Abstract
CaMKII plays a critical role in long-term potentiation (LTP). The kinase is a major component of the postsynaptic density (PSD); however, it is also contained in the spine cytoplasm. CaMKII can now be monitored optically in living neurons, and it is therefore important to understand the contribution of the PSD and cytoplasmic pools to optical signals. Here, we estimate the size of these pools under basal conditions. From EM immunolabeling data, we calculate that the PSD/cytoplasmic ratio is ~5%. A second independent estimate is derived from measurements indicating that the average mushroom spine PSD contains 90 to 240 holoenzymes. A cytoplasmic concentration of 16 µM (~2590 holoenzymes) in the spine can be estimated from the total measured CaMKII content of hippocampal tissue, the relative volume of different compartments, and the spine-dendrite ratio of CaMKII (2:1). These numbers yield a second estimate (6%) of the PSD/spine ratio in good agreement with the first. The CaMKII bound to the NMDAR is important because preventing the formation of this complex blocks LTP induction. We estimate that the percentage of spine CaMKII held active by binding to the NMDAR is ~0.2%. Implications of the high spine concentration of CaMKII (> 100 micromolar alpha subunits) and the small fraction within the PSD are discussed. Of particular note, the finding that the CaMKII signal in spines shows only transient activation (open state) after LTP induction is subject to the qualification that it does not reflect the small but important pool bound to the NMDAR.
Keywords: Ca2+/calmodulin-dependent protein Kinase II, postsynaptic density, spine, calmodulin, actin
Introduction
CaMKII is one of the most abundant brain proteins and comprises a major fraction of the protein in the postsynaptic density (PSD) (Cheng et al., 2006; Erondu and Kennedy, 1985; Kennedy et al., 1983). CaMKII has been strongly implicated in the induction of LTP, a cellular correlate of learning and memory. Biochemical studies show that CaMKII is activated during LTP induction (Fukunaga et al., 1993). Recent work shows that, when LTP is induced by two-photon uncaging at single identified spines, CaMKII activation is specific to the stimulated spine (Lee et al., 2009) and can therefore account for the synapse specificity of LTP. Consistent with a causal role for CaMKII activation, LTP is blocked if the activation of CaMKII is prevented by genetic or pharmacological methods (Giese et al., 1998; Ito et al., 1991; Silva et al., 1992). Moreover, if activated CaMKII subunits or holoenzymes are introduced into the cytoplasm, synaptic strength is increased and LTP is occluded (Lledo et al., 1995; Pi et al., 2010). Taken together, these results strongly implicate CaMKII in LTP induction.
The investigation of CaMKII function is complicated by the fact that there are different pools of the kinase, even within dendritic spines. CaMKII is a major component of the PSD (Kennedy et al., 1983), but there is also CaMKII in the spine cytoplasm, some of which is bound to actin (Lin and Redmond, 2008; Okamoto et al., 2007; Sanabria et al., 2009; Shen et al., 1998). CaMKII in the PSD can phosphorylate many synaptic proteins (Barria et al., 1997; Vinade and Dosemeci, 2000; Yoshimura et al., 2000). Notably, the phosphorylation of GluR1 increases the conductance of these channels (Derkach et al., 1999; Kristensen et al., 2011) and can contribute to the enhanced transmission during LTP (Benke et al., 1998; Poncer et al., 2002). Furthermore, phosphorylation of TARPs can enhance the number of AMPARs within the synapse (Opazo et al., 2010; Tomita et al., 2005). At least part of the PSD pool of CaMKII is bound to NMDARs (Leonard et al., 1999), an interaction that can itself lead to activation of the kinase (Bayer et al., 2001). The importance of this pool is indicated by the fact that overexpression of mutant NMDARs that cannot bind to CaMKII blocks the induction of LTP (Barria and Malinow, 2005). A recent paper indicates that the phosphorylation of stargazin is due to the pool of CaMKII bound to the NMDAR (Tsui and Malenka, 2006). There have been no previous estimates of the relative size of the different pools of CaMKII. Such estimates would be useful for understanding the role of different pools and interpreting the optical measurements of CaMKII activation in spines (Lee et al., 2009; Takao et al., 2005). Here, we combine data from several sources to estimate the size of the total pool, the cytoplasmic pool, the PSD pool, the pool bound to NMDAR, and the pool activated by the NMDAR.
