Abstract
To determine if previous exposure to bovine viral diarrhea virus (BVDV) and bovine herpes virus 1 (BHV-1) type 2 affects the onset of disease caused by Mycoplasma bovis, 6- to 8-month-old beef calves were exposed to BVDV or BHV-1 4 d prior to challenge with a suspension of 3 clinical isolates of M. bovis. Animals were observed for clinical signs of disease and at necropsy, percent abnormal lung tissue and presence of M. bovis were determined. Most animals pre-exposed to BHV-1 type 2 but not BVDV developed M. bovis-related respiratory illness. In a second trial, we determined that a 100-fold reduction in the number of M. bovis bacteria administered to BHV-1 exposed animals reduced the percentage of abnormal lung tissue but not the severity of clinical signs. We conclude that previous exposure to BHV-1 but not BVDV type 2 was a necessary cause of M. bovis-related respiratory diseases in our disease model.
Résumé
Une maladie respiratoire causée par Mycoplasma bovis est exacerbée par l’exposition à l’herpès-virus bovin type 1 (BHV-1) mais non par l’exposition au virus de la diarrhée virale bovine de type 2 (BVDV). Afin de déterminer si l’exposition antérieure au virus de la diarrhée virale bovine (BVDV) et à l’herpès-virus bovin 1 (BHV-1) de type 2 affecte l’apparition de la maladie causée par Mycoplasma bovis, des veaux de boucherie âgés de 6 à 8 mois ont été exposés au BVDV ou au BHV-1 4 jours avant l’exposition à une suspension de 3 isolats cliniques de M. bovis. Les animaux ont été observés pour des signes cliniques de maladie et, à la nécropsie, le pourcentage de tissus pulmonaires anormaux et la présence de M. bovis ont été déterminés. La plupart des animaux préalablement exposés au BHV-1 de type 2 mais non au BVDV ont développé la maladie respiratoire associée à M. bovis. Dans un deuxième essai, nous avons déterminé qu’une réduction de 100 fois du nombre de bactéries de M. bovis administrées aux animaux exposés au BHV-1 réduisait le pourcentage de tissus pulmonaires anormaux mais non la gravité des signes cliniques. Nous avons conclu que l’exposition préalable au BHV-1 mais non au BVDV de type 2 était une cause nécessaire des maladies respiratoires associées à M. bovis dans notre modèle de maladie.
(Traduit par Isabelle Vallières)
Introduction
Mycoplasma bovis is an important pathogen in the beef feedlot and dairy industries. Infections with M. bovis are related to the occurrence of chronic pneumonia and polyarthritis syndrome (CPPS), otitis media, conjunctivitis, meningitis in feedlot cattle and mastitis in dairy cattle. Research on M. bovis-related disease involves the use of large animals and the costs associated with purchase, husbandry, and disposing of the carcasses are high. Studies on pathogenesis and on experimental vaccines for M. bovis-associated respiratory diseases have usually used beef cattle not more than 4 mo old (1–5). While the choice of this animal model is valid to study pathogenesis, it does not reflect what happens in the feedlot.
A survey of exposure to M. bovis and other respiratory pathogens in Canadian feedlots suggested that CPPS is associated with previous exposure to BVDV. In western Canada, M. bovis was demonstrated in 71% of lungs and 45% of joints of animals with chronic disease. In 40% of these cases, BVDV was also present, suggesting an association between these 2 pathogens (6). The prevalence of M. bovis and BVDV was also studied in 2 groups of animals showing chronic antibiotic-resistant pneumonia and polyarthritis. Mycoplasma bovis was found in almost 92% and 93% of the cases in retrospective and prospective studies, whereas BVDV was present in 64.5% and 56% of the same cases, leading the authors to conclude that there was synergism between these 2 pathogens (7).
There seems to be a similar picture in Ontario feedlots. Prevalence of titers to M. bovis and BVDV were reported to be 50% and 40%, respectively at the time of arrival at the feedlot and seroconversion against these 2 pathogens occured in about 40% of the animals (8) and were predictive of increased respiratory disease rates (9). In another study, calves euthanized within 60 d of arrival at the feedlots presented lesions typical of CPPS in 54.5% of the animals with BVDV also present (10). Investigations in Ontario indicated that there was no association between M. bovis and BHV-1 in bovine respiratory diseases (11). Taken together, these studies suggested that a previous exposure to BVDV made the animals more susceptible to infections with M. bovis.
As part of our development of novel vaccines to protect cattle against CPPS we needed to develop a challenge model that reflects the health status of cattle arriving at the feedlot. While many risk factors may be related to the occurrence of CPPS, we focused on potential importance of previous exposure to BVDV or BHV-1.
