Abstract
The recommended treatment for soft tissue sarcomas (STS) and mast cell tumors (MCT) is complete surgical removal, provided that the tumor is amenable to surgical excision. The objective of this study was to evaluate possible risk factors for incomplete surgical excision of skin and subcutaneous STS and MCT in 100 dogs treated with wide excision with curative intent. Decreased body weight was a risk factor (P = 0.03, odd’s ratio = 0.96) as well as increased tumor size (1.4% increase in risk of incomplete excision per cm2; P = 0.02). Gender, age, breed, location, grade, tumor type, re-excision, and level of surgeon’s training (P = 0.0711) were not significant. Veterinary surgery residents were at increased risk of incompleteness of excision compared with ACVS surgeons and ACVS surgeons with additional training in surgical oncology.
Résumé
Facteurs influençant l’excision complète des mastocytomes et des sarcomes des tissus mous : étude rétrospective chez 100 chiens. Le traitement recommandé pour les sarcomes des tissus mous (STM) et les mastocytomes (SM) est l’enlèvement chirurgical complet, pourvu que la tumeur se prête à l’excision chirurgicale. L’objectif de cette étude était d’évaluer les facteurs de risque possibles pour une excision chirurgicale incomplète des STM et SM cutanés et sous-cutanés chez 100 chiens traités avec une excision large dans un but curatif. Un poids corporel réduit était un facteur de risque (P = 0,03, ratio d’incidence rapproché = 0,96) ainsi que la taille accrue de la tumeur (hausse de 1,4 % du risque pour l’excision incomplète par cm2; P = 0,02). Le sexe, l’âge, la race, l’emplacement, le degré de différenciation, le type de tumeur, une ré-excision et le niveau de formation du chirurgien (P = 0,0711) n’étaient pas significatifs. Les résidents vétérinaires présentaient un risque accru d’excision incomplète comparativement aux chirurgiens agréés par l’ACVS et aux chirurgiens agréés par l’ACVS possédant une formation additionnelle en oncologie chirurgicale.
(Traduit par Isabelle Vallières)
Introduction
Skin tumors comprise the most common type of neoplasm in dogs, accounting for almost 30% of malignant tumors in this species (1–3). Of the malignant cutaneous tumors, mast cell tumors (MCT) are the most common, representing 11% to 27% (2–6). Cutaneous MCT are typically solitary lesions and their clinical appearance varies as well as their biological and clinical behavior, ranging from benign to highly malignant (7). Soft tissue sarcomas (STS) represent 15% of skin and subcutaneous tumors (8). A heterogeneous group of malignant tumors characterized by a similar behavior, these tumors are pseudoen-capsulated and locally aggressive with a risk of metastasis that is low overall, but proportional to tumor grade (9,10). Complete surgical removal when the tumor is located in an area amenable to surgical excision is the recommended and effective local treatment for many malignant cancers (1). Generally, similar wide lateral margins of excision are recommended for MCT and STS as well as one fascial plane deep (11). Completeness of excision has been previously shown to be a positive prognostic indicator for both types of tumors (9,12–15). Almost all MCTs (grades I and II) are treated successfully by surgery alone (7,11,13,16,17). For STS, incomplete margin increases the risk of local recurrence by 10.5χ (9). Re-excision, metronomic chemotherapy, and radiation are also recommended for local control of STS after an incomplete excision (18–21). A study by Michels et al (22) evaluated 31 dogs with cutaneous MCT and observed no statistical influence of age, weight, gender, tumor site, or histopathological grade on surgical margins. McSporran (23) evaluated 236 dogs with subcutaneous STS that had undergone surgical excision. In these dogs, all 30 tumors that had been completely excised did not recur, regardless of histological grade. Completeness of excision was predictive of tumor-free survival.
The objective of this study was to evaluate possible risk factors for incomplete surgical excision of skin and subcutaneous MCT and STS. We hypothesized that the location and grade of the tumor, body weight of the dog, and the level of the surgeon’s training would influence the completeness of excision of these tumors.
