Abstract
Background
Illness severity scores are commonly employed in critically ill patients to predict outcome. To date, prior scores for post-cardiac arrest patients rely on some event-related data. We developed an early, novel post-arrest illness severity score to predict survival, good outcome and development of multiple organ failure (MOF) after cardiac arrest.
Methods
Retrospective review of data from adults treated after in-hospital or out-of-hospital cardiac arrest in a single tertiary care facility between 1/1/2005 and 12/31/2009. In addition to clinical data, initial illness severity was measured using serial organ function assessment (SOFA) scores and full outline of unresponsiveness (FOUR) scores at hospital or intensive care unit arrival. Outcomes were hospital mortality, good outcome (discharge to home or rehabilitation) and development of multiple organ failure (MOF). Single-variable logistic regression followed by Chi-squared automatic interaction detector (CHAID) were used to determine predictors of outcome. Stepwise multivariate logistic regression was used to determine the independent association between predictors and each outcome. The Hosmer-Lemeshow test was used to evaluate goodness of fit. The n-fold method was used to cross-validate each CHAID analysis and the difference between the misclassification risk estimates were used to determine model fit.
Results
Complete data from 457/495 (92%) subjects identified distinct categories of illness severity using combined FOUR motor and brainstem subscales, and combined SOFA cardiovascular and respiratory subscales: I. Awake; II. Moderate coma without cardiorespiratory failure; III. Moderate coma with cardiorespiratory failure; and IV. Severe coma. Survival was independently associated with category (I: OR 58.65; 95% CI 27.78, 123.82; II: OR 14.60; 95% CI 7.34, 29.02; III: OR 10.58; 95% CI 4.86, 23.00). Category was also similarly associated with good outcome and development of MOF. The proportion of subjects in each category changed over time.
Conclusions
Initial illness severity explains much of the variation in cardiac arrest outcome. This model provides prognostic information at hospital arrival and may be used to stratify patients in future studies.
INTRODUCTION
The treatment of a patient with restoration of spontaneous circulation (ROSC) after cardiac arrest has evolved significantly. In particular, the syndrome of post-cardiac arrest illness has been described as consisting of several distinct, pathophysiological changes [1]. Severity of illness is an important determinant of the response to therapeutic interventions. Classifying post-cardiac arrest patients with historical features, such as initial cardiac rhythm, or event-related features, such as witnessed collapse or location of collapse, is a surrogate for the physiological state of the patient. These classifications suffer from modest reliability [2] and weak association with in-hospital clinical course [3]. While several illness severity scores have been developed for critically ill patients, prior scores for post-cardiac arrest patients rely on some event-related data. [4, 5] Data obtained after ROSC and prior to reaching target temperature were used to stratify post-cardiac arrest patients into clinically meaningful illness severity categories. Such categories permit tailoring of therapy for post-arrest patients. Rates of survival, neurologic outcome and development of multiple organ failure (MOF) are presented for each category.
METHODS
This study was approved by the University of Pittsburgh Institutional Review Board. Subjects did not provide written informed consent for this study as these data are part of an ongoing quality assurance/quality improvement initiative in our facility.
Study Population
Subjects were adults (≥18 years) admitted to the emergency department or intensive care unit at a single tertiary care center after ROSC following cardiac arrest between 1/1/2005 and 12/31/2009. Cardiac arrest was defined as receiving chest compressions or rescue shock by a professional healthcare provider. Subjects with both in-hospital cardiac arrest (IHCA) and out-of-hospital cardiac arrest (OHCA) were included. Cardiac arrests occurring in the emergency department were classified as IHCA. In 2007, our facility implemented a multi-disciplinary post-cardiac arrest care plan, including therapeutic hypothermia (TH), for this patient population [6]. The protocol involves rapid induction of TH to a goal temperature of 33 °C for 24 hours followed by gradual rewarming (0.25 °C) to normothermia. To optimize cerebral perfusion, mean arterial blood pressure is targeted to ≥80mmHg and ventilator settings are titrated for a pCO2 of 40. Fluid infusion along with vasopressor or inotropes were used to maintain a urine output of ≥0.5 mL/kg/hr. Patients with STEMI or a new left-bundle branch block on EKG receive emergent coronary angiography. Patients with a history and symptoms consistent with acute myocardial infarction, cardiogenic shock, or focal wall motion abnormality on echocardiogram are also considered for urgent angiography. In 2009, continuous EEG monitoring was added to this post-arrest bundle given the high incidence of seizures in the post-arrest population. [7]
All patient records between 1/1/2005 and 12/31/2009 were abstracted for age, gender, initial rhythm of arrest, location of arrest (IHCA or OHCA), performance of coronary angiography, TH treatment, survival to hospital discharge, and rates of good outcome. Other prehospital variables, such as determination of witnessed collapse and bystander CPR, suffer from modest reliability and were not included in the data abstraction. [2] Multiple reviewers examined a subset of records to confirm data reliability. Discrepancies were resolved by consensus.
