Abstract
Objectives
To explore the experience of reproductive-age women in the French population with premenstrual syndrome (PMS) by estimating perceived symptom prevalence, identifying risk factors, and quantifying the burden of symptoms. This study also assesses the stability of the PMS diagnosis over a 1-year period of follow-up.
Methods
The prevalence of reported PMS was estimated from a population-based cohort of 2863 French women interviewed in 2003 and 2004. Multivariate logistic regressions were used to identify risk factors associated with PMS. PMS fluctuation was studied by comparing women's responses in 2003 and 2004.
Results
Results show that 4.1% of women qualified for severe PMS (six symptoms) and 8.1% qualified for moderate PMS (one to five symptoms), resulting in 12.2% of women who reported PMS symptoms that impacted their daily lives. Risk factors for PMS fell into three categories: hormonal, psychosocial, and physiological, with life stressors and exogenous hormonal exposure exerting the most substantial impact. Results also indicate a high level of intraindividual variation in PMS status over time; among women who qualified for PMS during 1 or both years of the study, 72% demonstrated fluctuation in their PMS status.
Conclusions
More women report suffering from distressing premenstrual symptoms than are captured by strict premenstrual dysphoric disorder (PMDD) diagnostic criteria. The impact of PMS symptoms on women appears to fluctuate over time, however, producing greater variability in the syndrome than previously recognized. Clinicians should be mindful of high intraindividual variability in the syndrome when advising patients about long-term management.
Introduction
Although it has been shown that the majority of reproductive-age women experience some premenstrual symptoms in the days leading up to menses,1 a minority of women report that these symptoms significantly reduce their quality of life. Prior studies have demonstrated that severe premenstrual symptoms can have consequences on social functioning, work productivity, and healthcare use,2–4 but the percentage of women in the general population who face these negative outcomes is still the subject of debate.
Much of the challenge in quantifying the burden of premenstrual symptoms rests in the lack of uniformity of premenstrual syndrome (PMS) definitions and study methodology. Epidemiological studies have historically reported PMS prevalence based on the criteria for premenstrual dysphoric disorder (PMDD) provided in the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV).5 To meet the criteria for this diagnosis, a woman must demonstrate the presence of at least five emotional and physical symptoms in two consecutive menstrual cycles by prospective report and note marked interference due to symptoms.5 Studies using these criteria cite a PMDD prevalence ranging from 3% to 9%.6
More recent work has tried to loosen the restrictive PMDD criteria to create a broader definition of PMS, placing emphasis on the negative ramifications of living with premenstrual symptoms.2,3 Wittchen et al.7 contributed to this effort by demonstrating that 18.6% of women met subthreshold criteria for PMDD but nevertheless demonstrated a significant increase in the risk of suicide attempt. Noting these findings, Halbreich et al.6 suggested that researchers substitute a more relevant PMS definition that would encompass both PMDD and the cases of moderate/severe PMS that cause recognizable “suffering, distress, and impairment” to women. This approach is also supported by the 2000 American College of Obstetricians and Gynecologists (ACOG) PMS guidelines, which base PMS diagnosis on the impact, rather than the number, of symptoms. Using this new definition of PMS, Halbreich et al.6 cite a lifetime PMS prevalence ranging from 13% to 19%. However, challenges remain, as ethnic/cultural differences in the reporting of premenstrual symptoms and differences in the questionnaires and methods used to conduct epidemiological studies further complicate the effort to obtain an accurate prevalence of PMS. Among the handful of population-based studies that tackle this question, the prevalence ranges from 5.3% to 50.2%.8–10
There is consensus that reliance on prospectively collected data in the form of a validated daily journal of symptoms is the gold standard for PMS diagnosis, such as is required for a diagnosis of PMDD.11 However, this approach does not allow for assessment of PMS/PMDD prevalence among a large, nationally representative sample of women. Cohen et al.12 confronted this challenge in their study of PMS/PMDD prevalence among American women, with only 12% of their original sample completing daily diaries. Moreover, even women who complete daily diaries rarely do so for more than one cycle.13,14 Many studies that have analyzed PMS/PMDD prevalence among the general population have, therefore, resorted to using retrospective data.8,10 Although retrospective studies may suffer from recall bias or reinterpretation of past events and, therefore, may not allow for precise data collection, they have the benefit of addressing women's personal experiences with symptoms over time.