Materials and methods
Medical records from the Veterinary Teaching Hospital (VTH) at the Ontario Veterinary College from 2002 to 2009 were reviewed retrospectively. One-hundred client-owned dogs met the following inclusion criteria: dogs that had a skin or subcutaneous STS or MCT treated with a wide excision (3 cm and one fascial plane deep) with curative intent as well as re-excision of a previously attempted excision with inadequate margins before referral. The data collected included age at time of diagnosis, gender, breed and weight of dog; tumor type, grade, size and location; histological report of diagnosis, grade and completeness of excision determined by a pathologist; level of the surgeon’s training, and whether or not advanced imaging was performed prior to surgery.
An incomplete excision was defined as one having neoplastic cells at the edge of the cut edge or within 1 mm of the cut edge. A clean but close excision was defined as one having neoplastic cells within 1 to 5 mm of the cut edge. A complete excision was defined as one having neoplastic cells > 5 mm from the cut edge of excision. A clean but close excision was evaluated as an incomplete excision, so that there were 2 groups: complete and incomplete excision.
The level of the surgeon’s training was divided into ACVS residents, board certified surgeons (ACVS), and board certified surgeons with additional training in surgical oncology (ACVS-SO).
The tumor size was presented in cm2. When 3 dimensions were available, the 2 largest were chosen for evaluation. When re-excision was performed, the measurements were made as length of scar × 1 cm wide or by the size of the tumor regrowth when a mass was present. The location of the tumor was divided into head and neck, trunk, extremities, and perineal and external genitalia. Advanced imaging included computed tomography (CT) and magnetic resonance imaging (MRI).
Statistical analysis
Exact conditional logistic regression on tumor type was used for univariate models in order to determine if the parameters of gender, weight, breed, surgeon, re-excision, tumor size, grade, or location could be risk factors for incomplete excision. Based on the univariate results and biological relevance, multiple exact conditional explanatory models were fit with up to 2 parameters at a time as allowed by the degrees of freedom for error of our model. Significance was set at 0.05. Statistical analysis was done using SAS OnlineDoc® 9.1.3 (SAS Institute, Cary, North Carolina, USA).
Results
There were 58 spayed females, 2 intact females, and 40 neutered males in the study. Mean age and body weight were 7.8 y (range: 0.75 to 15 y) and 27.7 kg (range: 3.8 to 65 kg), respectively. There were 77 MCT and 23 STS. Forty-eight (48%) of all tumors had complete excision (35 MCT and 13 STS) and 52 had inadequate margins of excision (42 MCT and 10 STS). Seven tumors (6 MCT and 1 STS) were classified as having clean but close excision where neoplastic cells were present from 1 to 5 mm from the surgical margins. Due to the low statistical strength of the clean but close group, they were evaluated with the incompletely excised tumors. Only 7 tumors were excised with advanced imaging performed prior to excision and 5 of these were STS. The mean tumor size for both MCT and STS was 26.2 cm2 (range: 0.25 to 600 cm2).
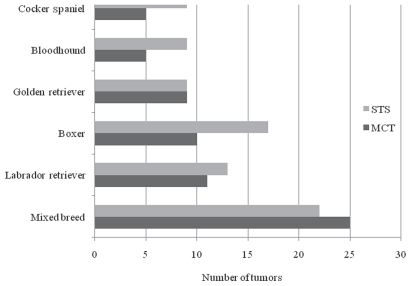
The breeds most commonly affected by MCT and STS are shown in Figure 1. When the affected breeds were compared to the hospital’s breed population at the time, the bloodhounds (P = 0.0001) and boxers (P < 0.0001) were at increased risk of developing both MCT and STS.
Figure 1.
Prevalence of mast cell tumors (MCT) and soft tissue sarcomas (STS) in various breeds of dogs presented at a veterinary teaching hospital (n = 100).
The frequency of grades I, II, and III for MCT were 28%, 58%, and 14%, respectively. The grades were not available for 20 MCT in this study, because these were considered to be subcutaneous MCT and the pathologists at our institution do not use the skin MCT grading scheme for subcutaneous MCT. Twenty-one of the 77 (27%) MCT excised were re-excisions.