Materials and methods
Bacterial and virus strains
The M. bovis isolates used in this study were Mb1, obtained by aspiration of synovial fluid of a steer with polyarthritis (12), and 2 field isolates, Mb160 and Mb163 that were recovered from calves with CPPS in feedlots in Alberta and Saskatchewan, respectively. The M. bovis isolates were grown in modified Hayflick’s medium at 37°C in 5% CO2. Bacteria were collected by centrifugation at 10 000 × g and suspended in the same medium at the highest concentration as determined by counting the bacteria in a hemocytometer. Challenge doses were prepared by serial dilutions of the original suspension in the same medium.
Non-cytopathogenic type 2 BVDV strain NY-1 was grown in Madin-Darby bovine kidney (MDBK) cells cultured in minimal essential medium (MEM-GIBCO, Invitrogen Burlington, Ontario) supplemented with 2% horse serum. Aliquots were diluted in 4 mL of tissue culture to 1 × 107 pfu/mL before the challenge. The BHV-1 strain 108 was grown in MDBK cells in MEM supplemented with 5% fetal bovine serum (FBS, Gibco). Cells were lysed and aliquots were diluted in 4 mL of tissue culture medium to 1 × 105.5 tissue culture infective dose 50 (TCID50)/mL.
Challenge of animals
Six- to 8-month-old beef calves, pre-screened for exposure to BHV-1 and BVDV, were transported to the VIDO research farm and acclimatized. Animals without titers to BVDV and BHV-1 and with titers against M. bovis whole cells [range between 4000 and 22 000 as determined by an enzyme-linked immunosorbent assay (ELISA) assay (12)] were used in our studies. Bovine viral diarrhea virus and BHV-1 infections were performed by intra-nasal aerosol exposure over 4 min, using a DeVilbis Nebulizer model 99 (DeVilbis, Somerset, Pennsylvania, USA).
Two challenge trials were conducted. In Trial #1 we studied the effect of M. bovis challenge of calves previously infected with BVDV or BHV-1. Twenty-four calves were assigned to 1 of 3 challenge groups (1, 2, and 3) of 8 animals (BVDV, BHV-1 or non-viral-exposed control) using a random numbers table. The groups were separated for the duration of the study. Animals were infected with BVDV type 2 or BHV-1 on day 0 and challenged, along with the controls, 4 days later by intra-tracheal injection of 3 isolates of M. bovis (1.5 × 1010 cfu/mL) suspended in 4 mL of culture medium (Table 1). Trial #2 was conducted to test different challenge routes and doses of the M. bovis strains in animals previously infected with BHV-1 (Table 1). Twenty-four calves were randomly assigned to 1 of 4 groups (A, B, C, D) of 6 animals and managed in the same pen. Four days after the BHV-1 infection, the animals were challenged with various concentrations of M. bovis strains suspended in 4 mL of culture medium (Table 1).
Table 1.
Group assignments for the animal trials
| Group | BVDV type 2 (Day 0) | BHV-1 (Day 0) | Route | M. bovis strains (Day 4)* | Route |
|---|---|---|---|---|---|
| Co-infection trial | |||||
| 1 | 1 × 107pfu/mL | None | IN | 1.5 × 1010/mL | IT |
| 2 | None | 1 × 105.5/mL TCID50 | IN | 1.5 × 1010/mL | IT |
| 3 | None | None | 1.5 × 1010/mL | IT | |
| Dose titration and route of challenge trial | |||||
| A | 1 × 105.5/mL TCID50 | IN | 2.6 × 1010/mL | IN | |
| B | 1 × 105.5/mL TCID50 | IN | 2.6 × 1010/mL | IT | |
| C | 1 × 105.5/mL TCID50 | IN | 2.6 × 109/mL | IT | |
| D | 1 × 105.5/mL TCID50 | IN | 2.6 × 108/mL | IT |
The groups and treatments for the co-infection and dose titration and route of challenge trials are shown.
This consists of an equal mixture of M. bovis Mb1 (RFLP 1), 160 (RFLP 2) and 163 (RFLP transitional). Where applicable, animals were challenged by the intratracheal (IT) or intranasal (IN) route with 4 mL of bacterial suspension 4 d after the viral challenge.