Organ system dysfunction was determined using the individual organ dysfunction subscales of the Serial Organ Function Assessment (SOFA) scale [8]. The SOFA score ranges from 0–4 in each of the following organ systems: cardiovascular, respiratory, nervous, liver, coagulation and renal. A higher score signifies greater impairment. The SOFA was selected over other scores such as the APACHE or Simplified Acute Physiology Score, which included measurements such as central venous pressure that are frequently not available [9]. Organ failure was defined as a score ≥3 (range 0–4) on one subscale of the SOFA, with MOF defined as a score of ≥3 on three or more subscales during the first 72 hours of hospitalization. As this model is designed to evaluate the utility of an early illness severity score, baseline organ dysfunction was determined using data obtained within the first 6 hours after cardiac arrest.
Neurological status was rated for subjects by using the Full Outline of Unresponsiveness (FOUR) score [10]. This 16 point score is designed to evaluate the comatose patient with greater texture than the Glasgow Coma Scale, which is one subscale in the SOFA. FOUR score is comprised of a 0–4 score for Motor, Brainstem, Respiratory, and Eye responses. A lower score signifies greater impairment. Data for the calculation of this score were considered valid only when determined in the absence of sedatives or paralytics and within 6 hours of arrest. Neurologic examinations were completed by one of the authors for 222/457 (49%) of subjects. The remainder was obtained from either the critical care attending note or the ICU nursing note. In prior work [11], the reliability of these examinations is good between various examiners and abstractors.
Records were considered adequate for analysis if data allowed calculation of an initial SOFA and FOUR score in the absence of sedation or paralysis within the first six hours after cardiac arrest. In subjects treated with TH, SOFA and FOUR scores were calculated prior to arrival at goal temperature (defined as ≤34°C). Prior work has demonstrated that most OHCA patients are mildly hypothermic on hospital arrival [12]. Only cases lacking these data, or for which specific data were not recorded until more than 6 hours after ROSC, were considered inadequate and excluded.
Outcome Measures
Patient outcomes included survival to hospital discharge, neurological status at death or hospital discharge, and development of MOF. For subjects surviving to hospital discharge, “good” neurological outcome was defined as discharged to home or acute rehabilitation and “poor” neurological outcome was defined as being discharged to a skilled nursing facility, long-term acute care facility, or death. “Good” status corresponds to cerebral performance categories (CPC) 1 or 2 and “poor” status corresponds to CPC 3–4 [6].
Statistical Analysis
Single-variable logistic regression was completed on each SOFA and FOUR subscore as well as age, gender, primary rhythm of arrest, location of arrest (IHCA or OHCA), treatment with TH, initial temperature, and year of treatment to determine if it was associated with survival, MOF, or good outcome. Individual subscores of SOFA and FOUR were used to determine the most significant predictors of outcome. Subscores were considered more appropriate for this analysis because aggregate SOFA and FOUR scores contain duplicative information (e.g. FOUR-motor and SOFA-nervous subscores) and the values of some subscores may be artificially distorted (e.g. FOUR-respiratory may be affected by controlled mechanical ventilation).
The Chi-Square automatic interaction detector (CHAID) was used to analyze interactions between candidate predictor variables from the single-variable logistic regression and three outcome measures: survival, good outcome, and MOF (Answer Tree 3.1; SPSS, Inc., Chicago, IL). Individual analyses were completed for each outcome measure. For each analysis, CHAID identified variables and split-points most strongly associated with outcome using an exhaustive automatic splitting method. This method tests all possible variables at each split (analysis of variance F test) and selects the variable with the strongest interaction.
