Abstract
Inflammation predicts risk of cardiovascular disease (CVD) events, but the relation of drugs that directly target inflammation with CVD risk is not established. Methotrexate is a disease-modifying anti-rheumatic drug broadly used for treatment of chronic inflammatory disorders. We performed a systematic review and meta-analysis of evidence for relationships of methotrexate with CVD occurrence. Cohorts, case-control studies or randomized trials were included if they reported an association between methotrexate and CVD risk. Inclusions/exclusions were independently adjudicated, and all data were extracted in duplicate. Pooled effects were calculated using inverse-variance-weighted meta-analysis. Of 694 identified publications, 10 observational studies, in which methotrexate was administered among patients with rheumatoid arthritis, psoriasis, or polyarthritis met inclusion criteria. Methotrexate was associated with 21% lower risk of total CVD (n=10 studies, 95% CI=0.73-0.87, p<0.001), and 18% lower risk of myocardial infarction (n=5, 95% CI=0.71-0.96, p=0.01), without evidence for statistical between-study heterogeneity (p=0.30, p=0.33, respectively). Among prespecified sources of heterogeneity explored, stronger associations were observed in studies that adjusted for underlying disease severity (RR=0.64, 95% CI=0.43-0.96, p<0.01), and for other concomitant medication (RR=0.73, 95% CI=0.63-0.84, p<0.001). Publication bias was potentially evident (funnel plot, Begg’s test, p=0.06); excluding studies with extreme risk estimates did not, however, alter results (RR=0.81, 95% CI=0.74-0.89). In conclusion, methotrexate use is associated with lower risk of CVD among patients with chronic inflammation. These findings suggest that a direct treatment of inflammation may reduce CVD risk.
Keywords: systematic review, meta-analysis, methotrexate, inflammation, cardiovascular disease
INTRODUCTION
Systemic inflammation is strongly linked to increased risk of cardiovascular disease (CVD) 1, 2. However, whether this relationship is causal or simply an association is not established; no randomized trials have directly addressed whether targeted anti-inflammatory agents that do not have concomitant lipid-lowering or anti-platelet effects also reduce CVD event rates. Methotrexate has received particular interest in that regard, as it is a disease-modifying anti-rheumatic drug broadly used for the treatment of systemic inflammatory disorders, such as rheumatoid arthritis (RA) and psoriasis. Although the underlying mechanisms are not fully understood, methotrexate is known to ameliorate inflammatory responses by altering nucleotide metabolisms and, at least in part, mitigating cytokine signaling 3. The anti-inflammatory properties of methotrexate have been hypothesized to be beneficial in reducing CVD risk among patients with chronic inflammatory disorders (e.g., RA) 4 or even among patients with persistent inflammatory responses (e.g., increased CRP levels) 5. Recently, the evidence for relationships between methotrexate use in patients with RA and CVD outcomes has been systematically reviewed 4. However, no systematic review and meta-analysis has been performed to critically and statistically evaluate heterogeneity among published studies in this field, and to quantify the effects of methotrexate on CVD, which would help elucidate whether direct treatment of inflammation could potentially reduce CVD risk. To address this important question, we performed a systematic review and meta-analysis of the evidence for relationships of methotrexate use with risk of CVD.
METHODS
The Meta-Analysis of Observational Studies in Epidemiology guidelines 6 were used as reference for all stages of design, implementation, and reporting of this systematic review and meta-analysis.
We searched for all clinical trials, or observational studies (prospective or retrospective or case-control studies) in which adults received methotrexate, the duration of follow-up was at least 3 months, and reported effect estimates on occurrence of “hard” CVD events (myocardial infarction (MI), coronary heart disease (CHD), sudden death, and/or stroke). We performed searches using multiple online databases, including MEDLINE (see Supplemental Methods), EMBASE, AGRIS, AMED, Web of Knowledge, CINAHL, CAB abstracts, Cochrane library, conference abstracts (ZETOC), Faculty of 1000, and grey literature sources (SIGLE). We additionally reviewed related articles, hand-searched reference lists, and performed direct author contact. For each database, the years searched included the earliest available online year of indexing up to June 2010, without language restrictions. Key words were methotrexate, mexate, amethopterin, and cardiovascular diseases. We excluded a priori studies that only had information on intermediate secondary endpoints (e.g., lipid or glucose levels) or “soft” CHD outcomes (e.g., angina, heart failure), studies in which methotrexate was administered as part of a combination therapy, and studies in which users were very different from non-users (e.g., diseased vs. non-diseased participants). We also excluded a priori ecological or cross-sectional studies; commentaries, general reviews, or case reports; duplicate publications from the same study; and studies reporting only crude risk estimates.