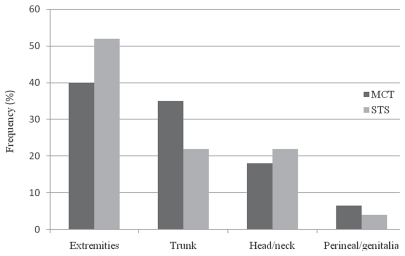
The frequency of grades I, II, and III for STS were 68%, 21%, and 10.5%. The grades were not available for 4 STS. Fifteen of 23 (65%) tumors were re-excisions. The most commonly affected locations for MCT and STS are presented in Figure 2.
Figure 2.
Frequency (%) of mast cell tumors (MCT) and soft tissue sarcomas (STS) affecting the extremities, trunk, head/neck, and perineal/genital areas of dogs presented at a veterinary teaching hospital (n = 100).
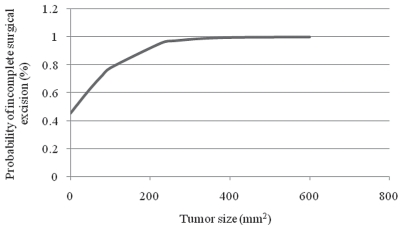
Decreased body weight was a significant risk factor for inadequate surgical margins when evaluating all tumors together (P = 0.03), but not when MCT and STS were evaluated separately. For each increase in 1 kg of body weight, there was a 4% reduced risk of incomplete excision. Increased tumor size was also a significant risk factor for inadequate surgical margins with a 1.4% increase in risk of incomplete excision per cm2 (P= 0.02) (Figure 3). The prognostic significance of tumor size, however, was valid only when both tumors were evaluated together. When tumor size was evaluated separately, it was significant for MCT (P = 0.013), but not for STS, which could be explained by the small sample size.
Figure 3.
Probability of incomplete surgical excision (%) of mast cell tumors and soft tissue sarcomas according to the size of the tumor (mm2) in dogs presented at a veterinary teaching hospital (n = 100).
There was no increased risk of inadequate margins if the tumor was a re-excision. The gender, age, breed, location, grade, and tumor type were not significant for the adequacy of surgical margins. The location was only significant when STS was evaluated individually and the tumors located in head/neck and extremities were compared (P = 0.04). All of the 5 STS affecting the head/neck had adequate margins, whereas 8 out of 12 STS affecting the extremities had inadequate margins.
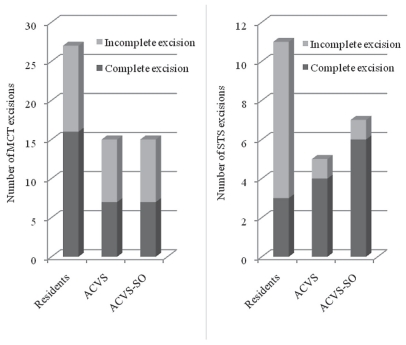
The surgeons in each group during the study consisted of 7 residents, 5 ACVS board certified surgeons, and 2 ACVS-SO board certified surgeons (Figure 4). There was no significant difference in risk of incomplete excision when the surgeons were evaluated for both tumors simultaneously (P = 0.0711) or separately. However, when compared to all ACVS and ACVS-SO board certified surgeons combined, there was a significant difference in the risk of an incomplete excision when the tumor was excised by a resident (P = 0.0123), with an odds ratio of 1.22 for an incomplete excision.
Figure 4.
Number of surgical excisions of mast cell tumors (MCT) and soft tissue sarcomas (STS) performed by ACVS residents, board-certified surgeons (ACVS), and board-certified with additional training in surgical oncology (ACVS-SO) in dogs presented at a veterinary teaching hospital (n = 100).
Discussion
The epidemiologic data from the present study were similar to those from previous reports. The mean age of 7.8 y was consistent with other studies for both MCT (7 to 9 y) (5,11–14,16,17) and STS (8 to 11 y) (9,14,18,20,24,25). Mean body weight of 27.7 kg was similar to that in previous reports for MCT (25.1 to 31.3 kg) (13,14,16,17) and STS (30 kg) (14,24). No statistical significance of gender predilection was observed here or in other studies on the development of MCT (11–14,16,17) or STS (18,21,24).
