi: 10.2106/JBJS.E.00377. [DOI] [PubMed] [Google Scholar]
- Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3(1):57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99(1–2):49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Rygh LJ, Svendsen F, Fiska A, Haugan F, Hole K, Tjolsen A. Long-term potentiation in spinal nociceptive systems--how acute pain may become chronic. Psychoneuroendocrinology. 2005;30(10):959–964. doi: 10.1016/j.psyneuen.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Sebastian D. Triangular interval syndrome: A differential diagnosis for upper extremity radicular pain. Physiother Theory Pract. 2010;26(2):113–119. doi: 10.3109/09593980802698040. [DOI] [PubMed] [Google Scholar]
- Stanton TR, Fritz JM, Hancock MJ, Latimer J, Maher CG, Wand BM, Parent EC. Evaluation of a treatment-based classification algorithm for low back pain: a cross-sectional study. Phys Ther. 2011;91(4):496–509. doi: 10.2522/ptj.20100272. [DOI] [PubMed] [Google Scholar]
- Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ., Jr Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003a;102(1–2):87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain. 2009;145(1–2):96–104. doi: 10.1016/j.pain.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8(11):893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Robinson ME, Vierck CJ, Jr, Cannon RC, Mauderli AP, Price DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003b;105(1–2):215–222. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Taylor-Gjevre RM, Gjevre JA, Nair B. Suspected carpal tunnel syndrome: Do nerve conduction study results and symptoms match? Can Fam Physician. 2010;56(7):e250–e254. [PMC free article] [PubMed] [Google Scholar]
- Tecchio F, Padua L, Aprile I, Rossini PM. Carpal tunnel syndrome modifies sensory hand cortical somatotopy: a MEG study. Hum Brain Mapp. 2002;17(1):28–36. doi: 10.1002/hbm.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle N. Do changes within a manual therapy treatment session predict between-session changes for patients with cervical spine pain? Aust J Physiother. 2005;51(1):43–48. doi: 10.1016/s0004-9514(05)70052-0. [DOI] [PubMed] [Google Scholar]
- Vicenzino B, Paungmali A, Buratowski S, Wright A. Specific manipulative therapy treatment for chronic lateral epicondylalgia produces uniquely characteristic hypoalgesia. Man Ther. 2001;6(4):205–212. doi: 10.1054/math.2001.0411. [DOI] [PubMed] [Google Scholar]
- Werner MU, Duun P, Kehlet H. Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. Anesthesiology. 2004;100(1):115–119. doi: 10.1097/00000542-200401000-00020. [DOI] [PubMed] [Google Scholar]
- Zanette G, Marani S, Tamburin S. Extra-median spread of sensory symptoms in carpal tunnel syndrome suggests the presence of pain-related mechanisms. Pain. 2006;122(3):264–270. doi: 10.1016/j.pain.2006.01.034. [DOI] [PubMed] [Google Scholar]