Abstract
Leptin is a pleiotropic hormone primarily secreted by adipocytes. A high density of functional Leptin receptors has been reported to be expressed in the hippocampus and other cortical regions of the brain, the physiological significance of which has not been explored extensively. Alzheimer’s disease (AD) is marked by impaired brain metabolism with decreased glucose utilization in those regions which often precede pathological changes. Recent epidemiological studies suggest that plasma Leptin is protective against AD. Specifically, elderly with plasma Leptin levels in the lowest quartile were found to be four times more likely to develop AD than those in the highest quartile. We have previously reported that Leptin modulates AD pathological pathways in vitro through a mechanism involving the energy sensor, AMP-activated protein kinase (AMPK). To this end, we investigated the extent to which activation of AMPK as well as another class of sensors linking energy availability to cellular metabolism, the sirtuins (SIRT), mediate Leptin’s biological activity. Leptin directly activated neuronal AMPK and SIRT in cell lines. Additionally, the ability of Leptin to reduce tau phosphorylation and β-amyloid production was sensitive to the AMPK and sirtuin inhibitors, compound C and nicotinamide, respectively. These findings implicate that Leptin normally acts as a signal for energy homeostasis in neurons. Perhaps Leptin deficiency in AD contributes to a neuronal imbalance in handling energy requirements, leading to higher Aβ and phospho-tau, which can be restored by replenishing low Leptin levels. This may also be a legitimate strategy for therapy.
Keywords: Leptin, AMPK, SIRT, Alzheimer’s, tau, β-amyloid
Introduction
Several pieces of evidence suggest that brain metabolic disturbances may precede the pathological cascades characteristic of AD. For example, functional neuroimaging studies, including 2-deoxy-2[(18)F]fluoro-D-glucose (FDG) positron emission topography (PET), have illustrated regional hypometabolism in the early AD brain [1; 2; 3], and that the pattern correlates with typical brain atrophy in AD [4]. Interestingly, pyramidal neurons of the hippocampus have particularly demanding energy needs [5], rendering the hippocampus a region more sensitive to states of metabolic distress.
Both genetic and environmental factors are likely contributorsin this interconnection between brain metabolic state and disease. For example, carriers of one copy of the APOE4 gene, involved in lipid metabolism, are three- to four-fold more likely to develop AD than APOE3 carriers [6]. Further, carriers of the very long isotype of the TOMM40 gene polyT region among APOE3 carriers develop AD, on average, seven years earlier than carriers of the short isotype [7].
Rodents fed high fat/caloric diets with limited exercise demonstrate impaired learning and memory performance compared to similar animals on lower energy diets [8]. In humans, a large longitudinal analysis showed a significant correlation between central obesity in midlife and an increased risk of dementia independent of diabetes and cardiovascular co-morbidities later in life [9].
Once AD pathological cascades are initiated because of these metabolic disturbances, these could further and cyclically exacerbate hypometabolic states regionally. Aβ oligomers induce oxidative stress [10], while activation of GSK-3β (promoted by Aβ and inhibited by Leptin), one of the many kinases that can phosphorylate tau, leads to decreased mitochondrial membrane potential and ATP production [11]. Studies utilizing animal models of aging and AD have shown that achieving optimal energy balance (i.e. through feeding and exercise) can improve cognitive function and prevent an age-related decline in learning [12; 13].
The main focus of our research efforts is to identify and characterize metabolic factors, the levels of which are altered during normal brain aging or during the conversion of a healthy brain to that with dementia and AD. Leptin, primarily secreted from adipocytes, can function as a modulator of energy metabolism [14]. Within the arcuate nucleus of the hypothalamus, Leptin acts on neuropeptide Y/agouti-related peptide (NPY/AgRP) and pro-opiomelanocortin (POMC) neurons to regulate food intake, energy expenditure and hepatic glucose production [15]. However, other larger regions in the brain known to express high levels of the Ob-Rb, the long isoform of Leptin receptor known to transduce signaling, include the cortex and the hippocampus [16; 17]. Peripherally, Leptin acts directly on fat and skeletal muscle to stimulate fatty acid oxidation, increase glucose uptake and inhibit lipogenesis [18]. In both settings, Leptin production is stimulated by a positive energy balance, and acts to restore energy homeostasis through suppressing anabolic and boosting catabolic pathways.