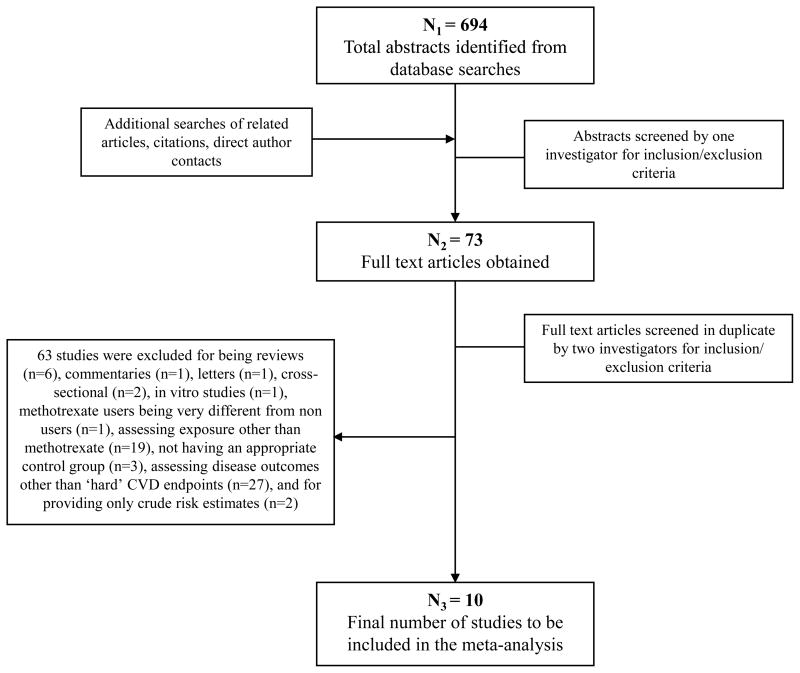
Of 694 identified articles, 621 were excluded based on review of the title and abstract (Figure 1). The remaining 73 manuscripts were reviewed in detail independently and in duplicate to determine inclusion/exclusion (95% concordant); differences were resolved by consensus or, if necessary, group consultation among all investigators. Sixty-three studies were excluded because they were reviews (n=6), commentaries (n=1), letters (n=1), cross-sectional (n=2), or in vitro (n=1); included methotrexate users which were very different from non users (n=1); assessed exposure other than methotrexate (n=19); did not have an appropriate control group (n=3); did not assess “hard” CVD endpoints (n=27); or reported only crude risk estimates (n=2) (see Supplemental Methods). In the end, 10 studies were included in this meta-analysis.
Figure 1.
Screening and selection process of studies of methotrexate use and cardiovascular disease risk.
For each study, data were extracted independently and in duplicate by 2 investigators, including year study was published and performed, study location, study design, sample size and number of events, inclusion and exclusion criteria, duration of follow-up, underlying disease (e.g., RA), duration of underlying disease, assessment of underlying disease severity, methotrexate comparison groups (e.g., initiators vs. non-initiators, ever users vs. never users), ascertainment of methotrexate use, treatment dose, disease outcome, disease incidence vs. recurrence, folate use, whether the reported analysis was primary or secondary and prespecified or posthoc in each paper, covariates adjusted for in the analysis, and adjusted risk estimates and confidence intervals (CIs). Accepted standardized quality scores are not available for observational studies. We performed quality assessment as previously described and used 7, by evaluating and scoring 6 design criteria on an integer scale (0 or 1, with 1 being better), including review of study design and inclusion and exclusion criteria, assessment of exposure, assessment of outcome, control of confounding, assessment of underlying disease severity, and evidence of bias. These scores were summed, and quality scores from 0 to 3 were considered lower quality, and scores from 4 to 6 higher quality. Differences in data extracted and quality assessment were very unusual, and if present, were resolved by group discussion and consensus. Missing data were obtained by direct author contact for only 1 of 10 studies, as described above.
All included studies were observational and reported rate ratios or odds ratios; because of the low incidence of CVD in all studies, we collectively refer to these measures using the general term “relative risk” (RR). Between-study heterogeneity of RRs was assessed using the Dersimonian and Laird Q-statistic, the I2 statistic, and meta-regression 8, 9. To calculate the overall pooled RR we used both a fixed effects and a random effects meta-analysis using the methods of Dersimonian and Laird 8, and reported the former if the point estimates were virtually equal. In one study 10, methotrexate was considered as the reference group to estimate the RRs of other RA medication on risk of MI or stroke hospitalization, including biologics, other cytotoxic agents, noncytotoxic agents, and glucocorticoids. Subsequently, to estimate the RR of methotrexate vs. other RA medication (the latter as the reference group) on CVD risk we pooled the inverse RRs of all other RA medication. Potential for publication bias was explored by visually inspecting a funnel plot of the effect size versus SE 11, and statistically using the Begg adjusted-rank correlation test 12. We explored potential prespecified sources of heterogeneity using stratified inverse-variance weighted fixed and random effects meta-analysis (and reported the latter if significant between-study heterogeneity was present, p < 0.1) and inverse-variance weighted meta-regression, including study design (prospective vs. retrospective cohorts), study location (America vs. Europe), years of follow-up, methotrexate comparison groups (initiators vs. non-initiators; ever vs. never users; current vs. non-current users), ascertainment of methotrexate use (physician vs. self-reported), underlying disease (RA; psoriasis; polyarthritis), disease outcome (CVD, MI, stroke), event (incident vs. recurrent), degree of covariate adjustment (most studies adjusted for sociodemographics and CVD risk factors, so further adjusting for underlying disease severity, other medication for underlying disease, or folate use), overall quality score (0-3 vs. 4-6), and whether the reported analysis was primary or secondary and prespecified or posthoc (to address potential publication bias of “positive” findings) in each publication. Analyses were performed using STATA 10.0 (StataCorp, College Station, Tex), with 2-tailed alpha<0.05.
