Abstract
Toll-like receptor (TLR) agonists are currently being examined as adjuvants for vaccines, with several lead candidates now in licensed products or in late-stage clinical development. Guinea pigs are widely used for preclinical testing of drugs and vaccines; however, evaluation of TLR agonists in this model is hindered by the limited availability of immunological tools and reagents. In this study, we validated the use of a branched-chain DNA (bDNA) assay known as the QuantiGene Plex 2.0 Reagent System for measuring innate cytokine and chemokine mRNA levels following TLR stimulation of guinea pig cells. Gene expression for T-helper-1 (Th1) polarizing cytokines (TNF-α, IL-1β, IL-12) and chemokines (CXCL1, CCL2) was upregulated following ex vivo stimulation of guinea pig splenocytes and whole blood with TLR-4 or TLR-7/8 agonists. These data confirm the utility of the QuantiGene system both as an alternative to RT-PCR for measuring transcript levels and as a high-throughput screening tool for dissecting the immunological response to TLR stimulation in guinea pigs. Overall, the QuantiGene platform is reliable, reproducible, and sensitive. These agonists have the potential to be used as adjuvant components in vaccines against various pathogens.
1. INTRODUCTION
Microbial detection by the host innate immune system hinges on pattern recognition receptors (PRRs), which sense conserved structural motifs, or pathogen-associated molecular patterns (PAMPs), common to many groups of microbes. One PRR family member is the Toll-like receptor (TLR) family, an evolutionarily conserved group of cell surface and intracellular molecules important for recognition of foreign lipids, lipoproteins, nucleic acids, and proteins. They play a fundamental role in pathogen recognition and mediate the production of cytokines and chemokines necessary for the development of effective immunity. At least 10 different TLRs and their ligands have been identified in humans (Zarember and Godowski, 2002; O’Neill, 2004). Both natural and synthetic ligands for well-defined PRRs are being evaluated as adjuvants. TLR-4 agonists such as bacterial lipopolysaccharides (LPS) have long been recognized as potent adjuvants. Monophosphoryl lipid A (MPL), a detoxified derivative of LPS, is now a component of licensed vaccines for Hepatitis B and papilloma virus and has proven to be both safe and effective (Giannini et al., 2006; Casella and Mitchell, 2008). TLR-7 and TLR-8 are expressed in endosomes and recognize guanosine- and uridine-rich ssRNA. TLR-7/8 agonists may be ideal for generating protective immune responses against intracellular pathogens and could effectively adjuvant vaccines designed to generate specific T-helper-1 (Th1) cells.
Quantification methods for gene expression have become an important tool both for evaluating the utility of novel TLR adjuvants and for dissecting their underlying immunological effects. Reverse-transcriptase PCR (RT-PCR) assays provide a “gold standard” approach (McMurray et al., 2005; Ly et al., 2008). RT-PCR is widely used for mRNA quantification and for examining changes in genetic expression over time. Conventional RT-PCR techniques, however, are time-consuming, entail tedious RNA purification protocols, and are encumbered by the need for costly equipment, personnel, and strict probe design criteria.
Here we validate an alternative gene expression profiling assay based on the QuantiGene Plex 2.0 platform (Affymetrix, Fremont, CA). The QuantiGene system is a sandwich nucleic acid hybridization method that uses branched DNA (bDNA) technology to directly quantify nucleic acid molecules at physiological levels. The basic approach is similar to that of an enzyme-linked immunosorbent assay (ELISA), whereby a target molecule is captured and detected by reporter signal. bDNA technology combines this approach with signal amplification chemistry to enable detection at a lower limit of ≤1,000-2,000 transcripts per well. Instead of using one kind of capture molecule which allows for detection of only a single target in a sample at a time, QuantiGene uses the convenience of a plexable bead-based platform to raise the muliplexity of the target analytes. Nucleic acid purification is also not a requirement, making QuantiGene an attractive option for high-throughput screening. bDNA technology has been validated extensively with human samples (Warrior et al., 2000; Canales et al., 2006; Knudsen et al., 2008) and is the basis of several clinically proven diagnostic viral load tests, including those for HIV-1 (Pachl et al., 1995; Todd et al., 1995; Kern et al., 1996; Nolte et al., 1998; Lubelchek et al., 2009; Pyne et al., 2009) and Hepatitis C (Alter et al., 1995; Detmer et al., 1996; Mayerat et al., 1996; Trimoulet et al., 2002; Elbeik et al., 2004).
We applied the bDNA signal amplification approach to detect innate cytokine and chemokine expression responses following TLR stimulation of guinea pig splenocytes and whole blood. The guinea pig (Cavia porcellus) is a widely accepted experimental animal model for studying infectious diseases and for assessing the safety and potency of candidate drugs, vaccines, and biological agents for use in humans. Dissecting the immunological response to experimental vaccines and to innate-inducing factors such as TLR agonists has been hampered by the dearth of suitable reagents and assays. Our ex vivo studies demonstrate that guinea pigs are responsive to the synthetic TLR-4 agonist glucopyranosyl lipid adjuvant (GLA-AF), to the TLR-7 agonist imiquimod (IMQ), to the TLR-7/8 agonists gardiquimod (GDQ) and resiquimod (R848), and to the TLR-8 agonist (CL075). Increases in transcriptional activity were observed for several innate Th1-polarizing cytokines, including chemokines involved in leukocyte and lymphocyte recruitment to sites of inflammation. These data demonstrate that QuantiGene is a reliable and sensitive assay for the detection of TLR-induced cytokine responses in guinea pigs. Several of these TLR agonists may represent potent additions to adjuvant formulations, given their ability to upregulate important innate immune responses in this animal model.
2. MATERIALS AND METHODS
2.1. Animals
Female Hartley guinea pigs (700-800g) were purchased from Charles River (Wilmington, MA). Animals were housed at the Infectious Disease Research Institute (IDRI) animal facility under specific pathogen-free conditions. All procedures were performed in accordance with the regulations and guidelines of the IDRI animal care and use committee.