Optimal combinations of SOFA and FOUR subscales for predicting survival, good outcome and MOF were determined using the CHAID algorithm [13, 14] implemented in SPSS (v. 17; SPSS, Inc., Chicago, IL). FOUR Motor and FOUR Brainstem were most consistently associated with survival and good outcome in both univariable and multivariable regression, while SOFA Cardiovascular and SOFA Respiratory were associated with survival and MOF. Therefore, these variables were combined into a neurologic variable (FOUR Motor-Brainstem = FOUR Motor + FOUR Brainstem) and organ failure variable (SOFA Cardiac-Respiratory = SOFA Cardiac + SOFA Respiratory).
Cross-validation of each analysis was conducted using the n-fold method. This established method [15–17] randomly partitions the original sample into subsamples with 90% of the cases (10 in this study), which then serve as test samples for cross-validation analyses. The misclassification risk estimates for each of the validation samples are then averaged and compared with the misclassification risk estimate for the original sample. The smaller the difference between risk estimates from the original sample and the average of the cross-validation samples, the lower the risk of misclassification in the original data-driven model. For categorical data, the risk estimate represents the proportion of misclassified cases; for continuous data, the risk estimate represents the potential variance around the mean of the measure.
The independent association between categories of early illness severity and outcomes were evaluated using multivariable logistic regression to adjust for other clinical variables. A stepwise approach was used with a p-value for inclusion of 0.15. Univariates that were dropped from the initial analysis were not reincorporated into the revised model. Interaction terms were examined as appropriate. The Hosmer-Lemeshow (HL) test was used to determine goodness-of-fit. [18] Regression results were presented using Odds Ratios (OR's). Category IV was used as the referent group for the logistic regression analysis given the low rates of survival and good outcome in this group. Chi-squared analyses were used to compare rates of survival, good outcome, and death. CHAID results were also presented using Chi-squared analyses. ANOVA was used to compare proportions of subjects in each category over time.
RESULTS
Of 495 subjects treated during this time period, 457 had valid data for analysis. Excluded subjects (N=38) more frequently experienced IHCA, were comatose, and were treated in 2008 (corresponding to a loss of data during the change in electronic medical record systems for the ICU; p<0.05). Excluded subjects less frequently experienced a primary rhythm of VF/VT, received TH, and received coronary angiography (p<0.05). (Table 1)
Table 1.
Characteristics of subjects deriving category of arrest. Therapeutic hypothermia achieved delineates group where temperature ≤34°C was achieved.
| Included (N=457) | Excluded (N=38) | |
|---|---|---|
| Age, in years (SD) | 61 (16) | 61 (14) |
|
| ||
| Male sex | 259 (57%) | 25 (66%) |
|
| ||
| OHCA* | 253 (55%) | 9 (24%) |
|
| ||
| Rhythm* | ||
| VF/VT | 185 (41%) | 8 (21%) |
| PEA | 131 (29%) | 9 (24%) |
| Asystole | 88 (19%) | 11 (29%) |
| Unknown | 52 (11%) | 10 (26%) |
|
| ||
| Year* | ||
| 2005 | 64 (14%) | 4 (10%) |
| 2006 | 86 (19%) | 8 (21%) |
| 2007 | 82 (18%) | 2 (6%) |
| 2008 | 103 (22%) | 20 (53%) |
| 2009 | 122 (27%) | 4 (10%) |
|
| ||
| Therapeutic Hypothermia Intended | 221 (48%) | 21 (55%) |
|
| ||
| Therapeutic Hypothermia Achieved | 202 (44%) | 13 (34%) |
|
| ||
| Coma on arrival (GCS<9)* | 287 (62%) | 31 (82%) |
|
| ||
| Coronary Angiography* | 160 (45%) | 3 (12%) |
|
| ||
| LOS (IQR) | 7 (4, 15) | 10 (4, 21) |
|
| ||
| Survival | 213 (47%) | 19 (50%) |
|
| ||
| Good Outcome | 144 (32%) | 7 (18%) |
p<0.05
Abbreviations: OHCA- out-of-hospital cardiac arrest, VF/VT- ventricular fibrillation/pulseless ventricular tachycardia, PEA- pulseless electrical activity, GCS- Glasgow coma scale, LOS-length of stay (days), SD- standard deviation, IQR- interquartile range.