RESULTS
The 10 identified investigations included 8 prospective and 2 retrospective cohort studies in America (n=6) and Europe (n=4), and included 66,334 individuals in whom 6,235 CVD events were identified 10, 13–21 (Table 1). We did not identify any randomized controlled trials that assigned methotrexate and assessed the occurrence of “hard” CVD events. One study 19 provided 2 separate estimates for patients receiving methotrexate with RA and polyarthritis as the underlying disease (total of 11 estimates). Therefore, 9 studies evaluated methotrexate use in RA as the underlying disease, 1 in psoriasis and 1 in polyarthritis.
Table 1.
Identified studies evaluating methotrexate use among patients with systemic inflammation and occurrence of cardiovascular disease.
| First Author (Year)s |
Country | Study Name | Under- lying disease |
Ascertainment of MTX use |
MTX comparison groups |
Disease outcome |
Disease ascertainment |
Sample size |
N of events |
Follow- up (y) |
Mean Age (y) |
Adjust- ments* |
Quality score† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective Cohort Studies | |||||||||||||
| Choi, HK et al. (2002)14 | US | Wichita Arthritis Center Cohort | RA | Arthritis medical record database | Initiators vs. non-initiators | CVD mortality|| | Medical records, death certificates, or national death registry | 1,240 | 84 | 6.0 | 57 | 1, 2, 3, 4 | 6 |
| Solomon, DH et al. (2006)10 | US | Pharmaceutical Assistance Contract for the Elderly | RA | Health-care utilization database | Initiators vs. non-initiators | MI or stroke hospitalization | Healthcare utilization database | 4770 | 398 | 2.0 | 82 | 1, 2, 4 | 3 |
| MI hospitalization | 4770 | Not reported | 2.0 | 82 | 1, 2, 4 | 3 | |||||||
| Stroke hospitalization | 4770 | Not reported | 2.0 | 82 | 1, 2, 4 | 3 | |||||||
| Suissa, S et al. (2006)15 | North America | PharMetrics Patient-Centric Outcomes Database Cohort | RA | Health-care utilization database | Current users vs. non-current users | AMI hospitalization § | Medical records | 5,118 | 476 | 1.2 | 65 | 1, 2, 4 | 2 |
| Troelsen, LN et al. (2007)16 | Denmark | N/A | RA | Clinical chart | Current users vs. non-current users | IHD hospitalization || | Medical records, death and patients registry databases | 178 | 29 | 9.5 | 62 | 1, 2, 3 | 5 |
| MI hospitalization | 178 | 12 | 1, 2, 3 | 5 | |||||||||
| Nadareishvili, Z et al. (2008)17 | US | National Data Bank for Rheumatic Disease longitudinal study | RA | Patients’ self-report | Ever-users vs. never-users | Ischemic stroke§ | Medical records and death certificates | 832 | 41 | 4.0 | 70 | 2, 3 | 4 |
| Wolfe, F et al. (2008)18 | US | National Data Bank for Rheumatic Disease longitudinal study | RA | Patients’ self-report | Ever-users vs. never-users | MI§ | Study questionnaires, Medical records | 3,974 | 198 | 3.0 | 41 | 1, 2, 3 | 4 |
| Edwards, CJ et al., (2008)20 | UK | UK General Practice Research Database | RA | General practice database | Ever-users vs. never-users | MI§ | General practice database | 34,364 | 966 | 7 | 53.5 | 1, 2 | 1 |
| Goodson, NJ et al., (2008)21 | UK | UK Norforlk Arthritis Register | Polyarthritis | Not reported | Current users vs. never users | CVD mortality|| | Not reported | 923 | 85 | 10.7 | 55 | 1, 2, 3, 4 | 3 |
| Retrospective Cohort Studies | |||||||||||||
| Prodanowich, S et al. (2005)19 | US | Miami Veterans cohort | Psoriasis | Pharmacy database | Ever-users vs. never-users | CVD§ | Medical records | 7,615 | 1,869 | Not reported# | 65 | 1, 2, 5 | 2 |
| RA | 6,707 | 2,017 | Not reported# | 66 | 1, 2, 5 | 2 | |||||||
| van Halm, VP et al. (2006)13 | Netherlands | Jan van Breemen Institute | RA | Medical record | Ever-users vs. never-users | CVD§ | Medical records | 613 | 72 | 9.2 | 64 | 1, 2, 3 | 3 |
Control of confounding: 1. Sociodemographic indicators; 2. Cardiovascular risk factors; 3. Severity of underlying disease; 4. Medications for underlying disease; 5. Use of folate.