Several studies across disciplines have sought to identify risk factors that predispose women to a personally distressing form of PMS, and such studies have informed a multi-factorial theory of PMS. Bancroft15 summarized the PMS risk factor profile as including a timing factor (controlled by neurotransmitters), a menstruation factor (involving physiological changes preceding menses), and a vulnerability factor (involving personality, predisposition to depression, attitude toward menstruation). However, as much of the previous work on PMS risk factors has been conducted on clinically derived cohorts, it is yet unknown how this risk factor profile applies to the general population.
The objective of our study is to quantify the burden of premenstrual symptoms based on women's subjective assessment, estimate the prevalence of PMS among French women, and identify factors associated with PMS. We also aim to assess the stability of the PMS diagnosis over a 1-year period of follow-up.
Materials and Methods
The COCON survey
The data for this study were drawn from the COhort CONtraception (COCON) survey, which was designed to gather information about contraceptive practices and abortion in France. The study has been described in detail elsewhere.16 The COCON survey followed a national, population-based cohort of reproductive-age women (18–44 years) over a 5-year period, from 2000 to 2004, using annual structured interviews to obtain data. The interviews were conducted over the telephone (Computer Assisted Telephone Interviewing (CATI)) by trained interviewers. In 2000, the initial sample of 2863 French-speaking women was identified through random telephone directory selection (response rate = 74.6%). An unequal probability of inclusion was used to overrepresent women who had had an abortion or an unintended pregnancy in the 5 years preceding the survey (sampling fraction = 100%, n = 1034), whereas only a fraction of the other women was selected at random (sampling fraction = 19%, n = 1829). Final results were weighted to reflect the sample design and the sociodemographic composition of French women according to the 1999 census.
After the initial interview in 2000 on entry into the cohort, women agreeing to participate were followed up once per year for 4 years (2001–2004) to explore any changes in contraceptive practices that had occurred since the previous interview. In 2003, questions about premenstrual symptoms were added to the original questionnaire. Data for the current study, therefore, were drawn from the last 2 years of follow-up (2003–2004.)
Of the initial cohort of 2863 women, 2217 women completed the first follow-up questionnaire in 2001, 1912 women completed the 2002 follow-up questionnaire, 1725 women completed the 2003 follow-up questionnaire, and 1569 completed the 2004 questionnaire. Although this substantial reduction in the sample size affects the precision of the statistical analysis, the attrition of the cohort between 2000 and 2002 did not suffer from selection bias on the main variable of interest, contraceptive practice.17 Furthermore, we found no differences in the proportion lost to follow-up (10% of the cohort) by PMS status between 2003 and 2004.
Study population
From the women who were interviewed in 2003 and 2004, a study population was identified to analyze PMS. Women were excluded from the study population if they had been told by their doctor that they were either menopausal or premenopausal and were having no/almost no more periods. Women who were pregnant or breastfeeding at the time of the survey were also excluded, as were women who had been breastfeeding for more than 10 months of the previous year. Women were further excluded if they had undergone hysterectomy or if they had been diagnosed with cancer in the past year, as they might experience with nonspecific symptoms similar to symptoms of PMS. Lastly, women were excluded from the study population if they did not answer the series of questions pertaining to PMS. The final study population included 1587 women in 2003 (92% of follow-up, with 141 women excluded) and 1440 women in 2004 (92% of follow-up, with 129 women excluded).
Questionnaires
The follow-up questionnaires (2001–2004) took a mean duration of 20 minutes to complete. Interviews were conducted over the telephone (CATI, converso) by trained interviewers. To ensure anonymity, contact information was automatically erased from the files in which the responses were recorded. Interviews were designed to document all changes over the past 12 months: sexual relationships, social and professional situation, stressful life events, pregnancies and contraceptive methods used during the year, gynecological problems, or serious illnesses. The following questions regarding PMS were introduced in the 2003 questionnaire and repeated in the 2004 interview:
During the year, in the few days preceding menses, have you experienced the following problems?