In a study from New Zealand (14), the mixed breed dogs and Rhodesian ridgebacks were at significantly greater risk of developing STS, and boxers, Australian cattle dogs, and Staffordshire bull terriers were at significantly greater risk of developing MCT. Boxers were over-represented in several studies. They were significantly prevalent over non-boxers and the hospital population in this and 2 other studies (2,22). To the authors’ knowledge, there is no report that the bloodhound is at increased risk of developing MCT or STS as seen in this study.
The most common locations of cutaneous MCT are reported here and elsewhere (11,12,16,17). This study indicates that tumor location does not have a significant effect on completeness of excision, similar to the results of a previous study (26). The authors of that study also reported that tumor location did not influence local recurrence and metastatic rates. A study by Simpson et al (11), that evaluated surgical margins in 21 dogs with cutaneous MCT, revealed that tumors located on the hind limbs were positively correlated with inadequate margins. However, in the present study tumors located in the extremities did not have an increased risk of incomplete excision.
The most common locations for STS in this study were also in agreement with the literature (18,20). In this study, location was not a risk factor for completeness of excision; however, when the tumors located in head/neck and extremities were compared, a significant statistical difference was observed where the STS located in the extremities had increased risk of incompleteness of excision. This finding is in contradiction with the literature which reports that STS behave differently in different locations and that tumors located in the head and neck carry a worse prognosis than those located in the extremities, where they are normally of low grade (9,10,27).
Increased tumor size was a risk factor for inadequate margins regardless of the tumor type. However, when MCT ranging in size from 3.3 to 4.7 cm were evaluated, this relationship was not observed (11,17). This difference between studies may be explained by the fact that MCT are usually small-sized tumors (1.3 to 3 cm) (11–13,16,17), making it easier to achieve adequate margins of excision. Increased tumor size has also been used as a prognostic indicator for local recurrence and metastasis for both MCT (17) and STS (15,21).
Success in complete excision of MCT varies substantially in different studies and has been reported to be between 42% and 91% (17,22,27). Herein, only 45% of MCT were completely excised, perhaps because these cases had an increased surgical challenge. All patients were referred from general practitioners, which is often the case due to recurrence and/or the referring veterinarian’s discomfort with the excision. In addition, mast cells in the surgical margins were presumed to be neoplastic and the few clean but close excisions were also considered as incomplete excisions. These tumors frequently have clusters of mast cells between normal tissue close to the mass. Therefore, it can be challenging to determine if mast cells at the histological margin of excision are normal mast cells that are at the tumor site due to chemoattractants produced by the tumor, or if they are neoplastic (11,28).
In our study, 56.5% of all STS were completely excised. Similar to the MCT group, if neoplastic cells were within 1 to 5 mm from the cut edge, the excision was considered incomplete. If this same consideration is taken for the study of Stefanello et al (25), the success rate in our study is superior. In that study (25), 35 low-grade STS in the extremities were evaluated for completeness of excision, and only 31% of the dogs had complete excision (34% of the surgical margins were assessed to be clean but close). The main difference between both studies is the treatment approach. All of the tumors in our study were excised with curative intent by wide excision whereas in Stefanello et al (25), marginal excision was performed. Also, the tumors were all classified as low grade, and as such, were less locally invasive (23).
The definition of complete, clean but close and incomplete excisions is not well established. According to Ehrhart et al (28), complete excisions are defined as having no neoplastic cells within 1 cm of the tumor and incomplete excisions as having tumor cells extending to the surgical edge or within 1 mm of the edge. Clean but close excision is, therefore, defined as having neoplastic cells > 1 mm away from the margin and < 1 cm from the tumor. These definitions are based on human literature, but to our knowledge, there have been no studies proposing a definition for surgical margins in veterinary medicine. There seems to be no consensus on the definition of clean but close margins. Bacon et al (18) defined clean but close margins as those having neoplastic cells within 3 mm of the inked margin, whereas Simpson et al (11) defined this as within 1 mm, and Stefanello et al (25), within 1 to 3 mm. Further evaluation of the prognostic significance of a clean but close margin is, therefore, warranted.