To date a number of reports have shown that there is a positive correlation between reduced levels of circulating Leptin and AD risk [19; 20], severity of dementia [21] and cognitive decline [22; 23]. Most notably, a study involving 785 cognitively-normal elderly followed for a median of 8.3 years showed that those with plasma Leptin levels in the lowest quartile at baseline were at four times greater risk for developing AD than those in the highest quartile [24]. At the physiological level, it is known that there is a high concentration of Leptin receptors in the hippocampus [16], which are functional, and direct injection of Leptin in that region can improve memory processing and modulate long term potentiation and synaptic plasticity [25]. Further, Leptin administration improves memory in SAMP-8 mice, an accelerated senescence rodent model that develops amyloid plaques [26]. Moreover, the diabetic/obese db/db mice, which lack a functional Leptin receptor exhibit cognitive impairment and impaired synaptic function and neurogenesis [27]. Interestingly, it has been suggested that one of Leptin’s roles could involve the prevention of excess accumulation of lipids in non-adipocytes, including neurons, which could be poisoning [28].
We have previously reported that Leptin reduces tau phosphorylation and Aβ production in neuronal cells and transgenic mice models of AD [29; 30; 31]. Leptin’s effects in vitro were dependent on activation of the cellular energy sensor, AMP-activated protein kinase (AMPK) [32]. AMPK is ubiquitously expressed throughout the body and is activated in states of low cellular energy by an elevated AMP/ATP ratio [33]. Besides ATP the only other small molecule in cells that indicates energy status is NAD+, which is necessary for activation of a family of evolutionarily conserved energy sensors, the sirtuins (SIRT) [34]. The sirtuins are histone deacetylases that play important roles in a number of physiological processes, including stress resistance [35], replicative senescence [36], aging and differentiation [37]. Notably SIRT1 has been associated with the anti-aging effects of caloric restriction and, most recently, inhibition of amyloidogenic pathways in laboratory models of AD [38; 39; 40]. Additionally, caloric restriction has been shown to indirectly activate SIRT1 through a linear pathway involving AMPK [41]. To this end, we investigated the extent to which activation of cellular energy sensors, involving AMPK and the sirtuins, is involved in Leptin’s beneficial effects on AD-related biochemical pathways.
Materials and Methods
Reagents and Antibodies
Minimum essential medium (MEM) was purchased from ATCC (Manassas, VA). Fetal bovine serum (FBS), all-trans retinoic acid, nicotinamide and recombinant human Leptin were purchased from Sigma-Aldrich (St. Louis, MO). Compound C was purchased from EMD Biosciences (San Diego, CA). Rabbit anti-tau (pThr181) was purchased from Abcam (Cambridge, MA). Tau (tau46) mAb was purchased from Cell Signaling.
Culture and Stable Transfection of Cell Lines
The human neuroblastoma cell line, SH-SY5Y, was purchased from ATCC. Cell culture was performed according to manufacturer’s specific guidelines. Cells were propagated in MEM containing 10% FBS. Neuronal differentiation was performed as described previously [29].
To generate SY5Y stably over-expressing amyloid precursor protein (APP), cells were transfected with a mammalian expression vector encoding the 751 amino acid isoform of human APP (APP751 – Accession # NM_201413) (Origene Technologies; Rockville, MD) using the FuGENE HD transfection reagent, according to manufacturer’s specific instructions (Promega; Madison, WI). Briefly, cells were transiently transfected with APP751 or vehicle for 48 h and then switched into selection medium containing a concentration range of the antibiotic G418 (100–600 μg/mL) to determine the optimal dose for stable selection. Selection media was changed every 3 days to remove non-viable cells. After 3 weeks, 200 μg/mL G418 yielded distinct colonies while all vehicle-transfected cells were non-viable. Cells were maintained in 10% FBS media containing 200 μg/mL G418 for expansion.