Results
PSD/cytoplasmic ratio (R): Method 1 based on immunocytochemistry
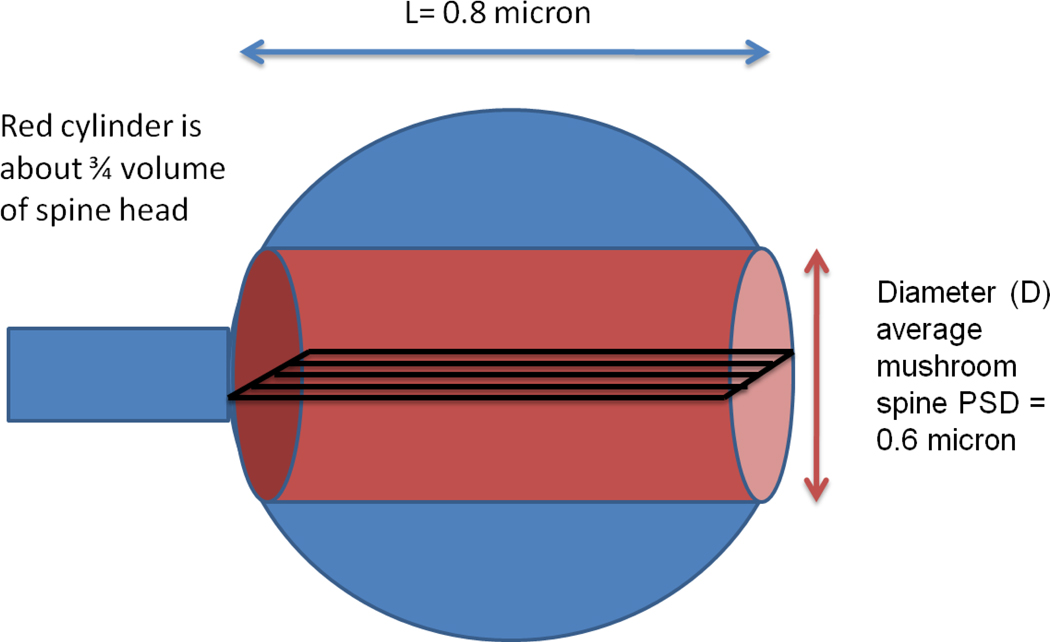
Measurements that provide a basis for calculating the relative size of the PSD and cytoplasmic pools of CaMKII in spines are presented in (Dosemeci et al., 2002). The CaMKII in spines of hippocampal pyramidal cells in primary culture was labeled by pre-embedding immunocytochemistry and was measured at the EM level using an antibody for the predominant alpha isoform. The spine cytoplasm labeling density (C) was 132 gold particles per square micron. The PSD labeling density (P) was 6.8 gold particles per micron length of PSD. These numbers have different units and thus do not give direct insight into the ratio (R) of PSD to cytoplasmic pools. However, R can be estimated from these numbers by using the geometrical framework shown in Fig.1. The spine head (represented by a sphere) contains an imaginary cylinder, the right end of which has the diameter (D) of the PSD. The spine head has the volume (0.27 µm3) of the average CA1 mushroom spine (Harris et al., 1992). Let R’ be the PSD/cytoplasm ratio in a thin section (black rectangle) of the cylinder. Then:
| eq. 1 |
where L is the length of the rectangle and equals diameter of the spine (0.8 µm) and D is the PSD diameter (0.6 micron) (Harris et al., 1992). Because D cancels out in eq.1 and L is approximately the same for any section within the cylinder (i.e., above or below the shown rectangle), the ratio, R’, applies to any section. It follows that R’ applies to the sum of all sections. Thus:
R’ = P/C*L=6.8(particles/µm)/(132(particles/µm2)*0.8 µm) =0.06
The volume of the spine in Fig.1 spine is 1.2 times the volume of the cylinder. Thus, the ratio (R) of CaMKII in the PSD to that in the spine is 0.05 (i.e., 5%).
Fig. 1.
Geometric framework used to calculate PSD/cytoplasmic CaMKII ratio by Method 1. Spine contains cylinder (red), the right side of which is the PSD (pink).
One concern regarding the above estimate is that pre-embedding labeling may underestimate the PSD CaMKII because the tight structure of the PSD prevents antibody penetration. However, the following calculations suggest that the underestimate is not large. From the direct mass measurements of the PSD, Chen et al. (Chen et al., 2005) found 80 holoenzymes in a 0.1 µm2 PSD. For the same area PSD, Petersen et al. (Petersen et al., 2003), using antibody labeling of isolated PSDs, found an average of 46 gold particles per PSD. Direct visualization of CaMKII “towers” showed that 80% of the non-occluded CaMKII “towers” were labeled. Thus, with 100% labeling efficiency (of accessible holoenzymes), 55 of the 80 holoenzymes of the average PSD would have been labeled. This suggests that only about 25 PSD CaMKII holoenzymes (~30% of the total) are inaccessible to antibody.