Animals were observed daily for clinical signs of disease and euthanized when there were severe signs, including depression, high fever or a body temperature dropping below 38°C. During the first trial, total blood (cell) counts (CBC) were performed at days 0, 2, 4 and 7. For both trials, body weights and temperatures were measured daily for 18 d after the viral challenge. At necropsy, gross pathologic examinations of the lungs and joints were done and blood, nasal, and synovial fluid samples were collected for isolation of M. bovis. At the end of the trial, the surviving animals were euthanized and examined. All clinical, clinico-pathologic, and gross pathology assessments were carried out by investigators who were unaware of the status of the animals. All animal experiments followed the guidelines of the Care and Use of Experimental Animals provided by the Canadian Council on Animal Care and protocols approved by the University of Saskatchewan Animal Research Ethics Board were followed.
Recovery of M. bovis from tissue samples
Nasal secretions, lung sections from grossly normal and diseased areas, synovial fluid from normal (carpal) and inflamed (carpal or stifle) joints and blood samples were collected and, after 48 h incubation in Hayflick’s medium, they were plated for isolation of M. bovis. Genomic DNA was prepared from colonies resembling M. bovis and confirmed as M. bovis by polymerase chain reaction (PCR) using specific primers, and analyzed by random amplified polymorphic DNA (RAPD) of the M. bovis gene encoding glyceraldehyde 3-phosphate dehydrogenase, GAPDH (12).
Statistical analysis
Statistical analyses [repeated measures analysis of variance (ANOVA), one-way ANOVA, and Fischer’s exact test] were performed by using the analytical software (Statistix 7.0 student edition; Analytical Software, Tallahassee, Florida, USA).
Results
Body weight
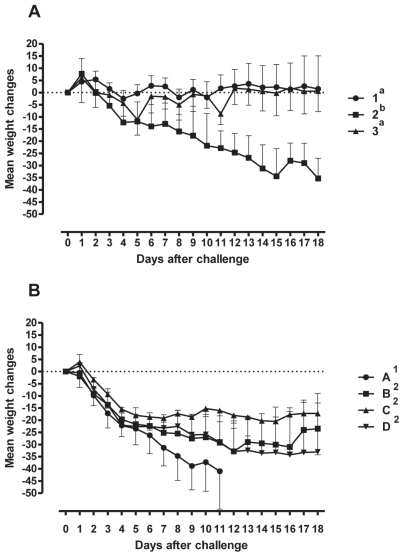
Changes in body weight over the duration of Trial #1 are presented in Figure 1A. Animals infected with BHV-1 and challenged with M. bovis lost an average of 35.3 kg [95% confidence interval (CI) = −56.0 to −14.6 kg] over the 18 d following viral infection. In comparison, animals infected with BVDV type 2 and challenged with M. bovis gained an average of 1.5 kg (95% CI = −4.0 to 20.6 kg) while animals challenged with M. bovis alone gained 0.7 kg (95% CI = −6.5 to 7.7 kg). The weight loss experienced by the BHV-1 infected group was significant compared with the other 2 groups (P = 0.002).
Figure 1.
Percentage weight gain of animals after the viral and bacterial challenges. The means ± SEM of the percentages are shown. Panel A — Animals (n = 8 animals/group) challenged with 1: BVDV type 1 and M. bovis; 2: BHV-1 and M. bovis; and 3: M. bovis. Panel B — Animals (n = 6 animals/group) challenged with A: BHV-1 and M. bovis by the intranasal route; B: BHV-1 (intranasal) and M. bovis (intratracheal) routes; C: Same as B but 10-fold less M. bovis; D: Same as B but 100 fold-less M. bovis. The changes in weight in groups with different superscripts are significantly different.
Changes in body weight during Trial #2 are presented in Figure 1B. Animals infected with BHV-1 and challenged intranasally with M. bovis (2.6 × 1010 cfu/mL), lost 41 kg (95% CI = −79 to −2.9 kg) in the 11 d post-challenge. Over time, this weight loss was significantly greater than the 16 to 29 kg weight loss observed in the groups challenged intratracheally with various doses of M. bovis (P < 0.05). Differences in weight loss among the groups receiving decreasing doses of M. bovis, were not significant (P = 0.08).