Protein Extraction and Immunoblotting
Neuronal cells were treated with Leptin (500 ng/ml) in the presence or absence of nicotinamide (5 mM) or compound C (20 μM) for 6 or 18 h, depending on readout, and then harvested by scraping. Preparation of lysates and immunoblotting were peformed as described [29].
AMPK Activity Assay
AMPK activity in cell lysates was determined using the CycLex AMPK Kinase Assay Kit (MBL International; Woburn, MA), as described previously [42; 43]. Briefly, “relative AMPK activity”, hereafter referred to as “AMPK activity”, is defined as Compound C-sensitive protein kinase activity in cell lysates. Titration of various Compound C doses identified 10 μM as the dose in which there was no further reduction in kinase activity upon increasing concentration (data not shown). Neuronal cell lysates were incubated in the presence or absence of 10 μM Compound C, and protein kinase activity determined by measuring phosphorylation of the Insulin Receptor Substrate-1 (IRS-1) through immunoassay and conversion of a chromogenic substrate at an absorbance of 450 nm (A450). Normalized AMPK activity in the lysates was defined as:
SIRT Activity Assay
“Total sirtuin”, hereafter termed “SIRT”, activity in cell lysates was determined using the HDAC Fluorimetric Cellular Activity Assay Kit (Enzo Life Sciences; Plymouth Meeting, PA), according to manufacturer’s specified guidelines. Briefly, SIRT activity is defined as nicotinamide-sensitive deacetylase (class III HDAC) activity in cell lysates. 5 mM nicotinamide was identified by the manufacturer as a dose in which there was no further reduction in deacetylase activity upon increasing concentration. Neuronal cell lysates were incubated in the presence or absence of 5 mM nicotinamide, and SIRT activity determined by adding the Flour de Lys Substrate for deacetylation followed by exposure to the Fluor de Lys Developer to generate a fluorescent signal for detection using a fluorimeter (Ex. 350–380 nm; Em. 440–460 nm). Normalized SIRT activity in the lysates was measured in units of fluorescence intensity (Fi) and defined as:
ELISAs
Aβ(1–40) levels in cell culture media were determined using the Human β-Amyloid 1–40 ELISA kit (Invitrogen; Carlsbad, CA), and phospho- and total tau levels in cell culture lysates were determined using the Human Tau pSer396, pThr231 and Total Tau ELISA kits (Invitrogen) according to manufacturer’s specific instructions.
Statistical Analyses
Statistical data analyses were performed with analysis of variance and Tukey-Kramer multiple comparisons test. Densitometric analyses were performed using the UN-SCAN-IT gel 6.1 software (Silk Scientific; Orem, UT). p<0.05 was considered statistically significant.