Quality assessment was performed by review of study design, including inclusion and exclusion criteria, assessment of exposure, assessment of outcome, assessment of underlying disease severity, control of confounding (any two plus disease severity was given a score of 1; otherwise, 0), and evidence of bias. Each of the six quality criteria was evaluated and scored on an integer scale (0 or 1; 1 being better) and summed; quality scores from 0 to 3 were considered lower quality, and 4-6 higher quality.
Incident events.
Not specified whether incident or recurrent event.
Upon independent review by 4 authors duration of follow-up was felt to exceed 3 months given the design and number of incident events. CVD, cardiovascular diseases; IHD, ischemic heart disease; MI, myocardial infarction; MTX, methotrexate; RA, rheumatoid arthritis.
Five studies 10, 13, 14, 16, 17 reported median duration of underlying disease, which ranged from 6 to 16 years. The majority of the studies did not report dose of methotrexate treatment, and for those which did 13, 14, 18 median dose ranged from 13-15 mg/week. With the exception of one study 19, all studies reported duration of follow-up; median duration across studies was 5.84 years. In the majority of the studies methotrexate use was ascertained by a physician (including prescription files and chart review); in only two studies 17, 18 methotrexate use was self-reported. Half of the studies 10, 13–15, 20 utilized health claims databases to ascertain methotrexate exposure. Most studies compared methotrexate ever users vs. never users (n=6 estimates), followed by current vs. non-current users (n=3 estimates), and initiators vs. non-initiators (n=2 estimates). Seven studies ascertained total or fatal CVD, 3 MI, and 1 ischemic stroke; 2 studies that evaluated CVD further provided separate estimates for MI 10, 16 and total stroke 10. Reported events were incident in 7 studies; case incidence vs. recurrence was not specified for 3 studies 14, 16, 21. Quality scores were in the lower range (0-3) for most studies, reflecting mainly study design limitations, such as lack of control for potentially important confounders and lack of assessment of underlying disease severity. Degree of covariate adjustment also varied among studies; all studies adjusted for CVD risk factors (e.g., blood pressure, blood cholesterol, smoking), and all but 1 study adjusted for socio-demographics (e.g., age, sex, socio-economic status, race); 6 adjusted for underlying disease severity; 4 for other medications used to treat the underlying disease; and 2 for folate use. For all studies the reported exposure-outcome assessment was a prespecified primary 10, 13, 15, 19, 21 or secondary 14, 16–18, 20 aim.
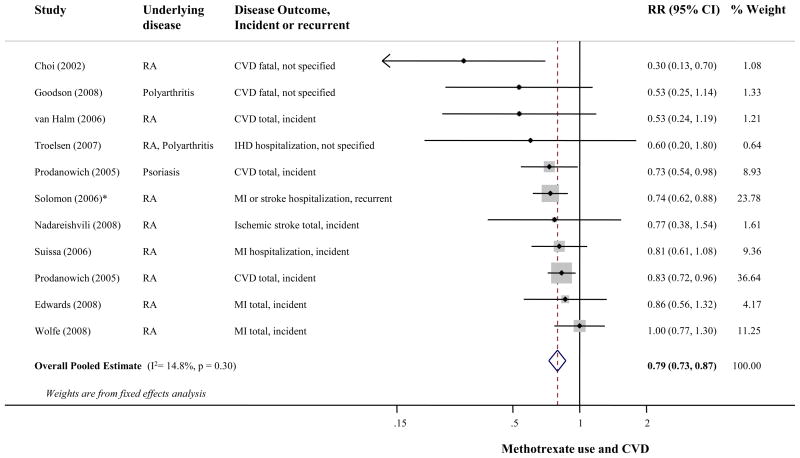
The p-value for between-study heterogeneity was 0.30 (I2 =15%). Figure 2 presents the RR of CVD events associated with methotrexate use, Combining all studies, methotrexate use was associated with 21% lower CVD risk (95% CI=0.73-0.87); results from the random-effects meta-analysis were very similar (RR=0.79, 95% CI=0.71-0.88). Visual inspection of the funnel plot (see Supplemental Figure) suggested possible publication bias (p=0.06), but excluding the 4 smallest studies 13, 14, 16, 21 with extreme risk estimates did not substantially alter the pooled estimate (RR=0.81, 95% CI=0.74-0.89). All p-values for heterogeneity for potential prespecified sources were >0.14 in separate meta-regression models (Table 2).
Figure 2.
Risk of cardiovascular disease associated with methotrexate use, including 8 prospective and 2 retrospective cohort studies, 66,334 participants and 6,235 events. Random effects meta-analysis was used to calculate the overall pooled relative risk (RR), in the presence of statistical between-study heterogeneity (p>0.1). Solid diamonds and lines are study-specific RRs and 95% CI, respectively; the size of each box is weighted by the inverse-variance of each study. Dashed line and open diamond are pooled RR and 95% CI, respectively, combining each study-specific RR.
* Assessed other RA medication vs. methotrexate as the reference group. The RR of methotrexate vs. other RA medication was calculated by pooling the inverse RRs of all other RA medication, using fixed effects meta-analysis.