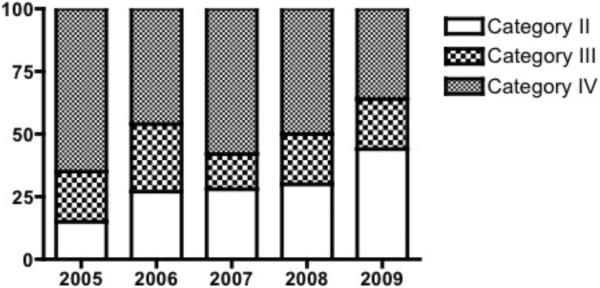
The proportion of comatose subjects in categories II and III increased between 2005–2009 (p=0.001). (Figure 1A) However, survival in the total comatose population (categories II, III, and IV) did not change over time (2005- 20%; 2006- 31%; 2007- 37%; 2008- 31%; 2009- 33%; p=0.52; Figure 1B).
Figure 1A).
Distribution of post-cardiac arrest category in comatose subjects by year.
Figure 1B).
Survival by post-cardiac arrest category in comatose subjects by year.
Derivation of illness severity categories
Regression Analysis
Predictors of survival, good outcome, and development of MOF are listed in Supplemental Table 1. Multivariable regression using the predictor variables outlined above demonstrated that survival was associated with FOUR Motor (OR 1.86; 95% CI 1.46, 2.36), FOUR Brainstem (OR 1.86; 95% CI 1.45, 2.37), SOFA Cardiovascular (OR 0.74; 95% CI 0.59, 0.93), and SOFA Renal (OR 0.74; 95% CI 0.55, 0.99). This model had good fit with a HL value of 0.60.
Good outcome was associated with FOUR Motor (OR 1.70; 95% CI 1.35, 2.14), FOUR Brainstem (OR 1.71; 95% CI 1.30, 2.24), age (OR 0.97; 95% CI 0.95, 0.99), and primary rhythm of VF/VT (OR 2.60; 95% CI 1.48, 4.59). This model demonstrated good fit with HL value of 0.88.
MOF was associated with FOUR Brainstem (OR 0.70; 95% CI 0.58, 0.85), SOFA Cardiovascular (1.69; 95% CI 1.36, 2.10), SOFA Respiratory (OR 1.48; 95% CI 1.15, 1.92), SOFA Renal (OR 1.54; 95% CI 1.12, 2.10), and SOFA Coagulation (OR 1.76; 95% CI 1.08, 2.86). This model had good fit with HL value of 0.46.
FOUR Motor-Brainstem had a univariable association with survival (OR 1.71; 95% CI: 1.55, 1.88), good outcome (OR 1.60; 95% CI: 1.44, 1.77) and MOF (OR 0.76; 95% CI: 0.70, 0.82). SOFA Cardiac-Respiratory had a univariable association with survival (OR 0.78; 95% CI: 0.71, 0.84), good outcome (OR 0.80; 95% CI: 0.73, 0.88) and MOF (OR 1.51; 95% CI: 1.37, 1.67).
CHAID Analysis
In CHAID analysis, FOUR Brainstem was the primary predictor of survival (Chi-Square 138.074, p<0.001). Good outcome was primarily predicted by FOUR Motor (Chi-Square 95.66, p<0.001). However, it was the combination variable of FOUR Motor-Brainstem that provided optimal categorization of the population for both survival (Chi-Square 172.47, p<0.001) and good outcome (Chi-Square 110.33, p<0.001). Both the survival and good outcome analyses, demonstrated data-driven models with a minimal risk of misclassification. The difference between risk estimates from the original sample and cross-validation sample were 0.041 and 0.079, respectively. SOFA Cardiac-Respiratory ≥ 4 was most predictive of development of MOF (Chi-Square 77.81, p<0.001). This difference between risk estimates from the original sample and cross-validation sample was 0.045, again demonstrating a data-driven model with a minimal risk of misclassification.
Grouping subjects according to neurological score (FOUR Motor-Brainstem 0–3, 4–7, 8) and cardiopulmonary dysfunction (SOFA Cardiac-Respiratory 0–3, 4–8) initially yielded six different categories of illness severity (Figure 2). However, subjects in the FOUR Motor-Brainstem <4 and in the FOUR Motor-Brainstem >7 groups experienced similar outcomes throughout the range of SOFA Cardiac-Respiratory scores. Therefore, these were combined for simplification, resulting in four categories with the following descriptions: awake (category I), moderate coma without cardiorespiratory failure (category II), moderate coma with cardiorespiratory failure (category III) and severe coma (category IV).