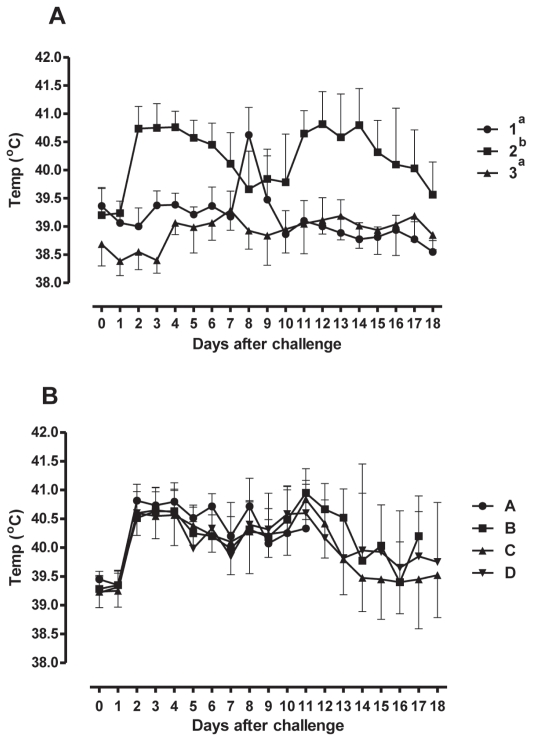
Rectal temperature
Over time, animals in Trial #1 infected with BHV-1 experienced significant increases in rectal temperature compared with controls, and animals infected with BVDV (P < 0.0001) (Figure 2A). Animals infected with BVDV type 2 experienced a spike in rectal temperature 8 d post-infection. In Trial #2, no difference was observed in rectal temperature increases amongst groups infected with BHV-1 and then challenged with various doses of M. bovis by different routes of administration (P = 0.20) (Figure 2B).
Figure 2.
Rectal temperatures of animals after the viral and bacterial challenges. The means ± SEM of the temperatures are shown. The symbols and legend are the same as for Figure 1.
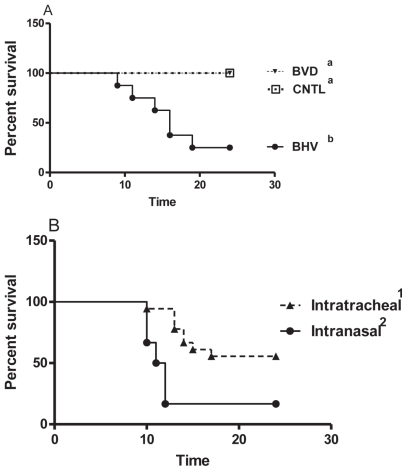
Survival
Animals were euthanized when they reached a predetermined endpoint defined by a combination of severe clinical signs, including depression, lethargy, and fever or a drop in temperature to below 38°C. In Trial #1 (Figure 3A), animals challenged with BHV-1 had significantly shorter survival times (P = 0.002, X2 = 17.3) compared to those challenged with M. bovis alone or infected with BVDV type 2 and challenged with M. bovis (survival = 3/8 for group 2, 8/8 for group 1, and 8/8 for group 3). Overall, in Trial #2 the survival times were significantly different (P = 0.046, X2 = 8.0) but there was no significant difference in survival (1/6 for group A, 2/6 for group B, 4/6 for group C, and 4/6 for group D) among the groups receiving decreasing doses of BHV-1 virus plus M. bovis (Groups B, C, and D, P = 0.632, X2 = 0.94). Therefore, these data were combined and the effects of the intranasal and intratracheal routes of challenge were compared (Figure 3B). Animals challenged via the intranasal route had significantly shorter survival times (P = 0.004, X2 = 8.3) compared with those challenged intratracheally (survival = 1/6 and 10/18, respectively).
Figure 3.
Survival of animals after the viral and bacterial challenges. A — BVD and CNTL: Animals challenged with BVDV type 1 and M. bovis or M. bovis alone respectively. BHV: Animals challenged with BHV-1 and M. bovis. B — IT and IN: Animals challenged by the intratracheal or intranasal route, respectively. The groups with different superscripts are significantly different from each other.
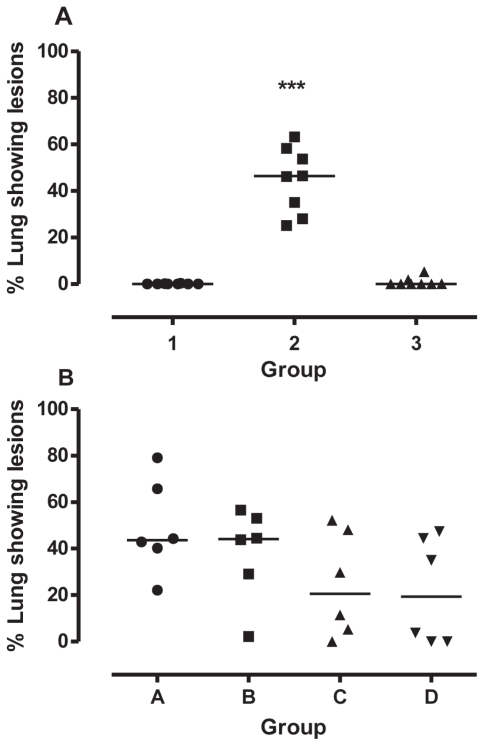
Lung lesions
Macroscopic examination of the lungs of the animals of the first trial revealed normal lungs for 6/8 and 5/8 animals in groups 1 (BVDV type 2) and 3 (Control), respectively (Figure 4A). All animals in group 2 (BHV-1) were affected. The median area of abnormal lung tissue in group 2 was 46.39% (Figure 4A); significantly greater (P < 0.001) than in the other groups. In the second trial, the areas of abnormal lung tissue were not significantly different (P = 0.13) among the 4 groups (medians = 44%, 44%, 21%, and 19%). The number of animals with gross lesions and the number that died were 6/5 in group A, 6/4 in group B, 6/2 in group C and 4/2 in group D.