The clinical significance of complete surgical excisions on local and distant recurrence or on metastatic rate has been questioned. While some authors claim that complete surgical excision is a positive prognostic indicator for local disease control (12,19,29) and longer survival time (13), others suggest that failure to achieve histopathologically tumor-free margins may not be a prognostic factor and does not prevent distant or local recurrence (7,16,17,22,27) for both MCT and STS. The required margins for complete excision for MCT have been evaluated by Simpson et al (11), who revealed that all grade I MCT were completely excised at the 1 cm lateral margin and at the deep margin (1 fascial plane deep to the mass) and that 75% and 100% of grade II MCT were completely excised at 1 cm and 2 cm lateral margins, respectively. Similar results were obtained by Fulcher et al (17), who evaluated surgical margins in 16 dogs with MCT and observed that all grade I MCT were completely excised at the 1 cm margin and 17 of 19 grade II MCT at the 2 cm margin. These are important findings that can help guide surgeons in surgical planning and allow them to be less aggressive with excision of small low- and intermediate-grade MCT since these tumors are generally benign and can usually be cured by surgical excision; the poorly differentiated, high-grade MCT are generally locally invasive, more likely to metastasize, and associated with a poor outcome (7,30,31). The same is true for STS where histological grading is prognostic for behavior (23,25). A recent study (23) evaluated 236 dogs with STS and concluded that histologic grade was a strong predictor for recurrence and that complete excisions were predictive for tumor-free survival.
Variations amongst pathologists for evaluation of tumor grade have been reported in several studies (7,13,27,32) and there is a need for a more complex tumor grading scheme or more consistent application of existing methods (21,32). In Northrup et al (32), 10 pathologists evaluated 60 MCT and only 4 tumors had the same classification, whereas 6 tumors were assigned all 3 grades. Also, pathologists who used a reference allowing inclusion of subcutaneous MCT in the low-grade category were more likely to assign a lower grade (39% probability) than pathologists using a reference that does not allow that. Some pathologists will grade tumors only as high or low. This can be a problem if the tumor has average malignancy features and the pathologists classify it as a low grade. In this situation, a less aggressive surgical approach would be performed, and risks of incomplete excision would increase as previously discussed by Simpson et al (11). In order to better address such conflict, Romansik et al (33) proposes a stratification of grade II MCT into 2 groups based on mitotic rate index, which, in their study, was a strong predictor of overall survival. A validation study for this proposal was then tested by Elston et al (34), who confirmed the prognostic importance of mitotic rate index but suggest stratification into 3 groups. The protocol suggested by Romansik et al (33) based on mitotic rate index is valid to help predict behavior and to guide clinicians to determine a therapy plan when managing grade II MCT. The significance of these findings is high and further validation studies are warranted in order to stimulate pathologists to adhere to this stratification protocol, whether it involves 2 or 3 groups.
The responsibility of surgeons, in aiding pathologists during evaluation of surgical margins, is to perform a careful description of the macroscopic appearance of the tumor and to immediately fix the tissue after removal (1). Once the tumor is excised, it should be handled with great care and the margins should be identified by ink or stay sutures in 3 dimensions so that pathologists can look for neoplastic cells in 6 margins for precise evaluation (29,35). For these reasons, the authors and others (7,9,11,18,25,36) strongly recommend preoperative biopsy for all skin and subcutaneous tumors, for better surgical planning and increased success rates, as well as for postoperative assessment of surgical margins for further adjuvant treatment and monitoring.
Advanced imaging is also a very important tool for surgical planning but is not extensively used. In the present study, only 7 dogs had CT or MRI prior to surgery; imaging is often declined for financial reasons. This problem is also encountered in human medicine, as observed in a study with 38 patients who presented for incomplete removal of STS at referral centers, only 6 of whom had had advanced imaging diagnostics prior to resection (37).
Our hypothesis was that a decreased level of training of the surgeon would be a risk factor for completeness of surgical margins. There is no report comparing surgeon level of training and success rates as done herein. The level of difficulty when performing a tumor excision can vary from simple small skin mass excision to an aggressive/invasive surgery involving important vital structures. The cases were all referrals and many were a recurrence or represented a surgical challenge. This possibly influenced the completeness of excision as well as all other variants in the study, such as the surgeon who would then perform the excision. We suggest that similar data be evaluated among general practitioners and referral centers to provide a more representative population.