Figure 2.
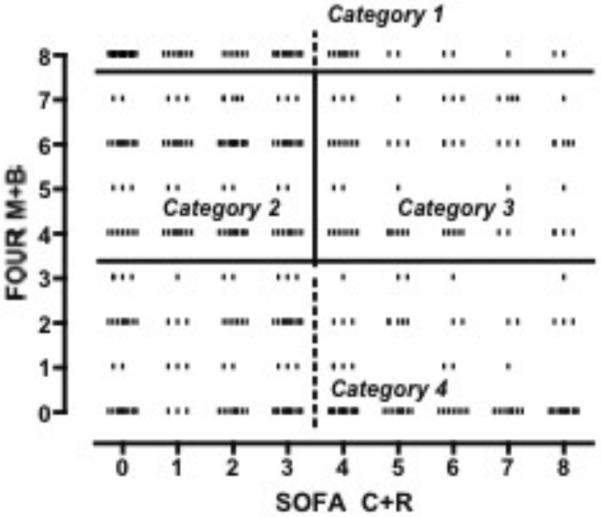
Categories of post-cardiac arrest early illness severity. Dashed lines delineate the cutoffs for the initial six-category model. Solid lines delineate cutoffs for the final four-category model. Abbreviations: FOUR M+B: full outline of unresponsiveness combined motor and brainstem subscores, SOFA C+R: serial organ function assessment combined cardiac and respiratory subscores.
Outcomes by illness severity category
Historical features and treatment differed between categories. (Table 2) An initial rhythm of VF/VT was less common in category IV subjects. OHCA was more common in category II and IV subjects. Temperature at initial examination was lowest in category IV subjects. TH was rarely applied to category I subjects (all prior to 2007). Coronary angiography was less commonly performed for category III and IV subjects.
Table 2.
Post cardiac arrest category, patient characteristics, treatments, and outcome. Therapeutic hypothermia achieved delineates group where temperature ≤34°C was achieved.
| Category I (N=141) | Category II (N=98) | Category III (N=63) | Category IV (N=155) | |
|---|---|---|---|---|
| Age, in years (SD) | 62 (15) | 61 (15) | 62 (16) | 60 (19) |
|
| ||||
| Male sex | 88 (62%) | 49 (50%) | 41 (65%) | 81 (52%) |
|
| ||||
| OHCA* | 52 (37%) | 68 (69%) | 24 (38%) | 109 (70%) |
|
| ||||
| Rhythm* | ||||
| VF/VT | 69 (49%) | 49 (50%) | 27 (43%) | 40 (26%) |
| PEA | 35 (25%) | 24 (25%) | 16 (25%) | 56 (36%) |
| Asystole | 23 (16%) | 10 (10%) | 14 (22%) | 42 (26%) |
| Unknown | 13 (10%) | 15 (15%) | 6 (10%) | 18 (12%) |
|
| ||||
| Temperature during initial clinical examination, °C (SD)*# | (N=106) 35.9 (1.5) |
(N=71) 35.5 (1.5) |
(N=48) 35.4 (2.3) |
(N=119) 35.1 (1.9) |
|
| ||||
| Therapeutic Hypothermia Intended* | 8 (6%) | 76 (78%) | 33 (52%) | 104 (67%) |
|
| ||||
| Therapeutic Hypothermia Achieved* | 4 (3%) | 71 (72%) | 29 (46%) | 98 (63%) |
|
| ||||
| Coronary Angiography* | 65 (56%) | 43 (64%) | 15 (31%) | 37 (30%) |
|
| ||||
| Death from poor neurologic prognosis | 5 (4%) | 29 (30%) | 16 (25%) | 66 (43%) |
p<0.01 between groups
initial temperature not available for all subjects.
Abbreviations: OHCA- out-of-hospital cardiac arrest, VF/VT- ventricular fibrillation/pulseless ventricular tachycardia, PEA- pulseless electrical activity, SD- standard deviation.