Method 2 for calculating R
The estimate of 5% derived above was based on immunolabeling of cells in primary culture. It would thus be useful to have an independent estimate based on measurements from actual brain tissue. Our second method is based on biochemical determinations of the number of CaMKII molecules in isolated PSDs and estimates of the total CaMKII concentration in spines.
PSDs can be isolated from mature brains. Although anoxia can lead to an enhancement of CaMKII in the PSD, rapid dissection and homogenization can minimize this and give an estimate of the CaMKII content of the PSD under basal conditions (Suzuki et al., 1994). According to experiments that determined the mass of CaMKII in the PSD compared to the total PSD mass (Chen et al., 2005), a 360 nm diameter PSD (area 0.1 µm2) contains an average of 80 CaMKII holoenzymes. Measurements by EM tomography (Petersen et al., 2003) directly visualized labeled CaMKII holoenzymes and yielded a somewhat lower value of 30–50. In both of these studies, tissue was prepared rapidly to avoid artifactual accumulation of CaMKII in the PSD. Taking 50 as an estimate for the holoenzyme content of a 0.1 µm2 PSD, there will be 150 holoenzymes (range 90–240) in the larger PSDs of the average mushroom spine, which have an area =0.30 µm2 (Harris et al., 1992).
The total number of CaMKII holoenzymes in the average mushroom spine can be estimated as follows from the measured average concentration of CaMKII in hippocampal tissue (1420 ng CaMKIIα subunit/100 µg protein ≈ 3.4 µM CaMKIIα holoenzymes) (Erondu and Kennedy, 1985). Considering that, in forebrain, there are three α subunits to every β subunit (Bennett et al., 1983; McGuinness et al., 1985), the total CaMKII holoenzyme concentration is 4.5 µM. This total CaMKII is distributed in tissue according to eq.2, which depends on the relative concentrations in different parts of the cell and the relative volume fraction of different parts. Let the concentration of CaMKII in spines = X. Based on fluorescence measurements of GFP-CaMKII (Otmakhov et al., 2004), the concentration in dendrites is half that in spines, i.e., = 0.5X (Zhang et al., 2008). Because these measurements were done using overexpressed kinase, there is concern that this value might not reflect that of endogenous kinase. However, two studies of endogenous CaMKII labeling (Liao et al., 2001; Merrill et al., 2005), though not quantified, show roughly equal fluorescence in large spines (~1 micron diameter) attached to small dendrites (~2 micron diameter). Given that the dendrite has a longer path length than spines, this is at least roughly consistent with the 0.5X value derived from overexpressed protein. Based on immunolabeling (Tao-Cheng et al., 2006), the concentration in axons (averaged over terminal and non-terminal regions) is low (we estimate this to be 0.2X). Interneurons and extracellular space contain very low concentrations of CaMKII. CaMKII is excluded from the nucleus but is contained in the soma cytoplasm (~20% of the cell body layer) at a concentration of 0.2X (Tao-Cheng et al., 2007); thus, the average concentration in the cell body layer (which has a volume fraction of ~0.15) is ~0.04X. Based on 3-D EM reconstruction of the molecular layer, the volume fraction of spines, dendrites, axons, and other (e.g., extracellular space) is 0.08, 0.30, 0.45, and 0.17, respectively (Mishchenko et al., 2010).
Thus, the total CaMKIIα concentration in hippocampal tissue:
| eq.2 |
This yields a value of X≈ 16 µM.
This value is in reasonable agreement with an estimate derived from antibody staining and absolute calibration of GFP fluorescence (personal communication, N. Otmakhov) that gives a value of 12 µM in spines (see also Lee, 2009). The average mushroom spine (0.27 µm3) (Harris et al., 1992) will thus contain ~2590 holoenzymes. As calculated above, the PSD of mushroom spines contains an average of 150 holoenzymes (range 90 to 240), meaning that the non-PSD pool is 2440. Thus, the PSD/cytoplasmic ratio (R) equals 0.06. Thus, 6% of spine CaMKII is in the PSD. The nearly exact agreement with Method 1 is probably fortuitous. It is difficult to quantify the errors of our estimates, but an uncertainty of +-30% seems likely.