The cases in this study were not randomly distributed based on surgeon’s level of training. During the course of the study, there were 2 surgeons who had advanced training in surgical oncology. Although these surgeons were working as general surgeons during this time, it is likely that, due to their area of expertise, the more challenging skin and subcutaneous tumors may have been referred to them. The cases that were managed by residents alone were more likely to be cases that were perceived to be less of a challenge in achieving complete surgical excisions. Even with this potential case distribution, there was an increase in the risk of incomplete excisions when the cases were treated by a resident, compared with an ACVS surgeon (with or without advanced training in surgical oncology). This finding highlights the need to ensure adequate supervision of residents and the need to adhere strictly to the principles of surgical oncology when performing a curative intent resection. The number of incompletely excised tumors that were excised by board-certified surgeons was relatively low. Only 11 MCT and 1 STS were not completely excised by ACVS-SO surgeons, and only 8 MCT and 1 STS were not completely excised by ACVS surgeons. The power calculation for this parameter was 6%, which made it difficult to show a difference between the 2 groups of board-certified surgeons.
Our data indicated that the gender, breed, age, grade, location, and type of tumor do not represent risk factors for incomplete surgical margins and that there is no increased risk in incomplete excision if the tumor is a re-excision. Increased tumor size and decreased body weight are indicative of a worse prognosis and, therefore, increased likelihood of incomplete excision. For completeness of excision, AVCS residents were at increased risk for incompleteness of excision compared with board-certified surgeons with or without additional training in surgical oncology. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Weiss E, Frese K. Tumors of the skin. Bull Wld Hlth Org. 1974;50:79–100. [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen D, Reif JS, Brodey RS, Keiser H. Epidemiological analysis of the most prevalent sites and types of canine neoplasia observed in a veterinary hospital. Cancer Research. 1974;34:2859–2868. [PubMed] [Google Scholar]
- 3.Kaldrymidou H, Leontides L, Koutinas AF, Saridomichelakis MN, Karayannopoulou M. Prevalence, distribution and factors associated with the presence and the potential for malignancy of cutaneous neoplasms in 174 dogs admitted to a clinic in northern Greece. J Vet Med. 2002;49:87–91. doi: 10.1046/j.1439-0442.2002.jv408.x. [DOI] [PubMed] [Google Scholar]
- 4.Finnie JW, Bostock DE. Skin neoplasia in dogs. Aus Vet J. 1979;55:602–604. doi: 10.1111/j.1751-0813.1979.tb07068.x. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell TLW, Howlettt CR, Middleton, Griffiths DA, Duff BC. Skin neoplasms of dogs in Sydney. Aus Vet J. 1987;64:161–164. doi: 10.1111/j.1751-0813.1987.tb09673.x. [DOI] [PubMed] [Google Scholar]
- 6.Macy DW. Canine and feline mast cell tumors: Biologic behaviour, diagnosis, and therapy. Sem Vet Med Surg. 1984;1:72–83. [PubMed] [Google Scholar]
- 7.Dobson JM, Scase TJ. Advances in the diagnosis and management of cutaneous mast cell tumours in dogs. J Small Anim Pract. 2007;48:424–431. doi: 10.1111/j.1748-5827.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- 8.Theilen GH, Madewell BR. Tumors of the skin and subcutaneous tissues. In: Theilen GH, Madewell BR, editors. Veterinary Cancer Medicine. 1st ed. Philadelphia, Pennsylvania: Lea & Febiger; 1979. pp. 123–191. [Google Scholar]