Marked depressive symptoms, despair, feeling undervalued
Marked anxiety, impression of being knotted up, tension, nervousness
Sadness, feeling of not being understood, wanting to cry
Anger, marked and persistent irritability• Lack of energy and motivation with regard to work, hobbies
Physical symptoms: tension, breast tenderness, headache, stomachache, bloating
The emotional symptoms addressed have high concordance with the premenstrual daily symptom diary recommended by the ACOG for diagnostic purposes,18 which includes 12 emotional symptoms and 9 physical symptoms rated on a scale from 1 to 6 (not at all to severe). In addition, the emotional symptoms asked about in the COCON survey are those given higher priority in the DSM-IV diagnostic criteria for PMDD and are worded similarly to the criteria listed in the DSM-IV.5
Following a model set forth by other authors,14 we developed a tiered system that was intended to capture the women's subjective evaluation of premenstrual symptoms. We defined a four-category PMS classification (Table 1), in which the definition of severe PMS corresponds most closely to the definition of PMDD in the DSM-IV. Under this classification, women were divided into two large categories, those who reported symptoms consistent with PMS and those who did not. Our PMS definition connotes a syndrome consisting of self-reported cyclic premenstrual symptoms that cause women disturbance in their daily functioning. Among the group of women who suffered from distressing PMS symptoms, those who reported the presence of all six emotional and physical symptoms were classified as having severe PMS. Women who did not meet criteria for PMS were subdivided into two groups, those with cyclic symptoms that did not cause disturbance in daily life and those without any cyclic symptoms.
Table 1.
Distribution of Observations by PMS Categories
| |
No PMS 2623 (87.8) |
PMS 404 (12.2) |
||
|---|---|---|---|---|
| n (%) Definition | No cyclic symptoms | Cyclic symptoms | Moderate PMS | Severe PMS |
| Number of cyclic symptoms | 0 | ≥1 | ≥1, <6 | All (6) |
| At least 2 consecutive cycles with symptoms | No | Yes | Yes | Yes |
| Symptoms remit following menses | No | Yes | Yes | Yes |
| Significant impact on daily life | No | No | Yes | Yes |
| n (%) | 1328 (47.3) | 1295 (40.5) | 277 (8.1) | 127 (4.1) |
Data analysis
We first examined the sociodemographic, physiological, behavioral, hormonal, and emotional factors associated with PMS, using the definition of PMS based on self-reported distressing symptoms and menstrual cyclicity. As no associations were found between the year of the interview and any of the factors analyzed, observations from the 2 years of data collection were integrated to produce a combined 2003–2004 dataset (n = 3027), with women contributing either one or two observations to the dataset. Data analysis was then performed on the combined dataset of observations. A cluster effect was introduced to take into account the fact that two observations could belong to the same woman. The multivariate analysis, which included variables with p values of <0.2 in the univariate analysis, was performed using two logistic regression models, one with the variables concerning menstrual complaints and one without these variables. We did so because we hypothesized that menstrual characteristics were associated with PMS, but we were unsure if an association between menstrual complaints and PMS would reflect a causal relationship or different manifestations of hormonal fluctuation throughout the menstrual cycle. We used the same strategy to examine factors associated with the more restrictive definition of severe PMS (all symptoms combined), but as only a small number of women qualified for severe PMS (n = 127), we present results solely from the univariate analysis.
In the second part of the analysis, we examined variation in PMS status over the 2-year period, using data provided by the subsample of women who answered both the 2003 and 2004 questionnaires and met study population criteria in both years (n = 1350). We also explored factors contributing to a change in PMS status, comparing women who did not meet criteria for PMS in either year with those who were PMS free in 2003 and developed PMS in 2004. However, the number of women included in this analysis was too small for a multivariate analysis.
Results
Determinants of PMS
Over the 2-year study period, 12.2% of women met criteria for PMS, reporting that they suffered from at least one of six cyclic premenstrual symptoms that was present for at least two cycles and caused significant impact on their lives (Table 1).These women reported an average of 4.3 symptoms, which did not differ between 2003 and 2004 (data not shown). Of these women, about one third (4.1% of the study population) qualified for the severe version of the syndrome, reporting the presence of all six emotional and physical symptoms. Women without PMS comprised 87.8% of the total study population, with just under half (40.5% of the total population) reporting cyclic menstrual symptoms that did not significantly impact their lives. Of the women who reported all six cyclic premenstrual symptoms, 42% reported that their symptoms did not impact their daily lives.
Both in the univariate analysis (Table 2)and in the multivariate regression models, we found no significant associations between PMS status and age, parity, employment status, living situation, educational achievement, age at menarche, or regularity of menses (Table 3).Conversely, contraceptive use was found to be highly correlated with PMS, as women using a hormonal contraceptive method were half as likely to demonstrate PMS as those using a nonhormonal method. Nevertheless, 29% of women with PMS were oral contraceptive pill users. Among women using a hormonal method, we found no difference between those using a method containing levonorgesterol and others. The protective effect of hormonal contraception held true even after excluding women reporting amenorrhea (data not shown). Women with a low BMI tended to be less likely than other women to meet criteria for PMS, with statistical significance achieved for the protective effect of a low BMI under 18.5. Finally, women with PMS were more likely than those without PMS to report a stressful event in the past year (OR = 2.3, p < 0.001).