2.2. Toll-Like Receptor (TLR) Agonists
TLR-4
The synthetic TLR-4 agonist, glucopyranosyl lipid adjuvant (GLA) was bulk manufactured by Avanti Polar Lipids, Inc. (Alabaster, AL) and formulated in our laboratory as an aqueous suspension (AF). GLA-AF is a hexaacylated lipid A derivative shown previously to have immunomodulatory activity similar to monophosphoryl lipid A (MPL) (Coler et al., 2011). Lipopolysaccharide (LPS) is a well-described and potent TLR-4 activator and was incorporated as a positive control. An ultra-pure lyophilized LPS preparation from the 0111:B4 strain of Escherichia coli was purchased from InvivoGen (San Diego, CA). All agonists were rehydrated in sterile, endotoxin-free water, vortexed, and stored at −20°C.
TLR-7/8
Resiquimod (R848), gardiquimod (GDQ), and imiquimod (IMQ) are synthetic imidazoquinoline compounds that activate cells expressing either TLR-7 and/or TLR-8. Resiquimod (R848) is a selective ligand to both TLR-7 and -8, whereas gardiquimod (GDQ) and imiquimod (IMQ) induce activation primarily through TLR-7. At high concentrations, GDQ slightly activates TLR-8. CL075, a thiazoloquinoline derivative, predominantly recognizes TLR-8, although low-level activation through TLR-7 is achievable at higher doses. All TLR-7/8 ligands were purchased from InvivoGen (San Diego, CA), except for gardiquimod (GDQ) which was purchased from Alexis Biochemicals (Plymouth Meeting, PA). Lyophilized products were rehydrated in sterile, endotoxin-free water with some heating (InvivoGen, San Diego, CA) and vortexed until complete solubilization was reached. Stock solutions were stored at −20°C.
2.3. Isolation and TLR Stimulation of Guinea Pig Spleens
Spleens were collected from guinea pigs and homogenized in 5 mL of complete RPMI (cRPMI) through a 3 μm cell strainer to obtain single cell suspensions. After a brief centrifugation, the pellet was re-suspended in red blood cell lysis buffer (2 mL) (eBioscience, San Diego, CA). Lysates were washed twice in cRPMI (40 mL) and centrifuged at 1200 rpm. The pellet was resuspended in 10 mL cRPMI for quantification and viability testing on a Guava ViaCount (Hayward, CA). After a final spin, cells were re-suspended in an appropriate volume of cRPMI to achieve a 1 × 106 cells/mL ‘master’ suspension. Splenocytes were plated at 5 × 105 cells/well by diluting 500 μL of the ‘master’ suspension in 500 μl of agonist: TLR-4 (GLA-AF), TLR-7 (IMQ), TLR-7/8 (R848, GDQ) and TLR-8 (CL075). Each stimulant was plated at three concentrations (1, 0.1, and 0.01 μg/mL) in duplicate and incubated with cells in 24-well plates (37°C, 4 h). PHA (100 μg/mL), LPS (0.01 μg/mL), and media controls were included. Next, plates were centrifuged (1200 rpm, 10 min) and supernatants were removed. 350 μL of RNeasy lysis buffer (RLT) (Qiagen, Valencia, CA) containing 1% beta-mercaptoethanol was added to cells. Samples were transferred to 1.5 mL RNase-free tubes, snap frozen in liquid nitrogen, and stored at −80°C until further analysis.
2.4. Isolation and TLR Stimulation of Guinea Pig Whole Blood
The same guinea pigs were terminally bled via cardiocentesis. Each guinea pig yielded approximately 24 mL of blood, collected in 50 mL heparinized conical tubes. Heparinized blood (450 μL) was placed in 5 mL polypropylene tubes containing 50 μL of each TLR agonist at three doses (1, 0.1, and 0.01 μg/mL). Whole blood stimulations were performed in duplicate at 37°C for 4 h. PHA (100 μg/mL), LPS (0.01 μg/mL), and media were included as control stimulants.
2.5. QuantiGene Plex 2.0 Reagent System - Branched DNA signal amplification (bDNA)
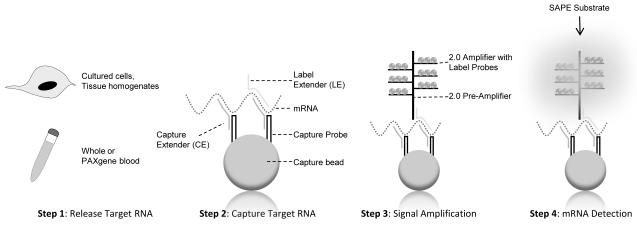
QuantiGene analysis was performed on purified RNA from TLR-stimulated guinea pig splenocytes or on stimulated whole blood without RNA purification. A schematic of the bDNA system is illustrated in Figure 1. All reagents were provided in the QuantiGene Sample Processing Kit Package purchased through Affymetrix (Fremont, CA).
Figure 1. QuantiGene Plex Reagent 2.0 Platform.
Samples are processed to release RNA (Step 1). The QuantiGene system does not require RNA purification. Target transcripts are bound to fluorescent microspheres via a Capture Extender (CE) - Capture Probe (CP) interaction (Step 2). Signal is amplified by the sequential hybridization of the 2.0 Pre-Amplifier, the 2.0 Amplifier, and the Biotinylated Label Probes to the tails of the LEs (Step 3). In the final step, a chemilumigenic substrate such as streptavidin-conjugated R-Phycoerythrin (SAPE) is added and the resulting fluorescence is measured on a Luminex flow instrument (Step 4). This figure was adapted from the QuantiGene Plex 2.0 user manual (Affymetrix, Fremont, CA).
Step 1: Sample Preparation and Release of Target RNA
RNA was isolated from thawed spleen lysates using the RNeasy Kit, according to the manufacturer’s instructions (Qiagen, Valencia, CA). Eluted samples were digested using two units of Turbo DNAse (Ambion, Austin, TX) in a total volume of 100 μL for 1 hr at 37°C. RNA was then purified using the MinElute RNA clean-up kit (Qiagen, Valencia, CA). The purity and concentration of each RNA sample was assessed by observing the 260/280 nm absorbance ratio on a NanoDrop full-spectrum spectrophotometer (Wilmington, DE).