Figure 4.
Percentage of lungs showing gross lesions consistent with CPPS. ***Significant differences between the lung scores (P < 0.001). The symbols and legend are the same as for Figure 1.
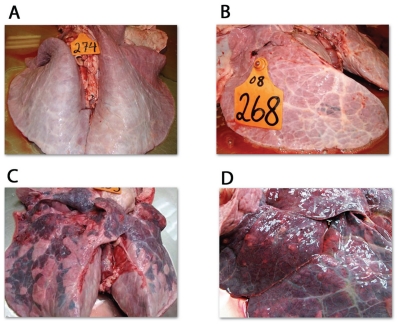
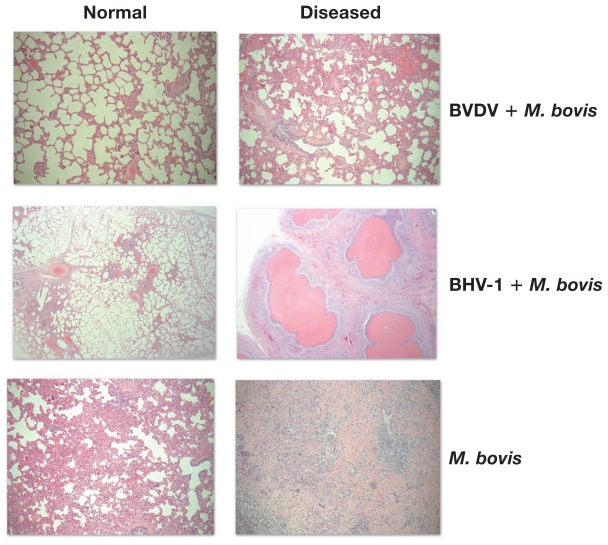
Representative images of lungs from both trials are shown in Figure 5. Lungs obtained from the animals challenged with BVDV type 2 and M. bovis (First trial, group 1) were grossly normal. Those from group 2 of the first trial (BHV-1 + M. bovis) had extensive areas of bronchopneumonia, consolidation, and multifocal white nodules containing caseous material, characteristic of M. bovis infections (Figures 5C and 5D) in all the animals. Those from group 3 of the first trial (M. bovis alone) had small consolidations (Figure 5B) without white nodules. In the second trial, white nodules and small areas of consolidation were found in 1/6 animals from group B and 3/6 animals from groups C and D. Lungs with extensive areas of bronchopneumonia, consolidation, and multifocal white nodules containing caseous material were found in 15 of the calves in groups A, B, C, and D. Lung tissues from normal and diseased areas of the lungs were stained and the results are shown in Figure 6. These sections show characteristic areas of focal necrosis with eosinophilic foci and infiltration of mostly mononuclear cells in the diseased lungs of animals infected by BHV-1 and M. bovis (Figure 6, diseased lungs right column). The animals of the groups challenged with either, BVDV and M. bovis or M. bovis alone did not show lesions typical of M. bovis pneumonia. The normal areas of the lungs from the same animals showed typical normal lung architecture (Figure 6, normal lungs left column).
Figure 5.
Gross lung lesions. A — Normal lung; B — Lung showing a small area of consolidation; C — lung showing large involvement of the cranial and caudal lobes; D — Intermediate lobe showing the typical lesions due to M. bovis infection.
Figure 6.
Lung tissue sections of challenged animals. Lung tissue sections stained with hematoxylin & eosin show normal (left column) and diseased (right column) lungs of the same animals challenged with BVDV type 2 and M. bovis, BHV-1 and M. bovis, or M. bovis alone. In all cases the magnification is 400×.