- 9.Kuntz CA, Dernell WS, Powers BE, Devitt C, Straw RC, Withrow SJ. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986–1996) J Am Vet Med Assoc. 1997;211:1147–1151. [PubMed] [Google Scholar]
- 10.Liptak JM, Forrest LJ. Soft tissue sarcomas. In: Withrow SJ, Vail DM, editors. Small Animal Clinical Oncology. 4th ed. St Louis, Missouri: Saunders Elsevier; 2007. pp. 425–454. [Google Scholar]
- 11.Simpson AM, Ludwig LL, Newman SJ. Evaluation of surgical margins required for complete excision of cutaneous mast cell tumors in dogs. J Am Vet Med Assoc. 2004;224:263–240. doi: 10.2460/javma.2004.224.236. [DOI] [PubMed] [Google Scholar]
- 12.Weisse C, Shofer FS, Sorenmo K. Recurrence rates and sites for grade ii canine cutaneous mast cell tumors following complete surgical excision. J Am Anim Hosp Assoc. 2002;38:71–73. doi: 10.5326/0380071. [DOI] [PubMed] [Google Scholar]
- 13.Mullins MN, Dernell WS, Withrow SJ, Ehrhart EJ, Thamm DH, Lana SE. Evaluation of prognostic factors associated with outcome in dogs with multiple cutaneous mast cell tumors treated with surgery with and without adjuvant treatment: 54 cases (1998–2004) J Am Vet Med Assoc. 2006;228:91–95. doi: 10.2460/javma.228.1.91. [DOI] [PubMed] [Google Scholar]
- 14.Baker-Gabb M, Hunt GB, Frances MP. Soft tissue sarcomas and mast cell tumours in dogs; Clinical behavior and response to surgery. Aust Vet J. 2003;81:732–738. doi: 10.1111/j.1751-0813.2003.tb14601.x. [DOI] [PubMed] [Google Scholar]
- 15.Bell RS, O’sullivan B, Liu FF, et al. The surgical margin in soft-tissue sarcoma. J Bone Joint Surg. 1989;71:370–375. [PubMed] [Google Scholar]
- 16.Séguin B, Leibman NF, Bregazzi VS, et al. Clinical outcome of dogs with grade-II mast cell tumors treated with surgery alone: 55 cases (1996–1999) J Am Vet Med Assoc. 2001;218:1120–1123. doi: 10.2460/javma.2001.218.1120. [DOI] [PubMed] [Google Scholar]
- 17.Fulcher RP, Ludwig LL, Bergman PJ, Newman SJ, Simpson AM, Patnaik AK. Evaluation of a two-centimeter lateral surgical margin for excision of grade I and grade II cutaneous mast cell tumors in dogs. J Am Vet Med Assoc. 2006;228:210–215. doi: 10.2460/javma.228.2.210. [DOI] [PubMed] [Google Scholar]
- 18.Bacon NJ, Dernell WS, Ehrhart N, Powers BE, Withrow SJ. Evaluation of primary re-excision after recent inadequate resection of soft tissue sarcomas in dogs: 41 cases (1999–2004) J Am Vet Med Assoc. 2007;230:548–554. doi: 10.2460/javma.230.4.548. [DOI] [PubMed] [Google Scholar]
- 19.Elmslie RE, Glawe P, Dow SW. Metronomic therapy with cyclophosphamide and piroxicam effectively delays tumor recurrence in dogs with incompletely resected soft tissue sarcomas. J Vet Intern Med. 2008;22:1373–1379. doi: 10.1111/j.1939-1676.2008.0179.x. [DOI] [PubMed] [Google Scholar]
- 20.McKnight JA, Mauldin GN, McEntee MC, Meleo KA, Patnaik AK. Radiation treatment for incompletely resected soft-tissue sarcomas in dogs. J Am Vet Med Assoc. 2000;217:205–10. doi: 10.2460/javma.2000.217.205. [DOI] [PubMed] [Google Scholar]
- 21.Heller DA, Stebbins ME, Reynolds TL, Hauck ML. A retrospective study of 87 cases of canine soft tissue sarcomas, 1986–2001. Intern J Appl Res Vet Med. 2005;3:81–87. [Google Scholar]
- 22.Michels GM, Knapp DW, DeNicola DB, Glickman N, Bonney P. Prognosis following surgical excision of canine cutaneous mast cell tumors with histopathologically tumor-free versus nontumor-free margins: A retrospective study of 31 cases. J Am Anim Hosp Assoc. 2002;38:458–466. doi: 10.5326/0380458. [DOI] [PubMed] [Google Scholar]