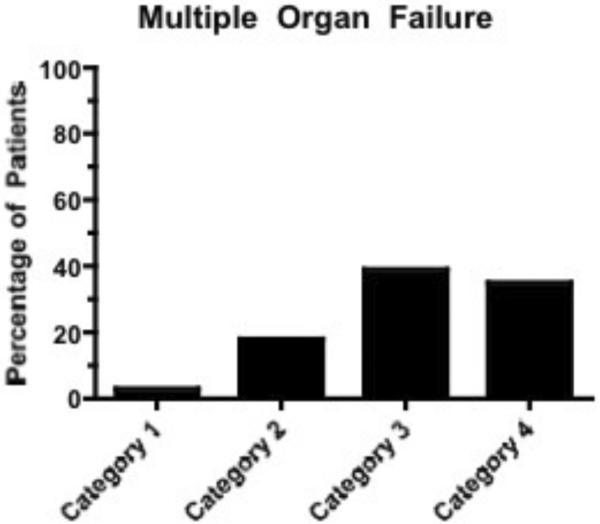
Survival was independently associated with category (I: OR 58.65; 95% CI 27.78, 123.82; II: OR 14.60; 95% CI 7.34, 29.02; III: OR 10.58; 95% CI 4.86, 23.00) as was OHCA (OR 2.03; 95% CI 1.22, 3.37). (Figure 3A) This model demonstrated good fit with an HL value of 0.27. Likewise, good outcome was associated with category (I: OR 52.31; 95% CI 21.87, 125.14; II: OR 11.22; 95% CI 4.81, 26.18; III: 10.76; 95% CI 4.14, 27.95), age (OR 0.98; 95% CI 0.96, 0.99), and OHCA (OR 2.71; 95% CI 1.58, 4.66). Again, appropriate fit was noted with an HL value of 0.70. Development of MOF was associated with category (I: 0.05; 95% CI 0.02, 0.15; II: 0.32; 95% CI 0.17, 0.58) alone. (Figure 3B) This final model was robust, with an HL value of 0.99.
Figure 3A).
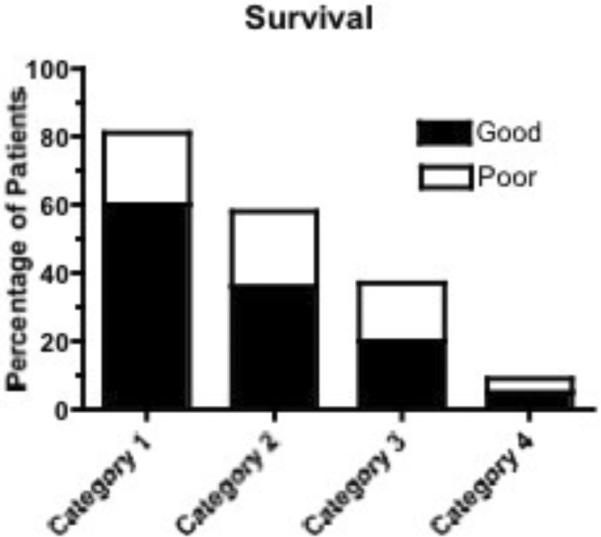
Survival and good outcome by post-cardiac arrest category.
Figure 3B).
Development of multiple organ failure by post-cardiac arrest category.
DISCUSSION
This study identified four distinct categories of post-cardiac arrest illness severity based on neurological dysfunction combined with cardiopulmonary dysfunction during the first few hours after ROSC. Survival, good outcome, and MOF varied greatly between categories, and the proportions of patients in different categories varied over time. The present results emphasize that illness severity should be carefully measured and accounted for in future studies of therapies.
The category of early illness severity had a stronger association with survival, good outcome and cause of death than did primary rhythm or location of arrest. Many patient characteristics are associated with survival with ROSC after cardiac arrest. For example, witnessed collapse, use of epinephrine, lidocaine, or dopamine are negatively associated with the hazard of death during the first day after OHCA [3]. However, only age and use of epinephrine are associated with hazard of death after the first day [3, 19], suggesting that event-related variables such as witnessed collapse, which are important for predicting restoration of pulses, have less influence on post-cardiac arrest course. Prior work has shown that coronary angiography is associated with good outcome following cardiac arrest. [20] However, receipt of coronary angiography covaried with OHCA and depth of coma. In this study, most subjects received coronary angiography more than one day following ROSC, limiting the utility of this variable in a model designed to predict outcomes based on variables found in the first 6 hours following ROSC.