CaMKII bound to NMDAR
The total number of NMDARs in the synapse has been estimated by various types of measurements (Table 1). A PSD contains 16–25 NMDA receptors, as determined by EM tomography (Chen et al., 2008). Protein measurement of the PSD (Cheng et al., 2006; Sheng and Hoogenraad, 2007) yields values of 20. Labeling with NMDAR antibodies by postembedding EM immunocytochemistry shows that small PSDs have 6–10 NMDARs (Racca et al., 2000). In the mouse hippocampus, related methods indicate that the average PSD contains 5–20 NMDA receptors (Shinohara et al., 2008). Computational models based on electrophysiological measurements estimated that a synapse may contain ~12 active NMDAR (Santucci and Raghavachari, 2008).
Table 1.
The number of NMDAR in postsynaptic density
| Author | No. of NMDAR |
Diameter of PSD (nm) |
Animal | Tissue |
|---|---|---|---|---|
| Chen, X | 20(16–25) | 400 | rat | hippocampus |
| Racca, C | 8(6–10) | 1151 | rat | hippocampus |
| Sheng, M | 20 | 360 | rat | forebrain |
| Shinohara, Y | 5–202 | 100–4002 | mouse | hippocampus |
| Santucci, DM | 12 | - | Computational model that bases on rat data | - |
Number based on synapse area in Table 2 of (Racca et al., 2000).
Numbers based on Fig. 4 of (Shinohara et al., 2008).
Assuming that there are 15 NMDA channels that contain NR2B and that each can bind to two CaMKII holoenzymes (through two NR2B subunits), 30 holoenzymes will be bound to the NMDAR. The CaMKII not bound to NMDAR is presumably bound to other CaMKII-binding proteins that include actinin, densin (Walikonis et al., 2001), and CaMKII itself (Khan et al., 2011; Hudmon et al., 2001; Petersen et al., 2003). Thus, of the 2590 holoenzymes in the average mushroom spine, about 150 are in the PSD, and a maximum of 50 of these (2%) will be bound to the NMDARs in the PSD. This estimate is an upper limit because not all NMDARs have CaMKII bound under basal conditions (see Discussion).
Discussion
We have derived the percentage of CaMKII in the spine that is contained in the PSD. This has been done using two independent methods. Method 1 is based on relative immunolabeling (Dosemeci et al., 2002). Method 2 is based on the determination of the absolute CaMKII content of isolated PSDs and the comparison of this value to our estimate of the total spine concentration, as calculated from the measured total concentration of CaMKII in the hippocampus (Erondu and Kennedy, 1985). These methods give 5% and 6%, respectively, but this very close agreement is probably fortuitous given that errors of least 30% are expected. We conclude that the fraction of total spine CaMKII that is in the PSD is small. In Method 1, we utilized results of immunolabeling of isolated cultured hippocampal neurons, whereas Method 2 depends on cells in intact tissue. The fact that these methods agree suggests that the state of CaMKII in isolated cells is a reasonable reflection of the intact system.
Fraction of tightly bound CaMKII, as measured by FRAP or photoactivation
Our conclusion that only a small fraction of spine CaMKII is in the PSD is consistent with estimates of the pool of very tightly bound CaMKII in spines. These estimates were obtained by using FRAP and photoactivation methods to determine the kinetics of unbinding of different CaMKII pools in the spine. FRAP measurements show several time constants of exchange for spine CaMKII, the fastest component being on the order of minutes. The slowest component shows no exchange in 30 minutes and is ~15% of the total (Sharma et al., 2006). Another study (Sturgill et al., 2009) used photoactivation methods and found that, at 30 minutes, ~15 % of the CaMKII still remained in the spine. It is clear that CaMKII in the PSD is tightly bound, as indicated by the fact that purified PSDs are tight-packed with CaMKII (Petersen et al., 2003) despite the fact that they have spent several hours in dilute solution during the procedures used to purify PSDs. From this, it can be concluded that the unbinding rate of CaMKII from the PSD structure must certainly be slower than 1 hour. Thus, the most tightly bound pools determined by FRAP and photoactivation place an upper limit on the size of the PSD pool of ~15%.