Results
Leptin Activates AMPK and SIRT in Neuronal Cells
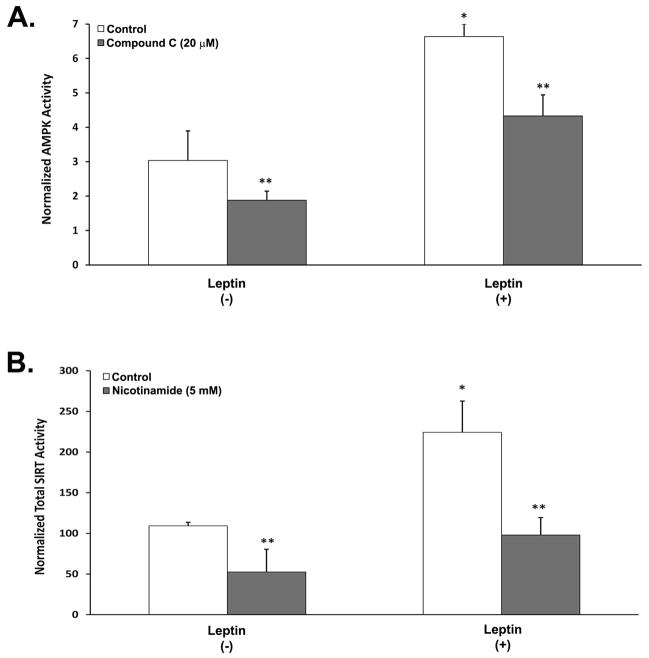
The initial set of studies investigated whether treatment of neuronal cells with Leptin can boost cellular metabolism by directly increasing AMPK kinase activity and total sirtuin (SIRT) deacetylase activity (Figure 1). We have previously shown that Leptin induces phoshorylation of AMPK in neurons [32], but have yet to determine its effects on AMPK kinase activity. SY5Y neuroblastoma cells were differentiated with retinoic acid (RA-SY5Y) and then treated with Leptin for 6 h in the presence or absence of the AMPK inhibitor, compound C, and AMPK activity measured. Leptin produced a significant increase in kinase activity (p<0.05) that was approximately 2-fold greater than in cells treated without Leptin (Fig. 1A, white bars). The increase in kinase activity was attenuated by co-treatment with compound C, which significantly (p<0.05) reduced AMPK activity (right gray bar) to levels similar to the non-treated control group (left white bar).
Fig. 1.
Effect of Leptin on AMPK and SIRT activity in RA-SY5Y. Cells were treated for 6 h with or without Leptin (500 ng/mL) in the presence or absence of the AMPK inhibitor, compound C (20 μM), or SIRT inhibitor, nicotinamide (5 mM), and (A) AMPK or (B) SIRT activity measured. All activity values were normalized to total protein, n=3.
*p<0.05 vs. no Leptin
**p<0.05 vs. control
Leptin has been reported to increase gene expression of SIRT-1 in models of cerebral ischemia [44], yet no studies to date have ascribed an ability of Leptin to increase SIRT deacetylase activity in neurons. Cells treated with Leptin for 6 h showed a significant (p<0.05) increase in total SIRT activity that was approximately 2.5-fold greater than in cells treated without Leptin (Fig. 1B, white bars). The increase in deacetylase activity was blunted by co-treatment with the SIRT inhibitor, nicotinamide, which significantly (p<0.05) reduced SIRT activity (right gray bar) to levels similar to the non-treated control group (left white bar). These results suggest that Leptin can directly stimulate cellular metabolism in neurons through activation of endogenous energy sensors.
Leptin’s Effects on Tau Phosphorylation and 3-Amyloid are Dependent on SIRT and AMPK
The next set of studies examined whether modulating cellular energy metabolism through activation of SIRT and AMPK mediates Leptin’s reducing effects on tau phosphorylation (Figure 2) and β-amyloid production (Figure 3).