Table 2.
Factors Associated with PMS, Univariate Analysisa
| |
PMS |
Severe PMS |
||||||
|---|---|---|---|---|---|---|---|---|
| Sociodemographic, physiological, hormonal factors | PMS n | No PMS n | % with PMS | p value | PMS n | No PMS n | % with severe PMS | p value |
| Total | 404 | 2623 | 12.2 | 127 | 2900 | 4.1 | ||
| Age, years | ||||||||
| ≤30 | 65 | 575 | 8.8 | 0.08 | 17 | 623 | 2.7 | 0.37 |
| 30–40 | 180 | 1099 | 13.8 | 53 | 1226 | 4.8 | ||
| ≥40 | 159 | 949 | 13.7 | 57 | 1051 | 4.7 | ||
| Ever been pregnant | ||||||||
| No | 51 | 439 | 9.9 | 0.16 | 11 | 479 | 2.7 | 0.26 |
| Yes | 353 | 2184 | 13.2 | 116 | 2421 | 4.7 | ||
| Employment | ||||||||
| Working | 297 | 2176 | 11.3 | 0.11 | 91 | 2382 | 3.9 | 0.47 |
| Student/homemaker/retired | 68 | 292 | 15.6 | 27 | 333 | 5.7 | ||
| Unemployed | 39 | 154 | 17.0 | 9 | 184 | 3.4 | ||
| Education | ||||||||
| No diploma | 61 | 295 | 15.8 | 0.29 | 20 | 336 | 6.2 | 0.36 |
| CAP/BEP | 118 | 661 | 11.9 | 37 | 742 | 3.0 | ||
| Bac/Bac+2 | 138 | 984 | 11.2 | 42 | 1080 | 3.7 | ||
| >Bac+2 | 87 | 683 | 10.9 | 28 | 742 | 4.0 | ||
| Living situation | ||||||||
| Married | 231 | 1493 | 13.2 | 0.65 | 69 | 1655 | 3.9 | 0.94 |
| Cohabitating | 88 | 577 | 11.2 | 29 | 636 | 4.5 | ||
| Single | 85 | 553 | 11.5 | 29 | 609 | 4.2 | ||
| BMI | ||||||||
| <18.5 | 21 | 210 | 6.1 | 0.06 | 4 | 227 | 0.8 | 0.14 |
| ≥18.5 to <30 | 355 | 2240 | 12.6 | 114 | 2481 | 4.4 | ||
| ≥30 | 27 | 167 | 15.3 | 9 | 185 | 4.4 | ||
| Stressful event in the past year | ||||||||
| No | 194 | 1774 | 9.4 | <0.001 | 59 | 1909 | 3.1 | 0.001 |
| Yes | 210 | 948 | 17.7 | 68 | 991 | 6.2 | ||
| Contraception | ||||||||
| Nonhormonal methodb | 250 | 1174 | 16.5 | 0.003 | 85 | 1339 | 6.1 | 0.02 |
| Progestin-only methodc | 46 | 440 | 8.7 | 16 | 470 | 2.8 | ||
| Combined | ||||||||
| Estrogen/progestin | 105 | 982 | 8.8 | 24 | 1063 | 2.3 | ||
| Menstrual characteristics | ||||||||
| Age at menarche | ||||||||
| <11 | 21 | 101 | 20.3 | 0.18 | 10 | 112 | 11.5 | 0.05 |
| ≥11 | 379 | 2469 | 12.0 | 116 | 2732 | 3.8 | ||
| Regular periods | ||||||||
| No | 81 | 537 | 14.0 | 0.35 | 24 | 594 | 6.4 | 0.06 |
| Yes | 323 | 2086 | 11.8 | 103 | 2306 | 3.6 | ||
| Periods lasting >1 week | ||||||||
| No | 328 | 2404 | 10.8 | <0.001 | 92 | 2640 | 3.1 | <0.001 |
| Yes | 76 | 219 | 24.0 | 35 | 260 | 12.3 | ||
| Dysmenorrhea | ||||||||
| No | 245 | 2180 | 8.6 | <0.001 | 65 | 2360 | 2.4 | <0.001 |
| Yes | 159 | 443 | 25.6 | 62 | 540 | 10.2 | ||
| Menorrhagia | ||||||||
| No | 256 | 1990 | 10.1 | <0.001 | 77 | 2169 | 2.8 | 0.003 |
| Yes | 148 | 633 | 19.0 | 50 | 731 | 8.1 | ||
Analysis was performed on the combined dataset of observations (2003–2004), using a cluster effect to take into account the intrawoman correlation.