For the whole blood assay, freshly stimulated blood was combined with PAXgene reagent for 2-3 h (Qiagen, Valencia, CA). PAXgene RNA Tubes contain a proprietary RNA stabilizing reagent that prevents degradation by RNases and minimizes ex vivo changes in gene expression. PAXgene blood was centrifuged (3000 g, 5 min), supernatant was removed, and the pellet was resuspended in a lysis buffer containing 68 μL QuantiGene lysis mixture, 124 μL nuclease-free dH20, and 8 μL proteinase K (60°C, 1 h).
Step 2: Capture Target RNA
To capture target RNA, a master “Working Bead Mix” was made in accordance with the manufacturer’s instructions (Affymetrix, Fremont, CA). Two principal reagents within this mix are the Capture Beads and the 2.0 Probe Set. Each capture bead is a fluorescent microsphere that binds specific RNA molecules. As many as 100 distinct assays within a single sample can be measured simultaneously given the availability of many uniquely dyed beads. A guinea pig 7-plex was used to capture message for several cytokines and chemokines (TNF-α, IL-1β, CXCL1, CCL2, IL-12b) and house-keeping genes (β-actin and GAPDH) (Affymetrix, Fremont, CA). IL-12b encodes the common p40 subunit of IL-12/IL-23. The 2.0 Probe Set consists of several synthetic probes, including Capture Extenders (CEs) and Label Extenders (LEs). CEs capture and tether target RNA to a specific bead via cooperative hybridization. The “Working Bead Mix” also contains lysis mixture, blocking reagent, and water. For capture of RNA in whole blood lysates, proteinase K was added.
Assemblage of the “Working Bead Mix” varied slightly depending on the source material. To capture purified RNA, the capture beads (1 μl) and 2.0 Probe Set (5 μl) were combined with blocking reagent (2 μl), lysis mixture (33.3 μl), and nuclease-free water (38.7 μl) (total volume 80 μl), according to the manufacturer’s instructions (Affymetrix, Fremont, CA). Purified RNA (300 ng in 20 μl) was combined with 80 μl bead mix in a 96-well “hybridization” plate (Affymetrix, Fremont). To capture RNA from whole blood lysates, the capture beads (1 μl) and 2.0 Probe Set (5 μl) were combined with blocking reagent (2 μl), lysis mixture (6.6 μl), proteinase K (0.2 μl), and nuclease-free water (5.2 μl) in 20 μl total volume. For every 20 μl of bead mix, 80 μL of cell lysate was added. Plates were pressure sealed and incubated overnight in an environmental shaker (54°C, 600 rpm) (VorTemp, Labnet Int. Inc., Woodbridge, NJ).
Step 3: Signal Amplification
Signal amplification was mediated by the sequential hybridization of the 2.0 Pre-Amplifier, the 2.0 Amplifier, and the Biotinylated Label Probes. Each Pre-Amplifier is a DNA amplification molecule that hybridizes to the tails of the LEs. Signal amplification was achieved by hybridization of multiple amplifier and biotinylated label probes to each pre-amplification unit.
Signal amplification was completed with minimum alteration to the manufacturer’s protocol (Affymetrix, Fremont, CA). The hybridization mixture (Step 2) was centrifuged at room temperature (RT) (240 × g, 1 min) and transferred to a pre-wet filter plate. Unbound sample was washed (200 μl wash buffer) and filtered using a vacuum manifold. This process was repeated for a total of three washes. The 2.0 Pre-Amplifier Working Reagent (100 μl) was added and sealed. The bottoms of each plate were blotted with a clean paper towel to avoid fluid weeping and incubated for 1 hr in a shaker incubator (50°C, 600 rpm). Next, the plates were washed three times with wash buffer (200 μl). The 2.0 Amplifier Working Reagent (100 μl) was added. Again, the plates were sealed, blotted, and incubated for 1 hr in a VorTemp shaker (50°C, 600 rpm). The final signal amplification step involved another three washes (200 μl wash buffer) followed by the addition of the Label Probe Working Reagent (100 μl). Plates were sealed, blotted, and incubated for an additional hour at 50°C, 600 rpm on a shaker platform.
Step 4: Detection of RNA Targets
The addition of Streptavidin-conjugated R-Phycoerythrin (SAPE) in the final stage results in a fluorescent signal that can be detected on a Luminex 100/200 flow analyzer (Austin, TX). Labeled plates were washed three times (200 μl wash buffer) and SAPE Working Reagent (100 μl) was added. Plates were sealed, blotted, wrapped in foil, and incubated at RT on a shaker (30 min, 600 rpm). Unbound SAPE was removed with SAPE Wash Buffer (200 μl) for a total of two washes. Prior to analysis, 130 μl of SAPE Wash Buffer was added with shaking for 2-5 min, RT. Signal is reported as median fluorescence intensity (MFI) and is proportional to the number of target RNA molecules captured in a sample.
2.6. Real-Time Quantitative RT-PCR
Real-time quantitative RT-PCR analysis for TNF-α and IL-12b message was performed. RNA was purified from TLR-stimulated splenocytes and whole blood, as described previously (refer Step 1 of QuantiGene Platform). Complementary DNA (cDNA) was generated with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). RT-PCR was performed in accordance with the TaqMan Universal PCR Master Mix protocol (Applied Biosystems, Foster City, CA). In a single reaction, 1 μl of probe-primer set (Applied Biosystems, Foster City, CA), 10 μl TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA), and 10 μl cDNA (equivalent to 10-20 ng of RNA) were mixed with nuclease-free water (20 μL total volume). Reactions were analyzed on a Roche Light cycler in a 386-well plate under the following cycle conditions: 95°C, 10 min; 45 cycles of 95°C, 15 s; and 60°C, 1 min. Guinea pig primer and probe sequences for TNF-α or IL-12b are provided (Table 1).
Table 1.
RT-PCR Guinea Pig Primer Sequences
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| TNF-α | GCCCTCCTGGCCAATGG | ATCCGAAGGCACCACCAG |
| IL-12b | GGAGTGACTTGTGGAGAAGCA | TGACACTCCACGGAGTACTTATACT |
| β-actin | CGGGACCTGACAGACTACCT | GCCGTGGTGGTGAAACTGTA |
Sequences (5′→3′) of the primers and probes used for detection of mRNA specific for cytokines and housekeeping genes by real-time PCR in guinea pig spleenocytes and whole blood.