The FRAP and photoactivation studies show that the fastest component of CaMKII exit from spines has a time constant of <1 minute (Lee et al., 2009), ~3 minutes (Sharma et al., 2006), or <5 minutes (Sturgill et al., 2009). This fast component describes a relatively large fraction of the total CaMKII (~82% (Sharma et al., 2006); ~80% (Sturgill et al., 2009)). GFP has been found to leave with a much faster time constant of 1.2 seconds (Sharma et al., 2006) or 169 msec (Bloodgood and Sabatini, 2005). The difference between these latter two studies may be due to different spine neck size or spine size. Diffusion of CaMKII would be expected to be slower than for GFP. Given that the diffusion coefficient depends on the cube root of molecular weight, the expected diffusion of CaMKII out of the spine would be 500 msec or 3 sec (based on the different values of GFP diffusion above and given that the molecular weight of CaMKII is 25 times that of GFP). Because the observed fast component for CaMKII is much slower, we conclude that the fastest component of CaMKII exit from spines is rate-limited by an unbinding reaction rather than simple diffusion. Similar conclusions are nicely developed in (Byrne et al., 2010). Thus, the bulk of cytoplasmic CaMKII in spines is bound, albeit weakly. Beta CaMKII binds directly to actin (Sanabria et al., 2009; Shen et al., 1998), and since beta can be incorporated into holoenzymes that contain primarily alpha (Shen et al., 1998), beta-mediated binding to actin can affect alpha diffusion. In addition, binding of CaMKII to actin may be indirect via the binding of alpha CaMKII to alpha-actinin (Robison et al., 2005; Walikonis et al., 2001) and the binding of alpha-actinin to actin (Shirao and Sekino, 2001). The measured exit times for CaMKII are similar to the treadmilling rates of actin filaments in spines (Frost et al., 2010), raising the possibility that actin dynamics set the unbinding of CaMKII.
We calculated that the maximum of 2% of total spine CaMKII is bound to NMDARs. Coimmunoprecipitation studies show that there is basal CaMKII/NR2B and that the amount of complex is increased by about 2 fold during strong synaptic stimulation (Leonard et al., 1999). Thus, under basal conditions, 1% of CaMKII would be bound to NR2B, and this might double after LTP induction. An important observation is that, once bound to NR2B, CaMKII subunits are locked into an active state (Bayer et al., 2001). This can occur even without continued T286 autophosphorylation. However, this activation probably occurs only for the one of 12 subunits in a holoenzyme that is actually bound to NR2B. Thus, the pool of CaMKII subunits activated by binding to NR2B would be about ~1/10 of the total, i.e., ~0.2%.
Implications
These calculations have implications for the question of whether CaMKII is involved in LTP maintenance. There is general agreement that CaMKII activation plays a critical role in the induction of LTP (Lisman et al., 2002), but the question of whether the kinase has a role in LTP maintenance has been controversial (Buard et al., 2010; Lee et al., 2009; Lengyel et al., 2004; Otmakhov et al., 2004; Sanhueza et al., 2007; Sanhueza et al., 2011). Some studies based on biochemical assays of enzyme activity indicate that a small fraction (~10%) of CaMKII is persistently (>30 minutes) activated after LTP induction (Fukunaga et al., 1993; Lengyel et al., 2004) and thus could have a role in maintenance (Sanhueza et al., 2007). Moreover, persistently active CaMKII in the PSD can phosphorylate substrates in the PSD (Dosemeci et al., 2002; Dosemeci and Jaffe, 2010) and in the synaptic membrane (Opazo et al., 2010). However, a recent study used FRET to monitor the open (active) state of CaMKII in spines and found that activation caused by glutamate uncaging declined to baseline within one minute (Lee et al., 2009). This could be taken to imply that CaMKII is not involved in maintenance. Our results indicate that optical measurements on spines reflect primarily the cytoplasmic pool of CaMKII and thus may not reflect the important pool bound to NMDARs. The pool of CaMKII subunits bound to and activated by the NMDAR could be as low as 0.2%, much too small to be detected by current optical methods. The importance of this pool is underscored by the fact that mutations in NR2B that block the binding of CaMKII prevent LTP induction (Barria and Malinow, 2005).
Our results provide the first estimate of the bulk CaMKII concentration in spines and indicate that the concentration is enormously high; the holoenzyme concentration is ~10 micromolar, and thus the subunit concentration (each subunit is catalytic) is ~100 micromolar. Efficient activation of a high fraction of the enzyme would thus require a calmodulin concentration in the 100 micromolar range. Estimates of the average calmodulin concentration in hippocampal pyramidal cells put the level at ~100 µM (Faas et al., 2011). Moreover, it is possible that the calmodulin-binding protein, neurogranin, may further concentrate calmodulin in spines (Zhabotinsky et al., 2006).