Abbreviations: CI, Confidence interval; CVD, Cardiovascular disease; IHD; Ischemic heart disease; MI, Myocardial infarction; RA, Rheumatoid arthritis; RR, Relative risk.
Table 2.
Potential prespecified sources of heterogeneity explored among the studies evaluating methotrexate treatment among patients with systemic inflammation and risk of CVD.
| Prespecified source of heterogeneity | No of estimates | Stratified fixed effects meta-analysis RR (95% CI) | Meta-regression P-value for heterogeneity |
|---|---|---|---|
| Study design | |||
| Prospective cohort | 8 | 0.79 (0.70-0.89) | 0.97 |
| Retrospective cohort | 3 | 0.80 (0.70-0.91) | |
| Study location | |||
| America | 7 | 0.80 (0.73-0.88) | 0.51 |
| Europe | 4 | 0.71 (0.51-0.98) | |
| Duration of follow-up (yrs) | 9 | 0.79 (0.70-0.89) | 0.25 |
| MTX comparison groups | |||
| Initiators vs. non-initiators | 2 | 0.52 (0.22-1.23)* | |
| Ever vs. never users | 6 | 0.84 (0.75-0.93) | 0.22 |
| Current vs. non-current users | 3 | 0.76 (0.59-0.99) | 0.70 |
| Ascertainment of MTX use | |||
| Physician | 8 | 0.78 (0.71-0.85) | 0.14 |
| Self-reported | 2 | 0.97 (0.76-1.24) | |
| Underlying disease | |||
| Rheumatoid arthritis | 9 | 0.81 (0.73-0.88) | |
| Disease outcome | |||
| CVD (including IHD) | 7 | 0.76 (0.69-0.84) | |
| MI† | 3 | 0.82 (0.71-0.96) | 0.15 |
| Stroke† | 1 | 0.70 (0.56-0.87) | 0.94 |
| Event | |||
| Incident | 7 | 0.83 (0.75-0.92) | |
| Degree of covariate adjustment | |||
| Underlying disease severity | |||
| Yes | 6 | 0.64 (0.43-0.96)* | 0.88 |
| No | 5 | 0.79 (0.72-0.87) | |
| Other medication for underlying disease | |||
| Yes | 4 | 0.73 (0.63-0.84) | 0.22 |
| No | 7 | 0.83 (0.75-0.93) | |
| Folate use | |||
| Yes | 2 | 0.81 (0.71-0.92) | 0.83 |
| No | 9 | 0.78 (0.69-0.88) | |
| Quality score‡ | |||
| Lower (0-3) | 7 | 0.78 (0.71-0.86) | 0.59 |
| Higher (4-6) | 4 | 0.68 (0.40-1.15)* | |
| Reported analysis | |||
| Primary | 6 | 0.78 (0.71-0.86) | |
| Secondary | 5 | 0.87 (0.71-1.06) | 0.50 |
We identified 10 observational studies that provided 11 separate estimates for investigating the association between methotrexate use and CVD risk. When reported total number of estimates is <11 this represents either missing information or 1 study in other subgroups. Potential prespecified sources of heterogeneity were explored by using stratified inverse-variance weighted fixed effects meta-analysis and inverse-variance weighted meta-regression.
Significant between-study heterogeneity was present (p<0.1) in stratified meta-analysis, and random effects meta-analysis is reported.
Two studies that evaluated CVD further provided separate estimates for MI (n=2) and stroke (n=1). These additional estimates were included in the stratified meta-analysis, resulting in 5 estimates for MI and 2 estimates for stroke.
Quality assessment was performed by review of study design, including inclusion and exclusion criteria, assessment of exposure, assessment of outcome, assessment of underlying disease severity, control of confounding, and evidence of bias. Each of the six quality criteria was evaluated and scored on an integer scale (0 or 1; 1 being better) and summed; quality scores from 0 to 3 were considered lower quality, and 4 to 6 higher quality.
CVD, cardiovascular disease; MI, myocardial infarction; MTX, methotrexate; RR, relative risk; CI, confidence interval.