Copper IUD/barrier/traditional, or no method.
Mirena IUD, progestin-only pill, implant.
Table 3.
Factors Associated with PMS, Multivariate Analysisa
| |
PMS model 1 |
PMS model 2 |
||||
|---|---|---|---|---|---|---|
| Sociodemographic, physiological, hormonal factors | Adjusted OR | 95% CI | p value | Adjusted OR | 95% CI | p value |
| Total n = 3042 | ||||||
| Age, years | ||||||
| ≤30 | 1 | 0.06 | 1 | 0.09 | ||
| >30 | 1.6 | 1.0–2.5 | 1.5 | 1.0–2.4 | ||
| Ever been pregnant | ||||||
| No | 1 | 0.72 | 1 | 0.88 | ||
| Yes | 0.9 | 0.6–1.5 | 1.0 | 0.6–1.7 | ||
| Employment | ||||||
| Working | 1 | 0.07 | 1 | 0.26 | ||
| Not working | 1.5 | 1.0–2.2 | 1.3 | 0.8–1.9 | ||
| BMI | ||||||
| >18.5 | 0.5 | 0.3–1.0 | 0.09 | 0.6 | 0.3–1.1 | 0.19 |
| ≥18.5 to <30 | 1 | 1 | ||||
| ≥30 | 1.2 | 0.6–2.2 | 1.0 | 0.6–1.8 | ||
| Stressful event in the past year | ||||||
| No | 1 | <0.001 | 1 | <0.001 | ||
| Yes | 2.3 | 1.7–3.3 | 2.1 | 1.5–3.0 | ||
| Contraception | ||||||
| Nonhormonal methodb | 1 | 0.001 | 1 | 0.1 | ||
| Progestin-only methodc | 0.5 | 0.3–0.8 | 0.5 | 0.3–1.0 | ||
| Combined estrogen/progestin | 0.5 | 0.4–0.8 | 0.8 | 0.5–1.2 | ||
| Menstrual characteristics | ||||||
| Age at menarche | ||||||
| <11 | 1.9 | 0.8–4.5 | 0.13 | 1.6 | 0.7–3.4 | 0.27 |
| ≥11 | 1 | 1 | ||||
| Periods lasting >1 week | ||||||
| No | 1 | 0.01 | ||||
| Yes | 1.8 | 1.1–2.9 | ||||
| Dysmenorrhea | ||||||
| No | 1 | <0.001 | ||||
| Yes | 2.9 | 2.0–4.2 | ||||
| Menorrhagia | ||||||
| No | 1 | 0.43 | ||||
| Yes | 1.2 | 0.8–1.7 | ||||
Analysis was performed on the combined dataset of observations (2003–2004), using a cluster effect to take into account the intrawoman correlation.
Copper IUD/barrier/traditional, or no method.
Mirena IUD, progestin-only pill, implant.
When we included menstrual symptoms in the regression model, the correlations remained significant, with the exception of the protective effect of hormonal contraceptive use (Table 3).However, progestin-only methods remained protective in this second regression model. In addition, results of this model show a strong association between menstrual disorders and PMS, with women who reported painful menses being three times as likely to meet criteria for PMS and women who reported periods lasting for longer than 1 week being twice as likely to meet criteria, even after controlling for use of hormonal contraception.
Determinants of severe PMS
When restricting the analysis to severe PMS, the subgroup of women who reported all six physical and emotional symptoms, results showed consistent relationships with the factors identified in the analysis of clinically relevant PMS (Table 2).The small percentage (4.1%) of women with severe PMS were also more likely to be using nonhormonal contraception, to have had a stressful event in the past year, and to suffer from menstrual complaints, especially dysmenorrhea and periods lasting longer than 1 week (Table 2).