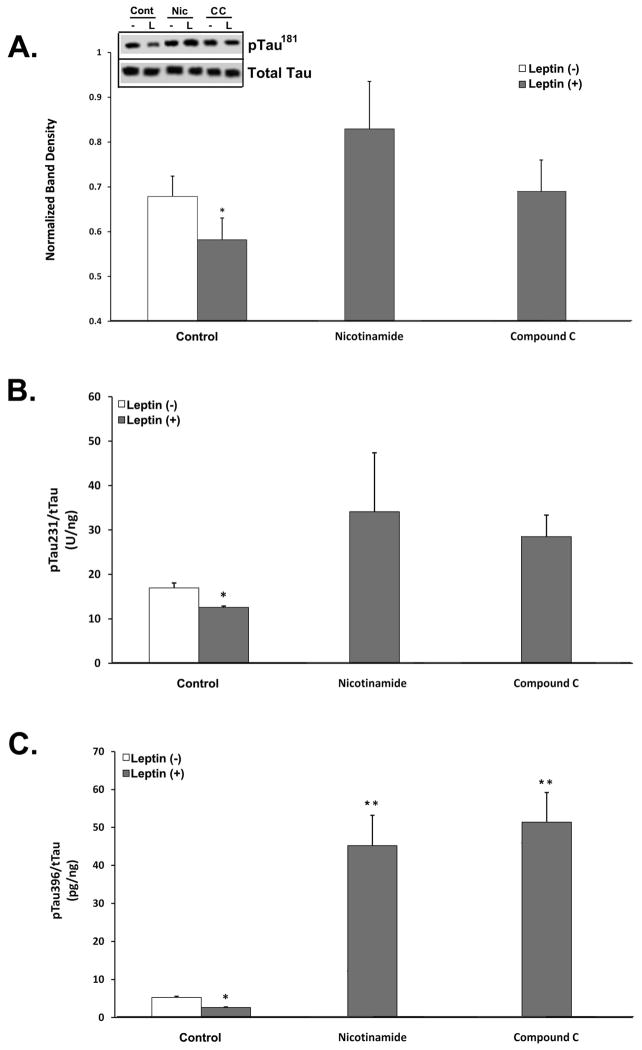
Fig. 2.
Inhibition of AMPK and SIRT negates Leptin’s effect on tau phosphorylation in RA-SY5Y. A. Cells were treated for 6 h with or without Leptin (500 ng/mL) in the presence or absence of nicotinamide (5 mM) or compound C (20 μM), and cell lysates probed for phosphorylated tau181 by immunoblot (inset). Blots were normalized by stripping and reprobing for total tau and densitometry analyses performed, n=3. Extracts from A were assayed by ELISA to quantify total and phosphorylated (B) tau231 and (C) tau396. All concentration values were normalized to total tau, n=3.
*p<0.05 vs. no Leptin
**p<0.05 vs. control
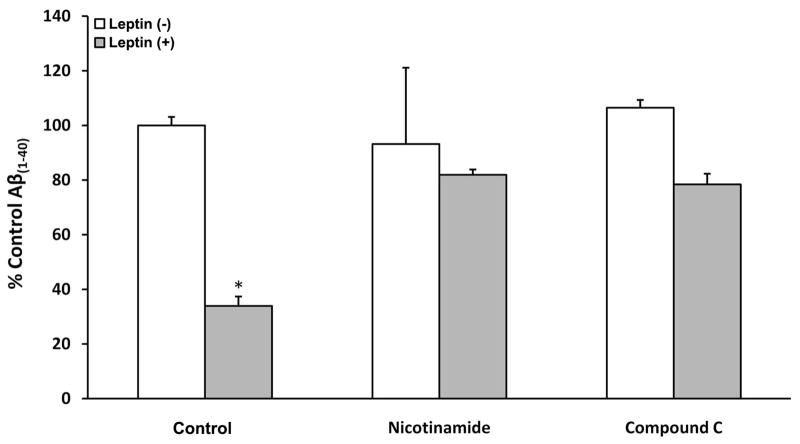
Fig. 3.
Inhibition of AMPK and SIRT negates Leptin’s effect on Aβ(1–40) in RA-SY5Y. Cells were treated overnight with or without Leptin (500 ng/mL) in the presence or absence of nicotinamide (5 mM) or compound C (20 μM), and culture media collected for determination of Aβ(1–40) levels by ELISA. Results were normalized to total protein and presented as a percentage relative to vehicle control, n=3.