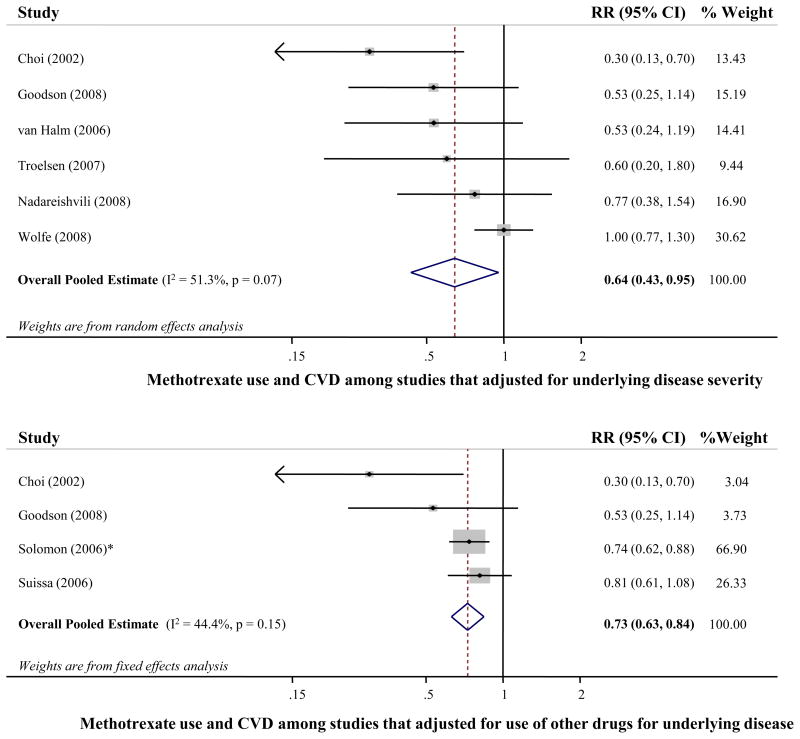
When prespecified sources of heterogeneity were explored in stratified meta-analyses models, the pooled RR (95% CI) was 0.71 (0.51-0.98) for studies conducted in Europe, 0.52 (0.22-1.23) for studies that compared methotrexate initiators vs. non-initiators, 0.68 (0.40-1.15) for studies with a higher quality score, and 0.97 (0.76-1.24) for studies with self-reported methotrexate use. Stronger associations were observed in studies that adjusted for underlying disease severity (RR=0.64, 95% CI=0.43-0.96), and studies that adjusted for concomitant medication use (RR=0.73, 95% CI=0.63-0.84). Figure 3 presents the forest plot for each of the latter 2 stratified meta-analyses performed. The pooled RR was 0.76 (95% CI=0.69-0.84) in studies that assessed only CVD, and 0.70 (95% CI=0.56-0.87) in those that assessed only stroke.
Figure 3.
Risk of cardiovascular disease associated with methotrexate use, among studies that adjusted for underlying disease severity (top; 5 cohort studies and 1 retrospective study, 7,760 participants and 509 events), and among studies that adjusted for other medication used for underlying disease (bottom; 3 cohort studies and 1 retrospective study, 12,051 participants and 1,043 events). Fixed effects meta-analysis was used to calculate the overall pooled relative risk (RR), in the absence of statistical between-study heterogeneity (p>0.1). Solid diamonds and lines are study-specific RRs and 95% CI, respectively; the size of each box is weighted by the inverse-variance of each study. Dashed line and open diamond are pooled RR and 95% CI, respectively, combining each study-specific RR.
* Assessed other RA medication vs. methotrexate as the reference group. The RR of methotrexate vs. other RA medication was calculated by pooling the inverse RRs of all other RA medication, using fixed effects meta-analysis.
Abbreviations: CI, Confidence interval; CVD, Cardiovascular disease; RR, Relative risk.
DISCUSSION
In this meta-analysis of observational studies, methotrexate use among patients with systemic inflammation (mainly rheumatoid arthritis) was associated with 21% lower CVD risk, with little evidence of between-study heterogeneity. A similar inverse association was observed for MI and stroke separately.
In general, findings in each of the sensitivity analyses performed were consistent and similar to the overall pooled estimate. For a few sources of heterogeneity, which in fact reflect potentially important study design limitations, results did differ. Lack of adjustment for underlying disease severity and other medication for underlying disease could lead to bias; sicker people would be more likely to get methotrexate and to also develop CVD, and thus methotrexate would appear less beneficial. This type of bias, “confounding by indication”, is a well recognized limitation of observational studies of treatment effects. Indeed, studies that controlled for underlying disease severity and medication for underlying disease (most of which had been carried out in Europe) were associated with 36% and 27% lower CVD risk, respectively compared with studies that did not. Furthermore, methotrexate comparison groups differed among studies, suggesting that combining them may be problematic. Ideally, the comparison should be among those who initiated vs. those who did not initiate methotrexate originally, which limits potential bias of studies of people who remain on the drug long-term, who could be doing that because they tolerate the drug (closer to an intention to treat analysis). When we restricted the analysis to the only two studies that assessed initiators vs. non-initiators, methotrexate use was associated with a trend towards 48% lower CVD risk (almost double that seen with all other comparison groups), but the CIs were wide. Ideally, such analyses should also adjust for underlying disease severity and use of other RA drugs, as described above. Ascertainment of methotrexate use could also be a potential study design limitation, as it could lead to exposure misclassification and attenuation of the observed relationship; in fact, when methotrexate use was self-reported it was not associated with CVD risk. The overall study design quality score reflects such design limitations; studies that were assigned a higher quality score were associated with lower CVD risk. In fact, the study by Choi et al. 14, which showed the largest reduction in RR, had taken into account such important study design limitations, further reflected by the highest attained quality score among the studies reviewed.