The very high concentration of CaMKII also has implications for the use of CaMKII inhibitors. It is often assumed that 50% inhibition should occur if inhibitor concentration equals the binding affinity of the inhibitor for the enzyme, as determined in vitro. This is true in dilute solution but is not the case if the total enzyme concentration is much higher than affinity of the inhibitor. Thus, high-affinity inhibitors used in the10 micromolar range may not be able to inhibit spine CaMKII simply because there is not enough inhibitor to bind the 100 micromolar of CaMKII subunits. When binding to CaMKII depletes inhibitor concentration, concentration may be restored by diffusion from the dendrite. However, such diffusion will be slowed by the spine neck (Svoboda et al., 1996), so delivery of inhibitor during short periods cannot be assured. These difficulties of inhibiting the high concentration of CaMKII may help to explain why KN62 produced only a modest inhibition of CaMKII activation when this activation was measured in spines (Lee et al., 2009). These considerations suggest that negative results with CaMKII inhibitors must be interpreted with caution: there may simply be insufficient inhibitor to bind to the high concentration of CaMKII present in spines.
Finally, it is of interest to ask why there is such a large pool of spine CaMKII that is not in the PSD. To the extent that CaMKII crosslinks actin, the kinase may serve a structural role to maintain spine size (Okamoto et al., 2007). The CaMKII beta that is activated by calmodulin is released from actin (Ohta et al., 1986) when Ca2+ is elevated, as occurs during LTP induction. Furthermore, Ca2+/calmodulin will release CaMKII bound to actinin (Robison et al., 2005). These reactions may generate the free pool of CaMKII that binds to the PSD and thereby initiates some of the processes that enhance transmission.
Highlights.
CaMKII is contained in both the spine cytoplasm and in the PSD
The fraction of CaMKII in the PSD under basal conditions is less than 10%
Formation of the complex of CaMKII with the NMDAR is necessary for LTP
The fraction of CaMKII activated by binding to the NMDAR is less than 1%
The small fraction of CaMKII activated by binding to the NMDAR is too small to detect by current methods
Acknowledgments
We gratefully acknowledge the support of The Ellison Foundation and NIH grants R01 DA027807 and R01 NS27337.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Benke TA, Luthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Erondu NE, Kennedy MB. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983;258:12735–12744. [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–869. doi: 10.1126/science.1114816. [DOI] [PubMed] [Google Scholar]
- Buard I, Coultrap SJ, Freund RK, Lee YS, Dell'Acqua ML, Silva AJ, Bayer KU. CaMKII "autonomy" is required for initiating but not for maintaining neuronal long-term information storage. J Neurosci. 2010;30:8214–8220. doi: 10.1523/JNEUROSCI.1469-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne MJ, Waxham MN, Kubota Y. The impacts of geometry and binding on CaMKII diffusion and retention in dendritic spines. J Comput Neurosci. 2010 doi: 10.1007/s10827-010-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Vinade L, Leapman RD, Petersen JD, Nakagawa T, Phillips TM, Sheng M, Reese TS. Mass of the postsynaptic density and enumeration of three key molecules. Proc Natl Acad Sci U S A. 2005;102:11551–11556. doi: 10.1073/pnas.0505359102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Winters C, Azzam R, Li X, Galbraith JA, Leapman RD, Reese TS. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci U S A. 2008;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, Xu P, Wijayawardana SR, Hanfelt J, Nakagawa T, Sheng M, Peng J. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A, Vinade L, Winters CA, Reese TS, Tao-Cheng JH. Inhibition of phosphatase activity prolongs NMDA-induced modification of the postsynaptic density. J Neurocytol. 2002;31:605–612. doi: 10.1023/a:1025735410738. [DOI] [PubMed] [Google Scholar]
- Dosemeci A, Jaffe H. Regulation of phosphorylation at the postsynaptic density during different activity states of Ca2+/calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 2010;391:78–84. doi: 10.1016/j.bbrc.2009.10.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas GC, Raghavachari S, Lisman JE, Mody I. Calmodulin as a direct detector of Ca(2+) signals. Nat Neurosci. 2011 doi: 10.1038/nn.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost NA, Shroff H, Kong H, Betzig E, Blanpied TA. Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron. 2010;67:86–99. doi: 10.1016/j.neuron.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K, Stoppini L, Miyamoto E, Muller D. Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1993;268:7863–7867. [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Kim SA, Kolb SJ, Stoops JK, Waxham MN. Light scattering and transmission electron microscopy studies reveal a mechanism for calcium/calmodulin-dependent protein kinase II self-association. J Neurochem. 2001;76:1364–1375. doi: 10.1046/j.1471-4159.2001.00119.x. [DOI] [PubMed] [Google Scholar]