*p<0.05 vs. no Leptin
We began by examining three different phospho-epitopes of tau by either immunoblot or ELISA (Fig. 2): pTau181 -used as an AD CSF clinical biomarker- (Fig. 2A) ; pTau231 – phosphoepitope within the microtubule-binding domain of tau and involved in microtubule destabilization (Fig. 2B); and pTau396 – implicated in oligomeric tau formation-(Fig. 2C). The levels of these specific phosphorylated tau forms were measured in RA-SY5Y following treatment with Leptin for 6 h, in the presence or absence of nicotinamide (middle group) or compound C (right group).. Leptin-treated cells in the absence of SIRT or AMPK inhibition showed a significant (p<0.05) reduction in all examined phosphor-tau species (left gray bars). However, co-treatment with either inhibitor negated these effects and returned phospho-tau to levels observed for the control group (left white bars). Interestingly, inhibition of SIRT or AMPK significantly (p<0.05) increased tau396 phosphorylation compared to control, independent of Leptin treatment (Fig. 2C).
Next, we investigated whether inhibition of SIRT or AMPK also blunted Leptin’s ability to impede production of β-amyloid in neurons (Figure 3). SY5Y stably expressing human APP751 (SY5Y-APP751) were treated with Leptin for 18 h in the presence or absence of nicotinamide (middle group) or compound C (far right group) and the amount of Aβ(1–40) present in culture media was measured by ELISAs. Similar to the results observed for phosphorylation of tau (Figure 2), Leptin-treated cells in the absence of inhibitors showed a significant (p<0.05) reduction in Aβ(1–40) (left gray bar), while co-treatment with either inhibitor (middle and right gray bars) reversed these effects and returned Aβ(1–40) to levels observed for the vehicle group (left white bar).
In summary, our findings suggest that Leptin’s ability to reduce tau phosphorylation and β-amyloid in neurons is mediated through activation of the endogenous energy sensors AMPK and the sirtuins.
Discussion
The link between dysfunctional brain energy metabolism and AD has become progressively clearer with the advent of sophisticated technologies that enable functional neuroimaging. Studies using FDG-PET have illustrated that areas of the brain sequentially presenting with the AD pathology are preceded by a matching distribution of regional hypometabolism. The etiologic basis for such cascade of events in AD is not entirely clear. It is possible that metabolic diseases, such as obesity and diabetes may independently contribute, as they are commonly detected as co-morbidities with AD. For example, these diseases are marked by central resistance to hormones involved in lipid and glucose metabolism, respectively. Administration of insulin-sensitizing agents, such as rosiglitazone, or central delivery of Leptin has proven beneficial in improving cognitive performance in animal models of AD [31; 45]. In obesity and diabetes, deprivation of these hormones’ central actions over a number of years could negate their preventative effects on AD pathophysiological pathways, albeit directly or indirectly.
Presently we report on Leptin’s ability to modulate AD pathways through activation of endogenous cellular energy sensors in neurons. Leptin activated the AMP- and NAD+-dependent sensors, AMPK and SIRT, respectively, in RA-SY5Y. Leptin’s ability to reduce both tau phosphorylation and Aβ(1–40) production was sensitive to AMPK and SIRT pharmacological inhibition – suggesting a role for these enzymes in mediating Leptin’s effects.
Prolonged states of positive energy balance, as observed in obesity, inhibit brain AMPK and SIRT1 activity [46; 47]. The contribution of chronic inhibition of these energy sensors towards increased AD risk is unknown; however, dysfunction of the Leptin system in obesity may abrogate its preventative effects on AD pathways via decreased activity of these enzymes.
We did observe that inhibition of AMPK and SIRT with compound C and nicotinamide, respectively, in the absence of Leptin, produced an increase in tau phosphorylation compared to vehicle (Figure 2). One potential explanation for both is that they are inhibiting baseline enzyme activities, which normally could keep phosphorylation of tau at check. For example, acetylation of tau increases the stability of phosphorylated tau and contributes to tauopathy [48]. SIRT1 has been shown to deacetylate phosphorylated tau to facilitate its degradation, and its expression to correlate inversely with the accumulation of tau in AD [48; 49]. Additionally, SIRT1 has been shown to suppress β-amyloid production by activating the alpha secretase pathway [39].