Rheumatoid arthritis is a chronic inflammatory degenerative disease characterized by substantial loss of functioning and mobility over time. Furthermore, patients with RA are at increased risk for CVD, and have substantially shorter life expectancy compared with the general population, mainly attributable to death due to CVD 22, 23. Methotrexate is the most commonly prescribed disease-modifying antirheumatic drug, which has serious side effects, but its long-term safety has been established among patients with RA 3, 24. Methotrexate improves the mobility of RA patients, as assessed for example by health assessment questionnaires and other global measures of life quality 25, 26. Methotrexate has also been shown to reduce inflammatory biomarkers, such as CRP, IL-6, and tumor necrosis factor (TNF)-α in patients with RA and psoriasis, without major concomitant effects on platelet function 27, 28, as well as in animal models 29. Regarding methotrexate’s potential effects on other intermediate risk factors for CVD, including lipid levels and insulin resistance, these have been recently systematically reviewed 4, and limited current evidence suggests lack of an association between methotrexate use and changes in either lipid profiles or insulin resistance. Therefore, it could be argued that methotrexate may potentially reduce CVD risk mainly through its anti-inflammatory properties, and not by acting on other traditional CVD risk factors. Our findings of a significant inverse association between methotrexate use in systemic inflammation and occurrence of CVD support the need for randomized clinical trials to test the effects of MTX on CVD events, such as the planned Cardiovascular Inflammation Reduction Trial (CIRT) 5, as well as additional experimental studies to elucidate possible underlying mechanisms of effect.
There were several strengths to our analysis. We performed a systematic literature search and directly contacted several authors making it likely that we identified all major published relevant reports. Study review (inclusion/exclusion) and data extraction was performed independently and in duplicate by at least two investigators increasing validity of results. We carefully identified several potential prespecified sources of heterogeneity, which were formally explored, including methotrexate comparison groups, study design, and degree of covariate adjustment, highlighting the importance of the consistency in reporting findings from observational studies of treatment effects. The 10 identified publications were performed in several countries increasing generalizability.
As in all meta-analyses, analyses are restricted to available data and their inherent limitations. No randomized controlled trials were identified that evaluated effects of methotrexate use in systemic inflammation on CVD events. Each of the included observational studies has potential limitations, which should be kept in mind in the interpretation of the findings. To evaluate the possible impact of these important limitations, we performed multiple sensitivity analyses to explore whether findings differed by study design (prospective vs. retrospective cohort), geographical location (America vs. Europe), methotrexate comparison groups, ascertainment of methotrexate use, control for potential confounders, and other indentified prespecified sources of heterogeneity. Findings from each of the sensitivity analyses performed were generally consistent with the overall pooled estimate (inverse association). Although every effort was made in directly contacting the authors to request for either additional data or clarifications with regard to the identified sources of heterogeneity, responses were received for only 1 13 of 10 identified publications resulting in missing data. Because of that, some of the identified prespecified sources of heterogeneity, such as methotrexate dose and duration of underlying disease could not be explored statistically. In all but one study the underlying disease was rheumatoid arthritis; compared with very high doses that can be used for cancer treatment, the range of methotrexate dose is relatively narrower for RA treatment (i.e., 10-25 mg per week). Since all studies were observational residual confounding by poorly measured or unmeasured confounders remains a possibility. Most studies did not adjust for folate use that could protect against harms and side effects of methotrexate use 30, and several studies did not adjust for underlying disease severity; these would generally cause underestimation of protective associations. Furthermore, the majority of the studies did not adjust for steroid use that might also attenuate the observed risk estimates; methotrexate has steroid-sparing properties (lower steroid use among methotrexate users and thus potentially lower CVD risk), and this could possibly be one of the mechanisms of effect. Publication bias cannot be completely excluded.
In summary, our findings provide support for the inflammatory hypothesis of atherothrombosis. Given the heterogenous methodology identified, future observational studies should ideally compare methotrexate initiators vs. non-initiators and also adjust for confounding by underlying disease severity. Our findings also support the need for further experimental studies to elucidate likely mechanisms and randomized clinical trials to establish causality.
Supplementary Material
Acknowledgments
We thank Vokko.P. van Halm for providing additional unpublished data.
Funding Sources
Supported by NHLBI (RC2 HL101816), and NIH (R01 HL080644).
Footnotes
Financial disclosure:
Dr Ridker is listed as a co-inventor on patents held by the Brigham and Women’s Hospital that related to the use of inflammatory biomarkers in cardiovascular disease. All other authors declare no conflicts of interest.
Author Contributions
Renata Micha: Conception and design, data collection, statistical analysis, interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and approval of final manuscript for submission.
Fumiaki Imamura: Conception and design, data collection, interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and approval of final manuscript for submission.
Moritz Wyler von Ballmoos: Conception and design, data collection, interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and approval of final manuscript for submission.
Daniel H. Solomon: Interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and approval of final manuscript for submission.
Miguel A. Hernán: Statistical advice, interpretation of the data, critical revision of the manuscript for important intellectual content, and approval of final manuscript for submission.
Paul M Ridker: Interpretation of the data, critical revision of the manuscript for important intellectual content, and approval of final manuscript for submission.