- Ito I, Hidaka H, Sugiyama H. Effects of KN-62, a specific inhibitor of calcium/calmodulin-dependent protein kinase II, on long-term potentiation in the rat hippocampus. Neurosci Lett. 1991;121:119–121. doi: 10.1016/0304-3940(91)90663-e. [DOI] [PubMed] [Google Scholar]
- Kennedy MB, Bennett MK, Erondu NE. Biochemical and immunochemical evidence that the "major postsynaptic density protein" is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1983;80:7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Zou Y, Amjad A, Gardezi A, Smith CL, Winters C, Reese TS. Sequestration of CaMKII in dendritic spines in silico. J Comput Neurosci. 2011 doi: 10.1007/s10827-011-0323-2. [DOI] [PubMed] [Google Scholar]
- Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir R, Traynelis SF. Mechanism of Ca(2+)/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci. 2011;14:727–735. doi: 10.1038/nn.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel I, Voss K, Cammarota M, Bradshaw K, Brent V, Murphy KP, Giese KP, Rostas JA, Bliss TV. Autonomous activity of CaMKII is only transiently increased following the induction of long-term potentiation in the rat hippocampus. Eur J Neurosci. 2004;20:3063–3072. doi: 10.1111/j.1460-9568.2004.03748.x. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1999;96:3239–3244. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Scannevin RH, Huganir R. Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J Neurosci. 2001;21:6008–6017. doi: 10.1523/JNEUROSCI.21-16-06008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Redmond L. CaMKIIbeta binding to stable F-actin in vivo regulates F-actin filament stability. Proc Natl Acad Sci U S A. 2008;105:15791–15796. doi: 10.1073/pnas.0804399105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci U S A. 1995;92:11175–11179. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness TL, Lai Y, Greengard P. Ca2+/calmodulin-dependent protein kinase II. Isozymic forms from rat forebrain and cerebellum. J Biol Chem. 1985;260:1696–1704. [PubMed] [Google Scholar]
- Merrill MA, Chen Y, Strack S, Hell JW. Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol Sci. 2005;26:645–653. doi: 10.1016/j.tips.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Mishchenko Y, Hu T, Spacek J, Mendenhall J, Harris KM, Chklovskii DB. Ultrastructural analysis of hippocampal neuropil from the connectomics perspective. Neuron. 2010;67:1009–1020. doi: 10.1016/j.neuron.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Nishida E, Sakai H. Type II Ca2+/calmodulin-dependent protein kinase binds to actin filaments in a calmodulin-sensitive manner. FEBS Lett. 1986;208:423–426. doi: 10.1016/0014-5793(86)81061-4. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci U S A. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, Choquet D. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Otmakhov N, Tao-Cheng JH, Carpenter S, Asrican B, Dosemeci A, Reese TS, Lisman J. Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J Neurosci. 2004;24:9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JD, Chen X, Vinade L, Dosemeci A, Lisman JE, Reese TS. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. J Neurosci. 2003;23:11270–11278. doi: 10.1523/JNEUROSCI.23-35-11270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Otmakhov N, Lemelin D, De Koninck P, Lisman J. Autonomous CaMKII can promote either long-term potentiation or long-term depression, depending on the state of T305/T306 phosphorylation. J Neurosci. 2010;30:8704–8709. doi: 10.1523/JNEUROSCI.0133-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncer JC, Esteban JA, Malinow R. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by alpha-Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2002;22:4406–4411. doi: 10.1523/JNEUROSCI.22-11-04406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci. 2000;20:2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Bartlett RK, Bass MA, Colbran RJ. Differential modulation of Ca2+/calmodulin-dependent protein kinase II activity by regulated interactions with N-methyl-D-aspartate receptor NR2B subunits and alpha-actinin. J Biol Chem. 2005;280:39316–39323. doi: 10.1074/jbc.M508189200. [DOI] [PubMed] [Google Scholar]