- 23.McSporran KD. Histologic grade predicts recurrence for marginally excised canine subcutaneous soft tissue sarcomas. Vet Pathol. 2009;46:928–933. doi: 10.1354/vp.08-VP-0277-M-FL. [DOI] [PubMed] [Google Scholar]
- 24.Selting KA, Powers BE, Thompson LJ, et al. Outcome of dogs with high-grade soft tissue sarcomas treated with and without adjuvant doxorubicin chemotherapy: 39 cases (1996–2004) J Am Vet Med Assoc. 2005;227:1442–1448. doi: 10.2460/javma.2005.227.1442. [DOI] [PubMed] [Google Scholar]
- 25.Stefanello D, Morello E, Roccabianca P, et al. Marginal excision of low-grade spindle cell sarcoma of canine extremities: 35 dogs (1996–2006) Vet Surg. 2008;37:461–465. doi: 10.1111/j.1532-950X.2008.00408.x. [DOI] [PubMed] [Google Scholar]
- 26.Sfiligoi G, Rassnick KM, Scarlett JM, Northrup NC, Gieger TL. Outcome of dogs with mast cell tumors in the inguinal or perineal region versus other cutaneous locations: 124 cases (1990–2001) J Am Vet Med Assoc. 2005;226:1368–1374. doi: 10.2460/javma.2005.226.1368. [DOI] [PubMed] [Google Scholar]
- 27.Murphy S, Sparkes AH, Smith KC, Blunden AS, Brearley MJ. Relationships between the histological grade of cutaneous mastcell tumours in dogs, their survival and the efficacy of surgical resection. Vet Rec. 2004;154:743–746. doi: 10.1136/vr.154.24.743. [DOI] [PubMed] [Google Scholar]
- 28.Ehrhart EJ, Powers BE. The pathology of neoplasia. In: Withrow SJ, Vail DM, editors. Small Animal Clinical Oncology. 4th ed. St Louis, Missouri: Saunders Elsevier; 2007. pp. 54–67. [Google Scholar]
- 29.Heslin MJ, Woodruff J, Brennan MF. Prognostic significance of a positive microscopic margin in high-risk extremity soft tissue sarcoma: Implications for management. J Clin Oncol. 1996;14:473–478. doi: 10.1200/JCO.1996.14.2.473. [DOI] [PubMed] [Google Scholar]
- 30.Bostock DE. The prognosis following surgical removal of mastocytomas in dogs. J Small Anim Pract. 1973;14:27–40. doi: 10.1111/j.1748-5827.1973.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 31.Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumor: Morphologic grading and survival time in 83 dogs. Vet Pathol. 1984;21:469–474. doi: 10.1177/030098588402100503. [DOI] [PubMed] [Google Scholar]
- 32.Northrup NC, Harmon BG, Gieger TL, et al. Variation among pathologists in histologic grading of canine cutaneous mast cell tumors. J Vet Diagn Invest. 2005;17:245–248. doi: 10.1177/104063870501700305. [DOI] [PubMed] [Google Scholar]
- 33.Romansik EM, Reilly CM, Kass PH, Moore PF, London CA. Mitotic index is predictive for survival for canine cutaneous mast cell tumors. Vet Pathol. 2007;44:335–341. doi: 10.1354/vp.44-3-335. [DOI] [PubMed] [Google Scholar]
- 34.Elston LB, Sueiro FAR, Cavalcanti JN, Metze K. The importance of the mitotic index as a prognostic factor for survival of canine cutaneous mast cell tumors: A validation study. Vet Pathol. 2009;46:362–365. doi: 10.1354/vp.46-2-362. [DOI] [PubMed] [Google Scholar]
- 35.Rochat MC, Mann FA, Pace LW, Renderson RA. Identification of surgical biopsy borders by use of india ink. J Am Vet Med Assoc. 1992;201:873–8. [PubMed] [Google Scholar]
- 36.Ehrhart N. Soft-tissue sarcomas in dogs: A review. J Am Anim Hosp Assoc. 2005;41:241–246. doi: 10.5326/0410241. [DOI] [PubMed] [Google Scholar]
- 37.Hoshi M, Ieguchi M, Takami M, et al. Clinical problems after initial unplanned resection of sarcoma. Jpn J Clin Oncol. 2008;38:701–709. doi: 10.1093/jjco/hyn093. [DOI] [PubMed] [Google Scholar]