3. RESULTS
3.1. bDNA Signal Amplification Using Purified RNA from Guinea Pig Splenocytes
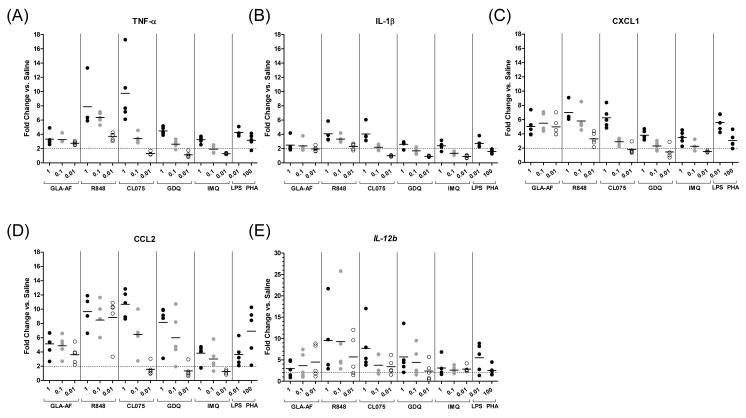
Initial assay performance for the QuantiGene Plex 2.0 platform was evaluated in the guinea pig model on TLR-stimulated spleen cells. To improve the likelihood of measuring a response, RNA was first purified similar to RT-PCR, a gold standard for measuring transcriptional activity. To optimize assay conditions, splenocytes from five guinea pigs were stimulated at three doses of agonist (1, 0.1, 0.01 μg/mL). Agonists to TLRs 4 (glucopyranosyl lipid adjuvant, or GLA-AF) and 7/8 (resiquimod, or R848; CL075; gardiquimod, or GDQ; imiquimod, or IMQ) were tested given their high expression levels on guinea pig cells. Phytohemagglutinin (PHA) and lipopolysaccharide (LPS) were included as positive controls. TNF-α, IL-1β, CXCL1, CCL2, and IL-12b transcripts were quantified using a custom-synthesized assay panel (Affymetrix, Fremont, CA). Included in this panel were two house-keeping genes, β-actin and GAPDH. For each target gene, the amount of RNA measured was normalized to the average of the β-actin and GAPDH housekeeping genes. Data is presented as the fold-induction in transcriptional activity over saline. The performance of this QuantiGene plex on TLR-stimulated guinea pig splenocytes is shown in Figure 2 and Supplemental Figure 1.
Figure 2. QuantiGene Assay on Splenocytes Using Purified RNA.
Dot plots showing relative gene expression, using RNA isolated from stimulated splenocytes. Message was quantified for several cytokine and chemokine analytes: (A) TNF-α, (B) IL-1β, (C) CXCL1, (D) CCL2, and (E) IL-12b, following stimulation with the TLR-4 agonist GLA-AF or with one of several TLR-7/8 agonists (R848, CL075, GDQ, and IMQ) at three doses (1, 0.1, and 0.01 μg/mL). LPS (0.01 μg/mL) and PHA (100 μg/mL) were included as positive controls. The amount of RNA measured for each gene was normalized to the average of two housekeeping genes, β-actin and GAPDH, and compared to saline to calculate the relative proportion of transcripts. Responses below a 2-fold induction relative to saline are indicated by the horizontal dotted line. Means are indicated by short horizontal bars (n=5 guinea pig spleens).
Measurable increases in TNF-α, IL-1β, CXCL1, CCL2, and IL-12b mRNA expression were observed for all subject samples as early as 4 h following TLR stimulation (Figures 2A-E, Supplemental Figure 1). GLA-AF, a synthetic TLR-4 agonist, induced on average ~2- to 5-fold increases in mRNA expression for all analytes at all doses. The highest responding cells were those stimulated with 1 μg/mL of TLR-7/8 agonists R848 and CL075, which induced on average ~8-10 fold higher TNF-α, CCL2, and IL-12b message and ~6-7 fold higher CXCL1 message compared to saline. At the highest dose of GDQ and IMQ (1 μg/mL), expression of TNF-α, CXCL1, and IL-12b was 3-6-fold higher on average than saline-treated controls (Figures 2A, 2C, 2E). CCL2 expression was responsive (~6-8 fold increase) to GDQ treatment at the highest agonist concentrations (0.1-1 μg/mL) (Figure 2D). Modest 2-4-fold gains in IL-1β transcript were observed across most agonists at 1 μg/mL (Figure 2B). In general, responses were higher with greater concentrations of stimulant (0.1-1 μg/mL) compared to the lower 0.01 μg/mL dosage. At the lowest concentration, particularly with the CL075, GDQ and IMQ agonists, TLR stimulation induced responses that fell below an arbitrary 2-fold cutoff relative to saline-treated controls. These ex vivo studies demonstrate proof in principle that (1) guinea pig splenocytes can be stimulated with a variety of TLR-4/7/8 agonists to induce acute cytokine responses, and (2) transcriptional message can be measured in high throughput using the QuantiGene system.