Bacteriology
Mycoplasma bovis was not isolated from nasal swabs of any animal prior to challenge. During the first trial, rates of isolation from diseased and normal tissues did not vary among the groups (Table 2) although the isolation of M. bovis from normal lung tended to be more common in animals in Group 2 (P = 0.07). Isolation of M. bovis from blood samples taken at necropsy occurred more frequently (P < 0.007) among animals in group 2 compared to animals in groups 1 and 3. In the second trial, M. bovis was isolated from most samples. At necropsy, the rates of isolation from nasal swabs, lung, and synovial tissue were not significantly different among the groups. There was no difference in the rates of isolation of M. bovis among calves infected with BHV-1 by the intranasal and intratracheal routes, although the rate of isolation from blood approached significance (P = 0.06).
Table 2.
Samples from infected and control animals that were positive for Mycoplasma bovis. The frequency of samples (positive/total samples) for M. bovis is shown. Tissue samples from the animals were collected at necropsy and analyzed for the presence of the challenge strains
| Group | Nasal | Lung | Synovial fluid | Blood Post | |||
|---|---|---|---|---|---|---|---|
| Pre | Post | Normal | Diseased | Normal | Diseased | ||
| Co-infection trial | |||||||
| 1 | 0/8 | 5/8 | 4/8 | 1/1 | 3/8 | 1/1 | 0/8 |
| 2 | 0/8 | 8/8 | 8/8 | 8/8 | 5/8 | 0/0 | 6/8 |
| 3 | 0/8 | 5/7 | 4/8 | 1/2 | 2/8 | 0/0 | 0/8 |
| Dose titration and route of challenge trial | |||||||
| A | 0/6 | 6/6 | 6/6 | 6/6 | 4/6 | 0/0 | 5/6 |
| B | 0/6 | 5/6 | 4/6 | 6/6 | 3/6 | 0/0 | 1/6 |
| C | 0/6 | 6/6 | 5/6 | 5/6 | 3/6 | 2/2 | 3/6 |
| D | 0/6 | 6/6 | 4/6 | 4/6 | 2/6 | 0/0 | 2/6 |
To determine if the recovered strains matched the strains used in the challenge, the RFLP profile of the gene encoding GAPDH was determined for at least 5 colonies obtained from random samples of each tissue. We isolated strains which matched RFLP profile 1 of M. bovis Mb1 Strains, with RFLP profile 2 characteristic of M. bovis Mb160, and with RFLP profile typical of M. bovis Mb163 (called transitional profile).
In the first trial, all the isolates recovered from animals challenged with BVDV type 2 and M. bovis (Group 1) fell into the RFLP profile 1, while all the isolates from the animals challenged with M. bovis alone (Group 3) were of the RFLP profile 2 (Table 3). In the animals challenged with BHV-1 and M. bovis (Group 2), we recovered isolates with RFLP profiles 1, transitional and 2 (Table 3). In the second trial, we recovered isolates belonging to the 3 RFLP profiles in all the challenge groups (Table 3). The proportion of RFLP1 strains was significantly higher in samples from animals of groups C and D compared with those of groups A and B (P-values ≤ 0.0198). A different result was obtained for strains of RFLP 2 pattern, where the proportion of RFLP 2 strains was significantly higher in the samples of animals of groups A and B compared to those of groups C and D (P-values ≤ 0.0053). The proportion of strains with the RFLP transitional pattern did not differ in any of the groups.
Table 3.
Number of isolates belonging to different RFLP types. The numbers of isolates (positive/total) for both trials possessing the RFLP 1, transitional, and 2 patterns of the gene encoding GAPDH are shown
| Group | RFLP 1 | RFLP Transitional | RFLP 2 |
|---|---|---|---|
| Co-infection trial | |||
| 1 | 37/37 | 0/37 | 0/37 |
| 2 | 8/18 | 2/18 | 8/18 |
| 3 | 0/24 | 0/24 | 24/24 |
| Dose titration and route of challenge trial | |||
| A | 2/35 | 1/35 | 32/35 |
| B | 5/30 | 2/30 | 23/30 |
| C | 18/40 | 6/40 | 16/40 |
| D | 19/35 | 2/35 | 14/35 |
Discussion
Beef cattle are moved from cow-calf operations to feedlots when they reach 6 to 8 mo of age and exposed to stresses such as transporting, changes in diet, and close contact with other animals. These stresses make the animals more susceptible to respiratory pathogens. Thus, to test vaccines designed to protect feedlot animals, it is necessary to have a challenge model that takes into account exposure to other respiratory pathogens. We developed a challenge model that takes into account exposure to BVDV type 2 and BHV-1. We showed here that animals challenged with BVDV type 2 prior to M. bovis challenge did not succumb to disease by M. bovis despite showing clear signs of BVDV infection such as lymphopenia, increase in body temperatures and no weight gain. Lung lesions were not present at the end of the trial (with the exception of 1 animal with a small lesion) despite the presence of the challenge M. bovis strains in most of the tissues examined. These results do not necessarily contradict previous findings that BVDV and M. bovis were the most common pathogens in western Canadian feedlots based on observations at necropsy of animals that died of respiratory diseases (6,7). These reports did not indicate whether both pathogens infected the host at the same time or not.