In contrast, the physiological state of the patient after ROSC appears to be highly associated with the subsequent clinical course. Initial examination and laboratory results available in the first 6 hours following ROSC may be used to provide immediate prognostic information to families and clinicians. Importantly, no category of initial illness severity excludes good neurologic outcome. We previously noted that patients in deep coma were less likely to receive coronary angiography and 4 patients with ST elevation myocardial infarction did not receive this therapy due to poor neurologic status. [20] These data do not support withholding aggressive post-arrest care for comatose patients.
The categories of early illness severity derived in this paper explain a variety of observations about post-cardiac arrest patients. For example, category III is largely comprised of IHCA cohort and is the category most likely to suffer from MOF. IHCA has previously been reported to have a higher rate of progression to MOF compared to OHCA. [21] Cardiac arrest with an initial rhythm of VF also is more likely than non-VF rhythm to result in category I or II patients, with excellent prognoses, versus category IV patients, with poor prognoses. [Table 2]
The secular trend noted in early illness severity between 2005–2009 may be due to several factors. (Figure 3A) First, this study occurred after implementation of the 2005 ECC guidelines [22], which may require several years to translate into practice [23]. Second, several local EMS agencies were involved in clinical trials that conducted targeted training to increase chest compression fraction [24]. Finally, the development of a comprehensive treatment plan at this referral center may have altered the pattern of patient referrals in the region.
Categories of post-cardiac arrest illness severity can be determined using objective data available to the clinician at the bedside during initial evaluation to predict the probability of survival, good outcome and MOF. Category can be used to counsel about prognosis, selecting patients for clinical trials, and comparing populations between different clinical trials or settings. Early illness severity explains much of the variation in outcome between IHCA and OHCA and between VF and non-VF cardiac arrest. Future interventional studies should carefully measure early illness severity using instruments such as SOFA, FOUR score or the post-arrest categories, and assure equal allocation of illness severity between treatment groups.
There are several limitations to this study. Subjects with greater illness severity had lower initial core temperatures on hospital arrival. This may represent an inability to maintain core temperature in subjects with most severe neurologic injury. While these findings are consistent with prior work [12], it may have confounded the initial neurologic examination. Some subjects did receive prehospital cooling, however, this is unlikely to have affected outcome as a recent clinical trial demonstrated no change in outcome with prehospital cooling. [25] It is also possible that the initial neurologic examination was less regimented prior to implementation of TH and a post-cardiac arrest care plan. However, we restricted this analysis to patients without sedation and with well-documented examinations by attending physicians. We believe the potential bias from excluded subjects is small as this group was only 8%, although there were some differences in baseline characteristics. Finally, the models could not adjust for other patient factors that were not measured. Thus, variations in illness severity over the first six hours, technical differences in delivery of TH, or other factors may have contributed to the results.
Advantages of this study include the fact that data were collected in a center with a multidisciplinary post-cardiac arrest care plan. Regimented neurological examinations were employed. The CHAID model both derived and validated optimal cutoffs used to differentiate between categories. Finally, the risk estimates of these models are small, demonstrating data-driven models with minimal risk of misclassification.
CONCLUSIONS
Initial illness severity explains much of the variation in cardiac arrest outcomes. This model provides prognostic information at hospital arrival and a universal nomenclature to stratify patients in future studies.
Supplementary Material
Acknowledgements
JCR and this project are supported by Grant Number 1 KL2 RR024154 from the National Center for Research Resources. The content is solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or the National Institutes of Health. JCR is also supported by an unrestricted grant from the National Association of EMS Physicians/Zoll EMS Resuscitation Research Fellowship.
The authors also would like to acknowledge Dr. Garrick Kwok for his assistance in chart review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest The authors have no relevant conflicts of interest to report.