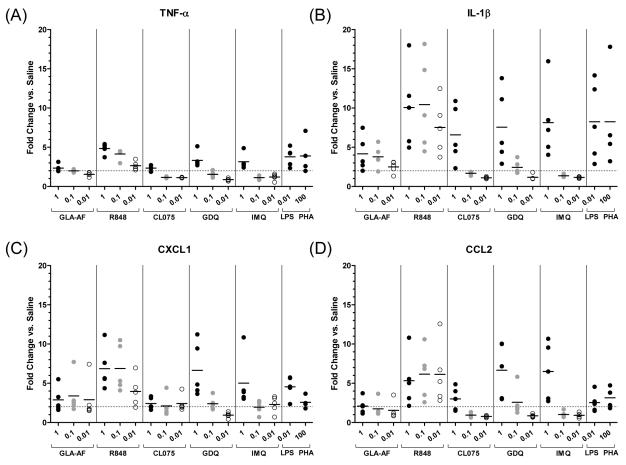
3.2. QuantiGene by Whole Blood Assay (WBA) without RNA Purification
To investigate the applicability of the branched DNA platform to other organ and tissue systems, QuantiGene performance was next evaluated on whole blood from the same five guinea pigs (Figure 3, Supplemental Figure 2). The whole blood assay was also performed without RNA extraction to examine the requirement for RNA purification. Blood was plated with three concentrations of agonist (1, 0.1, 0.01 μg/mL) and assayed for transcriptional activity using the plexed panel described previously. By whole blood assay, upregulation in mRNA expression was observed in response to all TLR agonists relative to saline (Figures 3). GLA-AF-induced responses were generally lower compared to TLR-7/8-induced responses. At lower doses of agonist, particularly with TLR-7/8 agonists CL075, GDQ, and IMQ, responses again fell below a 2-fold induction cutoff for most analytes. On average, a 4-fold increase in IL-1β transcript was observed following TLR-4 GLA-AF stimulation, and 6-10-fold higher IL-1β transcript was measured following stimulation with each TLR-7/8 agonist at the highest dose concentration (1 μg/mL) (Figure 3B). Induction of CXCL1 and CCL2 message followed a similar trend (Figures 3C and 3D). GLA-AF induced 2-3-fold higher levels of CXCL1 and CCL2, while TLR-7/8 agonists, with the exception of CL075, induced upward of 5-7-fold higher expression levels relative to saline. TNF-α and IL-12 responses were consistently low across all agonists and doses, with modest 2-4-fold gains in TNF-α activity (Figure 3A) and negligible changes in IL-12b activity (data not shown). These results demonstrate that QuantiGene is flexible and applicable to different tissue types including whole blood. Also, message for diverse analytes were measured in the absence of RNA purification, demonstrating high assay sensitivity.
Figure 3. QuantiGene Whole Blood Assay (WBA).
QuantiGene analysis was performed directly on stimulated whole blood lysates without RNA purification. Gene expression was quantified for the same cytokines and chemokines described previously: (A) TNF-α, (B) IL-1β, (C) CXCL1, and (D) CCL2. Data for IL-12b gene expression is not shown. Whole blood stimulations were performed with TLR-4/7/8 agonists (GLA-AF, R848, CL075, GDQ, and IMQ) at three doses (1, 0.1, and 0.01 μg/mL). LPS (0.01 μg/mL) and PHA (100 μg/mL) were included as controls. Data were normalized to the average of the β-actin and GAPDH housekeeping genes and depicted as the degree fold-change in transcriptional activity over saline. Responses below a 2.0-fold induction relative to saline are indicated by dot plots that fall below the horizontal dotted line. Means are indicated by short horizontal bars (whole blood from n=5 guinea pigs).
3.3. Validation of QuantiGene by Real-Time PCR (RT-PCR)
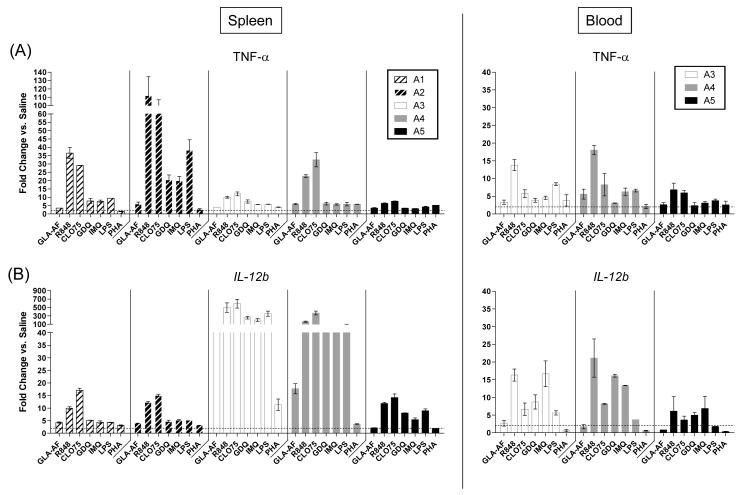
To validate responses by QuantiGene, quantitative real-time PCR was performed on the same pool of TLR-stimulated whole blood and spleen cells. RNA was purified from cells stimulated at the highest agonist dosage (1 μg/mL) and first-strand cDNA was synthesized for real-time amplification. TNF-α and IL-12b were selected for validation. TNF-α is a pro-inflammatory cytokine shown previously to be upregulated following GLA stimulation in other species (Bertholet et al., 2010; Coler et al., 2010; Coler et al., 2011). IL-12 was chosen out of interest, as it is a key cytokine for generating protective Th1 immunity. RT-PCR analysis was not performed for CXCL1, CCL2, or IL-1β. Table 1 lists the primers used to detect mRNA for all cytokine (TNF-α and IL-12b) and housekeeping (β-actin) genes. For each gene of interest, the amount of RNA measured was normalized to the β-actin housekeeping gene. Data is presented as the fold-induction in transcriptional activity over saline-treated controls.
Measurable increases in both TNF-α and IL-12b message were observed by RT-PCR for all guinea pigs (n=5) following TLR stimulation of guinea pig splenocytes (Figure 4A, left panel and Figure 4B, left panel). The TLR-4 agonist GLA-AF induced ~3.5 to 6-fold increases in TNF-α expression and ~2 to 18-fold increases in IL-12b gene expression, although it must be noted that more than 50-fold higher IL-12b expression levels were observed for animal A3. The greatest fold changes, as with QuantiGene, were measured in response to one or more TLR-7/8 agonists. The most notable increases for TNF-α were in response to R848 (6 to 110-fold higher) and CL075 (7 to 90-fold higher). IL-12b responses also varied, with most animals showing no greater than 20-fold increases (A1, A2, A5) but some animals (A3 and A4) showing greater than 50-fold higher induction of IL-12b message to every TLR-7/8 agonist.
Figure 4. Quantitative Real-time PCR (RT-PCR).
(A) TNF-α and (B) IL-12b mRNA expression. Cytokine expression was measured from guinea pig splenocytes (left panels, n=5 spleen samples) or whole blood (right panels, n=3 whole blood samples) following TLR-4/7/8 stimulation at a single dose of agonist (1 μg/mL). Data were normalized to the β-actin housekeeping gene and expressed relative to saline controls. Responses below a 2.0-fold induction relative to saline are indicated by the dotted horizontal line. The assay was performed in duplicate for each animal and is depicted as the mean + SEM.