In summary, we have demonstrated that Leptin boosts the activity of both cellular energy sensors, AMPK and SIRT, in a differentiated neuroblastoma cell line. These findings shall be investigated further using primary neurons harvested from various areas of the brain including hippocampus, cortex and hypothalamus, as well as transgenic animals to determine if Leptin’s beneficial effects in AD models [31] are related to this modulation.
Leptin treatment of neuronal cells reduces Aβ & phospho-tau levels via AMPK and SIRT1
Metabolic disturbances in neurons could precede pathological changes in AD
MPK or SIRT1 could be drug targets for AD
Acknowledgments
This work was supported by the National Institute on Aging (SBIR –1R43AG029670).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 10:187–98. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fouquet M, Desgranges B, Landeau B, Duchesnay E, Mezenge F, de la Sayette V, Viader F, Baron JC, Eustache F, Chetelat G. Longitudinal brain metabolic changes from amnestic mild cognitive impairment to Alzheimer’s disease. Brain. 2009;132:2058–67. doi: 10.1093/brain/awp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, Pirraglia E, De Santi S, Reisberg B, Wisniewski T, de Leon MJ. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36:811–22. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaManna JC, Harik SI. Regional comparisons of brain glucose influx. Brain Res. 1985;326:299–305. doi: 10.1016/0006-8993(85)90039-3. [DOI] [PubMed] [Google Scholar]
- 6.Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiology of aging. 2004;25:641–50. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Roses AD, Lutz MW, Amrine-Madsen H, Saunders AM, Crenshaw DG, Sundseth SS, Huentelman MJ, Welsh-Bohmer KA, Reiman EM. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. The pharmacogenomics journal. 2010;10:375–84. doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winocur G, Greenwood CE. The effects of high fat diets and environmental influences on cognitive performance in rats. Behav Brain Res. 1999;101:153–61. doi: 10.1016/s0166-4328(98)00147-8. [DOI] [PubMed] [Google Scholar]
- 9.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 10.Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997;17:1046–54. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukkur EA, Shimohata A, Akagi T, Yu W, Yamaguchi M, Murayama M, Chui D, Takeuchi T, Amano K, Subramhanya KH, Hashikawa T, Sago H, Epstein CJ, Takashima A, Yamakawa K. Mitochondrial dysfunction and tau hyperphosphorylation in Ts1Cje, a mouse model for Down syndrome. Hum Mol Genet. 2006;15:2752–62. doi: 10.1093/hmg/ddl211. [DOI] [PubMed] [Google Scholar]
- 12.Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol. 1987;42:78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- 13.Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–94. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morton GJ. Hypothalamic leptin regulation of energy homeostasis and glucose metabolism. J Physiol. 2007;583:437–43. doi: 10.1113/jphysiol.2007.135590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–9. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 16.Huang XF, Koutcherov I, Lin S, Wang HQ, Storlien L. Localization of leptin receptor mRNA expression in mouse brain. Neuroreport. 1996;7:2635–8. doi: 10.1097/00001756-199611040-00045. [DOI] [PubMed] [Google Scholar]
- 17.Heshka JT, Jones PJ. A role for dietary fat in leptin receptor, OB-Rb, function. Life Sci. 2001;69:987–1003. doi: 10.1016/s0024-3205(01)01201-2. [DOI] [PubMed] [Google Scholar]
- 18.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–83. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsson T, Nasman B, Rasmuson S, Ahren B. Dual relation between leptin and cortisol in humans is disturbed in Alzheimer’s disease. Biol Psychiatry. 1998;44:374–6. [PubMed] [Google Scholar]
- 20.Power DA, Noel J, Collins R, O’Neill D. Circulating leptin levels and weight loss in Alzheimer’s disease patients. Dement Geriatr Cogn Disord. 2001;12:167–70. doi: 10.1159/000051252. [DOI] [PubMed] [Google Scholar]
- 21.Ray S, Wyss-Coray A. Methods and Compositions for Diagnosis, Stratification, and monitoring of Alzheimer’s Disease and other neurological disorders in body fluids. WO 2005/052592 A2. Satoris, Inc; 2005
- 22.Holden KF, Lindquist K, Rosano C, Tylavsky FA, Harris TB, Yaffe K. Low Serum Leptin is Associated with Poor Cognitive Performance in the Elderly, American Academy of Neurology. 58th Annual Meeting; San Diego. 2006. p. S41.006. [Google Scholar]