Infections with BHV-1 cause disease in the bovine upper respiratory tract (13,14). This fact and the high cost of animals prompted us to not have a group of animals infected with BHV-1 only. On the first 4 d after the BHV-1 challenge, animals showed marked clinical signs such as increased rectal temperatures and weight losses. After a BHV-1 challenge, clinical signs usually last up to 6 d, after which the animals regain weight and show normal temperatures (15). In our study, however, animals infected with M. bovis after challenge with BHV-1 did not recover but developed clinical signs of increasing severity including continuous weight loss and elevated temperature, dyspnea, coughing, and in a few cases, lameness. The mortality rate was high, and significant lesions were observed in the lungs of most of the animals, an outcome that is not observed following experimental challenge with BHV-1. While we cannot stipulate that the BHV-1 infection had completely cleared prior to the time of infection by M. bovis, the clinical manifestations, gross lung pathology, and lung tissue sections observed at the end of the trial were consistent with CPPS and not BHV-1. Finally, a challenge with M. bovis alone resulted in no weight loss, short-lasting changes in temperature and small lung lesions despite the presence of the challenge strains in some of the tissues.
Despite the differences in mortality between the groups of the first trial, we were able to isolate M. bovis from tissues of animals in all the groups with the largest number of M. bovis isolated from group 2 animals. This group showed marked clinical signs and mortality. Similarly in trial 2, most of the tissue samples in all the groups contained M. bovis. These findings are consistent with those for the group 2 animals of the first trial; in both cases, the animals received BHV-1 and M. bovis. We recovered all 3 RFLP types in both trials. In the first trial, all the isolates from group 1 were of RFLP type 1 while all the isolates from group 3 were of RFLP type 1. This suggests that these strains in combination with BVDV or alone did not cause disease but were able to colonize the tissues. Animals that had had previous exposure to BHV-1 harbored the 3 RFLP types, which suggests that after a BHV-1 infection these strains were able to cause disease.
Animals from group 2 of the first trial and group B of the second trial received similar treatments (exposure to BHV-1) and challenge with a high dose of M. bovis by intratracheal injection (Table 1); the survival rates and percentages of lung involvement were comparable (16 versus 15 d and 46.39% versus 44.18%). This indicates the reproducibility of the challenge model. Lowering the challenge dose of M. bovis 10- or 100-fold did not result in significant differences in clinical signs, gross pathology of the lungs and recovery of M. bovis, but survival rates were higher.
The results presented here are consistent with those observed in BHV-1 related diseases where mixed bacterial infection, usually Mannheimia haemolytica or Pasteurella multocida (14) is present.
Infection of animals with BHV-1 followed by infection with M. bovis resulted in clinical and pathological signs consistent with CPPS whereas infection with M. bovis and the non-cytopathic strain of BVDV type 2 did not. The main defining features of CPPS are the chronicity of the disease, isolation of M. bovis from lungs, the tendency to have both pneumonia and polyarthritis, and the development of anteroventral pneumonia with caseous nodules. In this study, we reproduced all clinical signs of CPPS only after a combined BHV-1 and M. bovis challenge, although we saw very few cases of arthritis, probably due to the short duration of the trials. Other studies have found severe disease and pathological changes following infection with M. bovis alone in infant calves but not feedlot-age animals (3–5,16).
We believe that the difference between our results and those in the other studies is in the age of the animals. Previous studies all used animals 4-months-old or younger, while we used 6- to 8-month-old calves. As the immune status of the animal changes with age, we believe that older animals are more resistant to M. bovis and development of CPPS in these animals requires the contribution of BHV-1.