Whole blood stimulation induced significant increases in TNF-α and IL-12b message (Figure 4A, right panel and 4B, right panel). Insufficient levels of purified RNA from blood were available for animals A1 and A2, and thus, RT-PCR analysis was performed for animals A3, A4, and A5 only. By RT-PCR, GLA-AF induced ~3-6 fold higher TNF-α transcription compared to saline-treated controls, similar to responses assayed by QuantiGene. The pattern of TNF-α expression following TLR-7/8 stimulation is also consistent with QuantiGene, with R848 (7-18-fold) and CL075 (5-8-fold) inducing the highest responses. Most interesting was the expression profile of IL-12b. Although negligible changes in IL-12b transcription were detected by QuantiGene whole blood assay, by RT-PCR, we measured a modest 3-fold increase in IL-12b message following GLA-AF stimulation and upwards of a 20-fold change in IL-12b message following stimulation of blood with one or more TLR-7/8 agonists. Finally, although comparable expression profiles were generated for spleens and whole blood, a few differences were observed. In particular, IL-12b splenocyte expression was significantly higher compared to whole blood for animals A3 and A4. These results by RT-PCR confirm data generated by QuantiGene and validate this assay as a method for elucidating the transcriptional profile of TLR-stimulated guinea pig cells. These results also highlight differences in the sensitivities of the two assays as well as tissue-specific differences in transcriptional activity.
4. DISCUSSION
We evaluated the bDNA-based QuantiGene Plex 2.0 assay as an alternative to RT-PCR for measuring gene expression in the guinea pig model. Our results highlight several advantages. First, RNA can be measured directly from a sample source, circumventing the need for intricate RNA isolation and enzymatic processing procedures. In this study, QuantiGene performance was assessed using two formats, first by quantification from a purified RNA source (splenocytes) and then by direct measurement of mRNA message in whole blood. Responses to most analytes were detected even without purification, indicating good assay sensitivity overall, although it must be noted that each format yielded slightly unique profiles. Further work is needed to clarify the independent effects of RNA purification and tissue source on these expression differences. By RT-PCR, similar differences were observed when using equivalent RNA input, indicating that some of these differences are tissue-specific, possibly due to variations in cell composition, cell density, or TLR expression. Neutrophils, for instance, express cell-surface TLR-4 at low levels (Kurt-Jones et al., 2002) and do not respond to bacterial LPS as effectively as monocytes or macrophages. A benefit of the QuantiGene system is that users have the flexibility to choose either format, depending on one’s requirements. As a general screen, RNA purification may be unwarranted, while measurement of low expressing genes may benefit from purification. The ability to sample from a variety of tissues sources permits more comprehensive transcriptional profiling and targeting of tissues relevant to different disease models. The whole blood format also offers the practicality of sample isolation through a simple blood draw without animal sacrifice and the ability to harvest samples longitudinally from the same animal. Finally, with its bead-based design, multiple transcripts (as opposed to two with RT-PCR) can be measured simultaneously from a single well in high-throughput. Both analytes and housekeeping genes can be measured within the same well to reduce variability and operator error.
We also acknowledge several limitations in the current QuantiGene platform. First, although multiple transcripts can be profiled simultaneously, assay throughput is still encumbered by a tedious protocol involving numerous dilution, wash, and incubation steps. The current methodology, however, has the potential to be simplified and automated for true high throughput processing given its relatively simple work-flow. Another potential limitation is the lack of target enrichment due to the absence of a cDNA amplification step. While target pre-amplification is a source of error, it improves assay sensitivity, particularly for low abundance transcripts. Measurable increases in gene expression were observed with or without RNA purification for most of the analytes examined, suggesting that QuantiGene is, overall, still a highly sensitive assay even in the absence of preamplification. Comparatively, however, RT-PCR measured far greater fold increases in TNF-α and IL-12b message. In fact, QuantiGene (but not RT-PCR) failed to detect any increase in IL-12b transcript by whole blood assay.
Assay sensitivity may be improved using a combination of strategies. Larger sample input and longitudinal testing may, for example, provide a combined solution. One limitation in our current study design is that spleen and whole blood lysates were plated at a single cell density (5 × 105 cells/well for splenocytes) or volume (80 μ l/well for whole blood) and responses were tested at just a single time-point (4 h). Further optimization will require testing on a range of cell densities and over several intervals to assess peak responses. Another potential solution is through sample enrichment. Guinea pig whole blood is an amalgam of different cellular and non-cellular components. In addition to erythrocytes (red blood cells), thrombocytes (platelets), lymphocytes (B- and T-cells), and neutrophils which constitute most of the cellular component of whole blood, there are also clotting factors, immunoglobulins, and various other nutrients (i.e. glucose, amino acids, fatty acids) and waste products (i.e. urea, lactic acid, carbon dioxide) that may promote degradation of assay sensitivity. Ficoll enrichment for PBMCs permits accurate measurement of sample input size (cells/mL) and vastly improves QuantiGene performance and assay sensitivity (data not shown). Probe optimization represents a third solution. Affymetrix provides support for rapid custom design of bDNA CE- and LE-probe sets (refer to Experimental Procedures) to maximize specificity for target mRNA and minimize nonspecific interactions. The bDNA molecules used for signal amplification can also be modified to incorporate more binding sites for fluorescent molecules. Finally, bead loss may occur following the multiple washing steps involved in this protocol. To diminish loss of transcript and promote efficient washing, a magnetic option is available that could be used in place of polystyrene reagents.