- 23.Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, Seshadri S. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA : the journal of the American Medical Association. 2009;302:2565–72. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey J, Shanley LJ, O’Malley D, Irving AJ. Leptin: a potential cognitive enhancer? Biochem Soc Trans. 2005;33:1029–32. doi: 10.1042/BST20051029. [DOI] [PubMed] [Google Scholar]
- 26.Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–5. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nature neuroscience. 2008;11:309–17. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unger RH. Longevity, lipotoxicity and leptin: the adipocyte defense against feasting and famine. Biochimie. 2005;87:57–64. doi: 10.1016/j.biochi.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Greco SJ, Sarkar S, Johnston JM, Zhu X, Su B, Casadesus G, Ashford JW, Smith MA, Tezapsidis N. Leptin reduces Alzheimer’s disease-related tau phosphorylation in neuronal cells. Biochem Biophys Res Commun. 2008;376:536–41. doi: 10.1016/j.bbrc.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer’s Abeta. Faseb J. 2004;18:1870–8. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- 31.Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N, Casadesus G. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2010;19:1155–67. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greco SJ, Sarkar S, Johnston JM, Tezapsidis N. Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells. Biochem Biophys Res Commun. 2009;380:98–104. doi: 10.1016/j.bbrc.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 34.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 35.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–2. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 36.Chua KF, Mostoslavsky R, Lombard DB, Pang WW, Saito S, Franco S, Kaushal D, Cheng HL, Fischer MR, Stokes N, Murphy MM, Appella E, Alt FW. Mammalian SIRT1 limits replicative life span in response to chronic genotoxic stress. Cell metabolism. 2005;2:67–76. doi: 10.1016/j.cmet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annual review of biochemistry. 2004;73:417–35. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 38.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 39.Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–32. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Bonda DJ, Lee HG, Camins A, Pallas M, Casadesus G, Smith MA, Zhu X. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet neurology. 2011;10:275–9. doi: 10.1016/S1474-4422(11)70013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Developmental cell. 2008;14:661–73. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Nishi M, Doi A, Shono T, Furukawa Y, Shimada T, Furuta H, Sasaki H, Nanjo K. Ghrelin inhibits insulin secretion through the AMPK-UCP2 pathway in beta cells. FEBS letters. 2010;584:1503–8. doi: 10.1016/j.febslet.2010.02.069. [DOI] [PubMed] [Google Scholar]
- 43.Yeh CH, Chen TP, Wang YC, Lin YM, Fang SW. AMP-activated protein kinase activation during cardioplegia-induced hypoxia/reoxygenation injury attenuates cardiomyocytic apoptosis via reduction of endoplasmic reticulum stress. Mediators of inflammation. 2010;2010:130636. doi: 10.1155/2010/130636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avraham Y, Davidi N, Porat M, Chernoguz D, Magen I, Vorobeiv L, Berry EM, Leker RR. Leptin reduces infarct size in association with enhanced expression of CB2, TRPV1, SIRT-1 and leptin receptor. Current neurovascular research. 2010;7:136–43. doi: 10.2174/156720210791184943. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Experimental neurology. 2006;199:265–73. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 46.Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. Journal of neurochemistry. 2009;109(Suppl 1):17–23. doi: 10.1111/j.1471-4159.2009.05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R. Brain SIRT1: anatomical distribution and regulation by energy availability. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:9989–96. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–66. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Julien C, Tremblay C, Emond V, Lebbadi M, Salem N, Jr, Bennett DA, Calon F. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. Journal of neuropathology and experimental neurology. 2009;68:48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]