- Sanabria H, Swulius MT, Kolodziej SJ, Liu J, Waxham MN. {beta}CaMKII regulates actin assembly and structure. J Biol Chem. 2009;284:9770–9780. doi: 10.1074/jbc.M809518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, McIntyre CC, Lisman JE. Reversal of synaptic memory by Ca2+/calmodulin-dependent protein kinase II inhibitor. J Neurosci. 2007;27:5190–5199. doi: 10.1523/JNEUROSCI.5049-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, Fernandez-Villalobos G, Stein IS, Kasumova G, Zhang P, Bayer KU, Otmakhov N, Hell JW, Lisman J. Role of the CaMKII/NMDA Receptor Complex in the Maintenance of Synaptic Strength. J Neurosci. 2011;31:9170–9178. doi: 10.1523/JNEUROSCI.1250-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci DM, Raghavachari S. The effects of NR2 subunit-dependent NMDA receptor kinetics on synaptic transmission and CaMKII activation. PLoS Comput Biol. 2008;4:e1000208. doi: 10.1371/journal.pcbi.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Fong DK, Craig AM. Postsynaptic protein mobility in dendritic spines: long-term regulation by synaptic NMDA receptor activation. Mol Cell Neurosci. 2006;31:702–712. doi: 10.1016/j.mcn.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Subramanian K, Meyer T. CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Hirase H, Watanabe M, Itakura M, Takahashi M, Shigemoto R. Left-right asymmetry of the hippocampal synapses with differential subunit allocation of glutamate receptors. Proc Natl Acad Sci U S A. 2008;105:19498–19503. doi: 10.1073/pnas.0807461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirao T, Sekino Y. Clustering and anchoring mechanisms of molecular constituents of postsynaptic scaffolds in dendritic spines. Neurosci Res. 2001;40:1–7. doi: 10.1016/s0168-0102(01)00209-7. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Sturgill JF, Steiner P, Czervionke BL, Sabatini BL. Distinct domains within PSD-95 mediate synaptic incorporation, stabilization, and activity-dependent trafficking. J Neurosci. 2009;29:12845–12854. doi: 10.1523/JNEUROSCI.1841-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Okumura-Noji K, Tanaka R, Tada T. Rapid translocation of cytosolic Ca2+/calmodulin-dependent protein kinase II into postsynaptic density after decapitation. J Neurochem. 1994;63:1529–1537. doi: 10.1046/j.1471-4159.1994.63041529.x. [DOI] [PubMed] [Google Scholar]
- Svoboda K, Tank DW, Denk W. Direct measurement of coupling between dendritic spines and shafts. Science. 1996;272:716–719. doi: 10.1126/science.272.5262.716. [DOI] [PubMed] [Google Scholar]
- Takao K, Okamoto K, Nakagawa T, Neve RL, Nagai T, Miyawaki A, Hashikawa T, Kobayashi S, Hayashi Y. Visualization of synaptic Ca2+ /calmodulin-dependent protein kinase II activity in living neurons. J Neurosci. 2005;25:3107–3112. doi: 10.1523/JNEUROSCI.0085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Dosemeci A, Winters CA, Reese TS. Changes in the distribution of calcium calmodulin-dependent protein kinase II at the presynaptic bouton after depolarization. Brain Cell Biol. 2006;35:117–124. doi: 10.1007/s11068-007-9012-5. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Gallant PE, Brightman MW, Dosemeci A, Reese TS. Structural changes at synapses after delayed perfusion fixation in different regions of the mouse brain. J Comp Neurol. 2007;501:731–740. doi: 10.1002/cne.21276. [DOI] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Tsui J, Malenka RC. Substrate localization creates specificity in calcium/calmodulin-dependent protein kinase II signaling at synapses. J Biol Chem. 2006;281:13794–13804. doi: 10.1074/jbc.M600966200. [DOI] [PubMed] [Google Scholar]
- Vinade L, Dosemeci A. Regulation of the phosphorylation state of the AMPA receptor GluR1 subunit in the postsynaptic density. Cell Mol Neurobiol. 2000;20:451–463. doi: 10.1023/A:1007019030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walikonis RS, Oguni A, Khorosheva EM, Jeng CJ, Asuncion FJ, Kennedy MB. Densin-180 forms a ternary complex with the (alpha)-subunit of Ca2+/calmodulin-dependent protein kinase II and (alpha)-actinin. J Neurosci. 2001;21:423–433. doi: 10.1523/JNEUROSCI.21-02-00423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Aoi C, Yamauchi T. Investigation of protein substrates of Ca(2+)/calmodulin-dependent protein kinase II translocated to the postsynaptic density. Brain Res Mol Brain Res. 2000;81:118–128. doi: 10.1016/s0169-328x(00)00170-4. [DOI] [PubMed] [Google Scholar]
- Zhabotinsky AM, Camp RN, Epstein IR, Lisman JE. Role of the neurogranin concentrated in spines in the induction of long-term potentiation. J Neurosci. 2006;26:7337–7347. doi: 10.1523/JNEUROSCI.0729-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Holbro N, Oertner TG. Optical induction of plasticity at single synapses reveals input-specific accumulation of alphaCaMKII. Proc Natl Acad Sci U S A. 2008;105:12039–12044. doi: 10.1073/pnas.0802940105. [DOI] [PMC free article] [PubMed] [Google Scholar]