The ability to modulate gene expression for three cytokines (TNF-α, IL-1β, and IL-12) and two chemokines (CCL2 and CXCL1) with known immunomodulatory functions is encouraging to vaccine efforts. TNF-α and IL-1β are prototypic pro-inflammatory cytokines with dual roles in regulating the pathology of diseases like Mycobacterium tuberculosis (Mtb). IL-12 promotes a potent Th1 response and is a requisite innate cytokine for anti-mycobacterial immune responses. Chemokines and their receptors also play an essential role in host resistance. CCL2 is involved in chemotaxis and activation of monocytes, dendritic cells, and memory T-cells, while CXCL1 is a neutrophil chemoattractant. Previously, two Mtb subunit vaccines known as ID83 and ID93 were combined with GLA (TLR-4), GDQ (TLR-7), or CpG (TLR-9), alone or in combination. TLR stimulation enhanced the immunogenicity of co-administered recombinant antigens and produced strong cell-mediated and qualitative Th1 responses (Baldwin et al., 2009; Bertholet et al., 2010; Coler et al., 2011). Efforts to modulate vaccine responses by inclusion of TLR agonists will require careful monitoring. Systemic spillover of TNF-α, for example, may account for unwanted inflammatory effects like fever and wasting, and exorbitant upregulation of CCL2 can dampen IL-12 production in mice and promote a Th2-biased response (Chensue et al., 1996). QuantiGene provides a rapid method for monitoring agonists like these that are to be tested in guinea pig prophylactic and therapeutic vaccine studies.
Overall, our study highlights the reliability of the bDNA assay for measuring RNA in the guinea pig model. The assay is sensitive and flexible. The simple workflow facilitates high-throughput screening of multiple analytes from diverse sources. Moreover, our study supports previous findings that bDNA-based assays reproducibly detect responses observed by RT-PCR (Canales et al., 2006; Knudsen et al., 2008; Lubelchek et al., 2009), although additional steps may be taken to improve assay sensitivity further. We are currently optimizing these bDNA assays to measure antigen-specific immune responses in guinea pigs following immunization with ID93, our lead tuberculosis antigen. The ability to measure TLR-induced or antigen-specific upregulation of multiple cytokines in guinea pigs is encouraging given the relevance of this model for evaluating adjuvants and vaccines. With the recent completion of the guinea pig genome (http://www.genome.gov/10002154), novel probes can be designed to recognize any transcript of interest. Elucidation of vaccine-induced innate responses may provide insights into correlates of immune protection and reveal avenues for modulating the magnitude and quality of the adaptive response to maximize protection against specific pathogens.
Supplementary Material
Supplemental Figure 1: QuantiGene heat-map (spleen lysates). Enhanced heat-map depicting the degree fold change in transcriptional activity over saline (TNF-α, IL-1β, CXCL1, CCL2, and IL-12b) following TLR-4 or -7/8 stimulation of guinea pig spleen cells at three doses (1, 0.1, 0.01 μg/mL). RNA was purified for QuantiGene analysis. Data were normalized to β-actin and GAPDH mRNA. Red tones indicate higher levels of mRNA expression compared to saline controls. Blue tones indicate lower levels of transcriptional activity. Average-fold inductions in gene expression are reported for each animal (A1-A5). LPS (0.01 μg/mL) and PHA (100 μg/mL) were included as positive controls.
Supplemental Figure 2: QuantiGene heat-map (whole blood assay). QuantiGene analysis was performed directly on stimulated whole blood lysates without RNA purification (animals A1-A5). The heat-map illustrates the degree fold-change in TNF-α, IL-1β, CXCL1, and CCL2 transcriptional activity over saline following stimulation with the same TLR-4 and TLR-7/8 agonists described previously, at three doses (1, 0.1, 0.01 μg/mL). LPS (0.01 μg/mL) and PHA (100 μg/mL) were incorporated as positive controls. Data were normalized to β-actin and GAPDH mRNA. The level of transcriptional activity for each analyte is expressed as being either higher (red tones) or lower (blue tones) relative to saline controls. Average-fold inductions in gene expression are reported for each animal (A1-A5). Measurable changes in gene expression for the IL-12 p40 subunit, or IL-12b, were not observed (data not shown).
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health grant U01 AI070456, with additional support from grant 1U01 A1078054 and contract HHSN272200800045C. We acknowledge Tara Evers, Alison Bernard, Valerie Reese, and David Argilla for their technical expertise. We also thank Dr. Christopher Fox, Dr. Thomas Vedvick, and members of the IDRI Process Science and Formulations team who provided formulated reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST Dr. Reed is a founder of, and holds an equity interest in, Immune Design Corp., a licensee of certain rights associated with GLA.
REFERENCES
- Alter HJ, Sanchez-Pescador R, Urdea MS, Wilber JC, Lagier RJ, Di Bisceglie AM, Shih JW, Neuwald PD. Evaluation of branched DNA signal amplification for the detection of hepatitis C virus RNA. J Viral Hepat. 1995;2:121–32. doi: 10.1111/j.1365-2893.1995.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Baldwin SL, Bertholet S, Kahn M, Zharkikh I, Ireton GC, Vedvick TS, Reed SG, Coler RN. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine. 2009;27:3063–71. doi: 10.1016/j.vaccine.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, Coler RN, Reed SG. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2:53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales RD, Luo Y, Willey JC, Austermiller B, Barbacioru CC, Boysen C, Hunkapiller K, Jensen RV, Knight CR, Lee KY, Ma Y, Maqsodi B, Papallo A, Peters EH, Poulter K, Ruppel PL, Samaha RR, Shi L, Yang W, Zhang L, Goodsaid FM. Evaluation of DNA microarray results with quantitative gene expression platforms. Nat Biotechnol. 2006;24:1115–22. doi: 10.1038/nbt1236. [DOI] [PubMed] [Google Scholar]
- Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231–40. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue SW, Warmington KS, Ruth JH, Sanghi PS, Lincoln P, Kunkel SL. Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: relationship to local inflammation, Th cell expression, and IL-12 production. J Immunol. 1996;157:4602–8. [PubMed] [Google Scholar]
- Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS, Lu X, Devos J, Hancock K, Katz JM, Vedvick TS, Duthie MS, Clegg CH, Van Hoeven N, Reed SG. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One. 2010;5:e13677. doi: 10.1371/journal.pone.0013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer J, Lagier R, Flynn J, Zayati C, Kolberg J, Collins M, Urdea M, Sanchez-Pescador R. Accurate quantification of hepatitis C virus (HCV) RNA from all HCV genotypes by using branched-DNA technology. J Clin Microbiol. 1996;34:901–7. doi: 10.1128/jcm.34.4.901-907.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbeik T, Surtihadi J, Destree M, Gorlin J, Holodniy M, Jortani SA, Kuramoto K, Ng V, Valdes R, Jr., Valsamakis A, Terrault NA. Multicenter evaluation of the performance characteristics of the bayer VERSANT HCV RNA 3.0 assay (bDNA) J Clin Microbiol. 2004;42:563–9. doi: 10.1128/JCM.42.2.563-569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G, Martin MT, Dubin G, Wettendorff MA. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–49. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Kern D, Collins M, Fultz T, Detmer J, Hamren S, Peterkin JJ, Sheridan P, Urdea M, White R, Yeghiazarian T, Todd J. An enhanced-sensitivity branched-DNA assay for quantification of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1996;34:3196–202. doi: 10.1128/jcm.34.12.3196-3202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen BS, Allen AN, McLerran DF, Vessella RL, Karademos J, Davies JE, Maqsodi B, Kristal AR. Evaluation of the branched-chain DNA assay for measurement of RNA in formalin-fixed tissues. J Mol Diagn. 2008;10:169–76. doi: 10.2353/jmoldx.2008.070127. McMaster, G.K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones EA, Mandell L, Whitney C, Padgett A, Gosselin K, Newburger PE, Finberg RW. Role of toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood. 2002;100:1860–8. [PubMed] [Google Scholar]
- Lubelchek RJ, Max B, Sandusky CJ, Hota B, Barker DE. Reliability at the lower limits of HIV-1 RNA quantification in clinical samples: a comparison of RT-PCR versus bDNA assays. PLoS One. 2009;4:e6008. doi: 10.1371/journal.pone.0006008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly LH, Russell MI, McMurray DN. Cytokine profiles in primary and secondary pulmonary granulomas of Guinea pigs with tuberculosis. Am J Respir Cell Mol Biol. 2008;38:455–62. doi: 10.1165/rcmb.2007-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerat C, Burgisser P, Lavanchy D, Mantegani A, Frei PC. Comparison of a competitive combined reverse transcription-PCR assay with a branched-DNA assay for hepatitis C virus RNA quantitation. J Clin Microbiol. 1996;34:2702–6. doi: 10.1128/jcm.34.11.2702-2706.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray DN, Allen SS, Jeevan A, Lasco T, Cho H, Skwor T, Yamamoto T, McFarland C, Yoshimura T. Vaccine-induced cytokine responses in a guinea pig model of pulmonary tuberculosis. Tuberculosis (Edinb) 2005;85:295–301. doi: 10.1016/j.tube.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Nolte FS, Boysza J, Thurmond C, Clark WS, Lennox JL. Clinical comparison of an enhanced-sensitivity branched-DNA assay and reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1998;36:716–20. doi: 10.1128/jcm.36.3.716-720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA. TLRs: Professor Mechnikov, sit on your hat. Trends Immunol. 2004;25:687–93. doi: 10.1016/j.it.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Pachl C, Todd JA, Kern DG, Sheridan PJ, Fong SJ, Stempien M, Hoo B, Besemer D, Yeghiazarian T, Irvine B, et al. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:446–54. doi: 10.1097/00042560-199504120-00003. [DOI] [PubMed] [Google Scholar]
- Pyne MT, Konnick EQ, Phansalkar A, Hillyard DR. Evaluation of the Abbott investigational use only realtime HIV-1 assay and comparison to the Roche Amplicor HIV-1 monitor test, version 1.5. J Mol Diagn. 2009;11:347–54. doi: 10.2353/jmoldx.2009.080166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J, Pachl C, White R, Yeghiazarian T, Johnson P, Taylor B, Holodniy M, Kern D, Hamren S, Chernoff D, et al. Performance characteristics for the quantitation of plasma HIV-1 RNA using branched DNA signal amplification technology. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10(Suppl 2):S35–44. [PubMed] [Google Scholar]
- Trimoulet P, Halfon P, Pohier E, Khiri H, Chene G, Fleury H. Evaluation of the VERSANT HCV RNA 3.0 assay for quantification of hepatitis C virus RNA in serum. J Clin Microbiol. 2002;40:2031–6. doi: 10.1128/JCM.40.6.2031-2036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrior U, Fan Y, David CA, Wilkins JA, McKeegan EM, Kofron JL, Burns DJ. Application of QuantiGene nucleic acid quantification technology for high throughput screening. J Biomol Screen. 2000;5:343–52. doi: 10.1177/108705710000500506. [DOI] [PubMed] [Google Scholar]
- Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–61. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: QuantiGene heat-map (spleen lysates). Enhanced heat-map depicting the degree fold change in transcriptional activity over saline (TNF-α, IL-1β, CXCL1, CCL2, and IL-12b) following TLR-4 or -7/8 stimulation of guinea pig spleen cells at three doses (1, 0.1, 0.01 μg/mL). RNA was purified for QuantiGene analysis. Data were normalized to β-actin and GAPDH mRNA. Red tones indicate higher levels of mRNA expression compared to saline controls. Blue tones indicate lower levels of transcriptional activity. Average-fold inductions in gene expression are reported for each animal (A1-A5). LPS (0.01 μg/mL) and PHA (100 μg/mL) were included as positive controls.
Supplemental Figure 2: QuantiGene heat-map (whole blood assay). QuantiGene analysis was performed directly on stimulated whole blood lysates without RNA purification (animals A1-A5). The heat-map illustrates the degree fold-change in TNF-α, IL-1β, CXCL1, and CCL2 transcriptional activity over saline following stimulation with the same TLR-4 and TLR-7/8 agonists described previously, at three doses (1, 0.1, 0.01 μg/mL). LPS (0.01 μg/mL) and PHA (100 μg/mL) were incorporated as positive controls. Data were normalized to β-actin and GAPDH mRNA. The level of transcriptional activity for each analyte is expressed as being either higher (red tones) or lower (blue tones) relative to saline controls. Average-fold inductions in gene expression are reported for each animal (A1-A5). Measurable changes in gene expression for the IL-12 p40 subunit, or IL-12b, were not observed (data not shown).