Acknowledgements
We thank Drs. Murray Jelinski and Farshid Shahriar for the gift of M. bovis strains Mb160 and Mb163 and Ms. Mikaela Toovey for her help with the RFLP and PCR assays. This manuscript is published as number 580 in the Vaccine and Infectious Disease Organization journal series. CVJ
Footnotes
This research received financial support from the Beef Cattle Research Council (BCRC), Ontario Cattlemen’s Association (OCA), the Alberta Livestock Development Fund (ALIDF), the Saskatchewan Agriculture Development Fund (ADF), and the Advancing Agriculture and Agri-Food (ACCAF) Program.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Carroll EJ, Bennett RH, Rollins M, Jasper DE. The immune response of calves given Mycoplasma bovis antigens. Can J Comp Med. 1977;41:279–286. [PMC free article] [PubMed] [Google Scholar]
- 2.Howard CJ, Thomas LH, Parsons KR. Immune response of cattle to respiratory mycoplasmas. Vet Immunol Immunopathol. 1987;17:401–412. doi: 10.1016/0165-2427(87)90157-7. [DOI] [PubMed] [Google Scholar]
- 3.Vanden Bush TJ, Rosenbusch RF. Characterization of the immune response to Mycoplasma bovis lung infection. Vet Immunol Immunopathol. 2003;94:23–33. doi: 10.1016/s0165-2427(03)00056-4. [DOI] [PubMed] [Google Scholar]
- 4.Chima JC, Wilkie BN, Ruhnke HL, Truscott RB, Curtis RA. Immunoprophylaxis of experimental Mycoplasma bovis arthritis in calves. Protective efficacy of live organisms and formalinized vaccines. Veterinary Microbiology. 1980;5:113–122. [Google Scholar]
- 5.Nicholas RA, Ayling RD, Stipkovits LP. An experimental vaccine for calf pneumonia caused by Mycoplasma bovis: Clinical, cultural, serological and pathological findings. Vaccine. 2002;20:3569–3575. doi: 10.1016/S0264-410X(02)00340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haines DM, Martin KM, Clark EG, Jim GK, Janzen ED. The immunohistochemical detection of Mycoplasma bovis and bovine viral diarrhea virus in tissues of feedlot cattle with chronic, unresponsive respiratory disease and/or arthritis. Can Vet J. 2001;42:857–860. [PMC free article] [PubMed] [Google Scholar]
- 7.Shahriar FM, Clark EG, Janzen E, West K, Wobeser G. Coinfection with bovine viral diarrhea virus and Mycoplasma bovis in feedlot cattle with chronic pneumonia. Can Vet J. 2002;43:863–868. [PMC free article] [PubMed] [Google Scholar]
- 8.Martin SW, Bateman KG, Shewen PE, Rosendal S, Bohac JE. The frequency, distribution and effects of antibodies, to seven putative respiratory pathogens, on respiratory disease and weight gain in feedlot calves in Ontario. Can J Vet Res. 1989;53:355–362. [PMC free article] [PubMed] [Google Scholar]
- 9.Martin SW, Bateman KG, Shewen PE, Rosendal S, Bohac JG, Thorburn M. A group level analysis of the associations between antibodies to seven putative pathogens and respiratory disease and weight gain in Ontario feedlot calves. Can J Vet Res. 1990;54:337–342. [PMC free article] [PubMed] [Google Scholar]
- 10.Gagea MI, Bateman KG, Shanahan RA, et al. Naturally occurring Mycoplasma bovis-associated pneumonia and polyarthritis in feedlot beef calves. J Vet Diagn Invest. 2006;18:29–40. doi: 10.1177/104063870601800105. [DOI] [PubMed] [Google Scholar]
- 11.Martin SW, Nagy E, Armstrong D, Rosendal S. The associations of viral and mycoplasmal antibody titers with respiratory disease and weight gain in feedlot calves. Can Vet J. 1999;40:560–567. 570. [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Casal J, Prysliak T. Detection of antibodies against the Mycoplasma bovis glyceraldehyde-3-phosphate dehydrogenase protein in beef cattle. Microb Pathog. 2007;43:189–197. doi: 10.1016/j.micpath.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Meyer G, Lemaire M, Ros C, et al. Comparative pathogenesis of acute and latent infections of calves with bovine herpesvirus types 1 and 5. Arch Virol. 2001;146:633–652. doi: 10.1007/s007050170136. [DOI] [PubMed] [Google Scholar]
- 14.Ellis JA. Update on viral pathogenesis in BRD. Anim Health Res Rev. 2009;10:149–153. doi: 10.1017/S146625230999020X. [DOI] [PubMed] [Google Scholar]
- 15.van Drunen Littel-van den Hurk S, Parker MD, Massie B, et al. Protection of cattle from BHV-1 infection by immunization with recombinant glycoprotein gIV. Vaccine. 1993;11:25–35. doi: 10.1016/0264-410x(93)90336-v. [DOI] [PubMed] [Google Scholar]
- 16.Howard CJ, Stott EJ, Thomas LH, Gourlay RN, Taylor G. Protection against respiratory disease in calves induced by vaccines containing respiratory syncytial virus, parainfluenza type 3 virus, Mycoplasma bovis and M. dispar. Vet Rec. 1987;121:372–376. doi: 10.1136/vr.121.16.372. [DOI] [PubMed] [Google Scholar]