Dariush Mozaffarian: Conception and design, statistical advice, interpretation of the data, critical revision of the manuscript for important intellectual content, and approval of final manuscript for submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-Reactive Protein and Low-Density Lipoprotein Cholesterol Levels in the Prediction of First Cardiovascular Events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 2.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory Markers and the Risk of Coronary Heart Disease in Men and Women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 3.Cronstein BN. Low-Dose Methotrexate: A Mainstay in the Treatment of Rheumatoid Arthritis. Pharmacol Rev. 2005;57:163–172. doi: 10.1124/pr.57.2.3. [DOI] [PubMed] [Google Scholar]
- 4.Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E, Ostor AJK, Edwards CJ. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology. 2010;49:295–307. doi: 10.1093/rheumatology/kep366. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009;7:332–339. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 6.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 7.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121:2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Takkouche B, Cadarso-Suarez C, Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol. 1999;150:206–215. doi: 10.1093/oxfordjournals.aje.a009981. [DOI] [PubMed] [Google Scholar]
- 10.Solomon DH, Avorn J, Katz JN, Weinblatt ME, Setoguchi S, Levin R, Schneeweiss S. Immunosuppressive medications and hospitalization for cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3790–3798. doi: 10.1002/art.22255. [DOI] [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 13.van Halm VP, Nurmohamed MT, Twisk JW, Dijkmans BA, Voskuyl AE. Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther. 2006;8:R151. doi: 10.1186/ar2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi H, Hernan M, Seeger J, Robins J, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–1177. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 15.Suissa S, Bernatsky S, Hudson M. Antirheumatic drug use and the risk of acute myocardial infarction. Arthritis Rheum. 2006;55:531–536. doi: 10.1002/art.22094. [DOI] [PubMed] [Google Scholar]
- 16.Troelsen LN, Garred P, Madsen HO, Jacobsen S. Genetically determined high serum levels of mannose-binding lectin and agalactosyl IgG are associated with ischemic heart disease in rheumatoid arthritis. Arthritis and Rheumatism. 2007;56:21–29. doi: 10.1002/art.22302. [DOI] [PubMed] [Google Scholar]
- 17.Nadareishvili Z, Michaud K, Hallenbeck JM, Wolfe F. Cardiovascular, rheumatologic, and pharmacologic predictors of stroke in patients with rheumatoid arthritis: A nested, case-control study. Arthritis Care and Research. 2008;59:1090–1096. doi: 10.1002/art.23935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfe F, Michaud K. The risk of myocardial infarction and pharmacologic and nonpharmacologic myocardial infarction predictors in rheumatoid arthritis: A cohort and nested case-control analysis. Arthritis and Rheumatism. 2008;58:2612–2621. doi: 10.1002/art.23811. [DOI] [PubMed] [Google Scholar]
- 19.Prodanowich S, Ma F, Taylor JR, Pezon C, Fasihi T, Kirsner RS. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262–267. doi: 10.1016/j.jaad.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Edwards CJ, Fisher L, van Staa T, Cooper C, Arden N. Myocardial Infarction in Rheumatoid Arthritis: The Effects of DMARDs and Prednisolone [Abstract] Arthritis & Rheumatism. 2008;56 (Suppl 9):688. [Google Scholar]
- 21.Goodson NJ, Brookhart MA, Symmons DPM, Solomon DH. The mortality association with DMARD use in early inflammatory polyarthritis [Abstract] Rheumatology. 2008;47:ii49. [Google Scholar]
- 22.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 23.Prior P, Symmons DP, Scott DL, Brown R, Hawkins CF. Cause of death in rheumatoid arthritis. Br J Rheumatol. 1984;23:92–99. doi: 10.1093/rheumatology/23.2.92. [DOI] [PubMed] [Google Scholar]
- 24.Hirshberg B, Muszkat M, Schlesinger O, Rubinow A. Safety of low dose methotrexate in elderly patients with rheumatoid arthritis. Postgrad Med J. 2000;76:787–789. doi: 10.1136/pmj.76.902.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Genovese MC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Finck BK. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 26.Weinblatt ME, Kaplan H, Germain BF, Merriman RC, Solomon SD, Wall B, Anderson L, Block S, Irby R, Wolfe F. Low-dose methotrexate compared with auranofin in adult rheumatoid arthritis. A thirty-six-week, double-blind trial. Arthritis Rheum. 1990;33:330–338. doi: 10.1002/art.1780330305. [DOI] [PubMed] [Google Scholar]
- 27.Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, Canning C, Schneeweiss S. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheumatic Dis. 2006;65:1608–1612. doi: 10.1136/ard.2005.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roman MJ, Salmon JE. Cardiovascular Manifestations of Rheumatologic Diseases. Circulation. 2007;116:2346–2355. doi: 10.1161/CIRCULATIONAHA.106.678334. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Zhao P, Li A, Lv X, Gao Y, Sun H, Ding Y, Liu J. Effects of methotrexate on plasma cytokines and cardiac remodeling and function in postmyocarditis rats. Mediators Inflamm. 2009;2009:389720. doi: 10.1155/2009/389720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortiz Z, Shea B, Suarez Almazor M, Moher D, Wells G, Tugwell P. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2000:CD000951. doi: 10.1002/14651858.CD000951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.