References
- 1.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118(23):2452–83. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 2.Rittenberger JC, Martin JR, Kelly LJ, et al. Inter-rater reliability for witnessed collapse and presence of bystander CPR. Resuscitation. 2006;70(3):410–5. doi: 10.1016/j.resuscitation.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Wang HE, Min A, Hostler D, et al. Differential effects of out-of-hospital interventions on short- and long-term survival after cardiopulmonary arrest. Resuscitation. 2005;67(1):69–74. doi: 10.1016/j.resuscitation.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Adrie C, Cariou A, Mourvillier B, Laurent I, Dabbane H, Hantala F, Rhaoui A, Thoung M, Monchi M. Predicting survival with good neurological recovery at hospital admission after successful resuscitation from out-of-hospital cardiac arrest: the OHCA score. Eur Heart J. 2006;27:2840–5. doi: 10.1093/eurheartj/ehl335. [DOI] [PubMed] [Google Scholar]
- 5.Haukoos JS, Lewis RJ, Stratton SJ, Niemann JT. Is the ACLS score a valid prediction rule for survival after cardiac arrest? Acad Emerg Med. 2003;10:621–6. doi: 10.1111/j.1553-2712.2003.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 6.Rittenberger JC, Guyette FX, Tisherman SA, et al. Implementation of a hospital-wide plan to improve care of comatose survivors of cardiac arrest. Resuscitation. 2008;79:198–204. doi: 10.1016/j.resuscitation.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rittenberger JC, Popescu A, Guyette FX, Callaway CW. Neurocrit Care. Jun 3, 2011. Frequency and Timing of Nonconvulsive Status Epilepticus in Comatose Post-Cardiac Arrest Subjects Treated with Hypothermia. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pettila V, Pettila M, Sarna S, et al. Comparison of multiple organ dysfunction scores in the prediction of hospital mortality in the critically ill. Crit Care Med. 2002;30(8):1705–11. doi: 10.1097/00003246-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Knaus WA, Zimmerman JE, Wagner DP, et al. APACHE- Acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9(8):591–7. doi: 10.1097/00003246-198108000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Wijdicks EF, Bamlet WR, Maramattom BV, et al. Validation of a new coma scale: The FOUR score. Ann Neurol. 2005;58(4):585–93. doi: 10.1002/ana.20611. [DOI] [PubMed] [Google Scholar]
- 11.Rittenberger JC, Sangl J, Wheeler M, et al. Association between clinical examination and outcome after cardiac arrest. Resuscitation. 2010;81(9):1128–32. doi: 10.1016/j.resuscitation.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callaway CW, Tadler SC, Katz LM, et al. Feasibility of external cranial cooling during out-of-hospital cardiac arrest. Resuscitation. 2002;52(2):159–65. doi: 10.1016/s0300-9572(01)00462-2. [DOI] [PubMed] [Google Scholar]
- 13.Kass G. An exploratory technique for investigating large quantities of categorical data. Appl Statistics. 1980;29:119–27. [Google Scholar]
- 14.Biggs D, de Ville B, Suen B. A method of choosing multiway partitions for classification and decision trees. J Appl Statistics. 1991;18:49–62. [Google Scholar]
- 15.StataSoft I . Electronic statistics textbook. StatSoft; 2004. [Google Scholar]
- 16.Training Department . SPSS Answer Tree 3.0 training manual. SPSS, Inc.; 2001. [Google Scholar]
- 17.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Wadsworth International; Belmont, Ca: 1984. [Google Scholar]
- 18.Hosmer D, Lemeshow S. Applied logistic regression. Wiley-Interscience Publication; New York: 2000. [Google Scholar]
- 19.Hallstrom AP, Cobb LA, Swain M, et al. Predictors of hospital mortality after out-of-hospital cardiopulmonary resuscitation. Crit Care Med. 1985;13(11):927–929. doi: 10.1097/00003246-198511000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds JC, Callaway CW, El Khoudary SR, Moore CG, Alvarez RJ, Rittenberger JC. Coronary angiography predicts improved outcome following cardiac arrest: propensity-adjusted analysis. J Intens Care Med. 2009;24:179–86. doi: 10.1177/0885066609332725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laver S, Farrow C, Turner D, et al. Mode of death after admission to an intensive care unit following cardiac arrest. Int Care Med. 2004;30(11):2126–8. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 22.2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2005;112(Suppl.):IV, 19–34. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 23.Bigham BL, Koprowicz K, Augderheide TP, et al. Delayed prehospital implementation of the 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiac care. Prehosp Emerg Care. 2010;14(3):355–60. doi: 10.3109/10903121003770639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christenson J, Andrusiek D, Everson-Stewart S, et al. Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation. 2009;120(13):1241–7. doi: 10.1161/CIRCULATIONAHA.109.852202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard SA, Smith K, Cameron P, Masci K, Taylor DM, Cooper DJ, Kelly AM, Silvester W, the Rapid Infusion of Cold Hartmanns Investigators Induction of therapeutic hypothermia by paramedics after resuscitation from ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation. 2010;122:737–42. doi: 10.1161/CIRCULATIONAHA.109.906859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.