Variation in PMS symptoms between the 2 years (2003–2004) of data collection
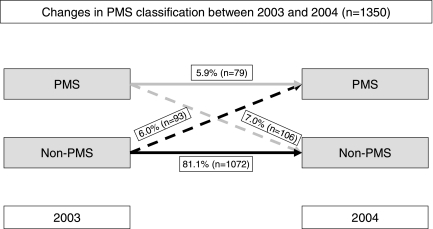
As illustrated in Figure 1, we found substantial fluctuation in the PMS status of women between 2003 and 2004. Among women who completed both years of follow-up, 81.1% did not qualify for the PMS definition in either year, 5.9% qualified for PMS in both years, and 13% met PMS criteria in only 1 of the years for which survey data were collected. Therefore, among women who qualified for PMS during 1 or both years of the study, 72% demonstrated fluctuation in their PMS status.
FIG. 1.
Changes in PMS classification between 2003 and 2004.
A more detailed comparison of the women's individual PMS status in 2003 and 2004 (Table 4)shows that the greatest variation from 1 year to the next was seen among women who qualified for moderate PMS in 2003 (women who reported at least one cyclic, distressing PMS symptom but reported fewer than six PMS symptoms). Of these women, about half continued to report cyclic premenstrual symptoms but noted that these symptoms no longer had a substantial impact on their lives. Only 36% of women with moderate PMS in 2003 reported symptoms that caused significant impact in 2004. These figures indicate that the predominant reason for variation in PMS status over time was a change in response to the question: Do these symptoms have a significant impact on your life? a requisite criterion for the PMS definition used in this analysis.
Table 4.
Variation in PMS Status Between 2003 and 2004 by PMS Category
| |
2003 |
2004 |
|
||
|---|---|---|---|---|---|
| No cyclic symptoms (%) | Cyclic symptoms (%) | Moderate PMS (%) | Severe PMS (%) | Total (%) | |
| No cyclic symptoms | 69.8 | 26.1 | 3.4 | 0.7 | 100 |
| Cyclic symptoms | 30.8 | 59.3 | 8.1 | 1.9 | 100 |
| Moderate PMS | 16.8 | 47.0 | 27.1 | 9.1 | 100 |
| Severe PMS | 9.8 | 25.9 | 18.6 | 45.7 | 100 |
| No PMS | PMS | ||||
| No PMS | 93.1 | 6.9 | 100 | ||
| PMS | 54.3 | 45.7 | 100 | ||
Less variation was seen among women who qualified for the most severe form of PMS. Of these women, 65% qualified for PMS in 2004 (46% with severe PMS, 19% with moderate PMS). Demonstrating even less variation, only 14% of women who did not qualify for PMS in 2003 met criteria for PMS in 2004. Of these women, 69% reported cyclic symptoms that did not impact their life in 2003 but did have an impact the following year.
To examine factors that potentially contributed to the development of PMS between 2003 and 2004, we compared women who met criteria for our definition for PMS after 2003 with those who remained PMS free in both years. Results indicate that menstrual symptoms and a body mass index (BMI) >30 were significantly associated with new onset of PMS (Table 5).Women reporting a stressful event in the past year also tended to be more likely to qualify for PMS in 2004 (9.8% vs. 5.5%, p = 0.06).
Table 5.
Factors Associated with PMS between 2003 and 2004
| |
Women without PMS in 2003 |
|||
|---|---|---|---|---|
| Sociodemographic, physiological, hormonal factors | PMS in 2004 (n) | No PMS in 2004 (n) | % with PMS in 2004 (n) | p value |
| Total | 93 | 1072 | 6.9 | |
| Age, years | ||||
| ≤30 | 14 | 206 | 5.3 | 0.4 |
| ≥40 | 79 | 865 | 7.5 | |
| Ever been pregnant | ||||
| No | 8 | 183 | 4.4 | 0.23 |
| Yes | 85 | 889 | 7.9 | |
| Employment | ||||
| Working | 72 | 908 | 6.4 | 0.32 |
| Not working | 21 | 163 | 9.0 | |
| Living situation | ||||
| Married | 54 | 608 | 7.4 | 0.85 |
| Cohabitating | 21 | 228 | 6.0 | |
| Single | 18 | 236 | 6.7 | |
| BMI | ||||
| <30 | 83 | 997 | 6.7 | 0.04 |
| ≥30 | 10 | 72 | 18.1 | |
| Stressful event in the past year | ||||
| No | 49 | 735 | 5.5 | 0.06 |
| Yes | 44 | 337 | 9.8 | |
| Contraception | ||||
| Nonhormonal methodb | 9 | 31 | 8.8 | 0.2 |
| Progestin-only methodc | 9 | 10 | 4.6 | |
| Combined pill estrogen/progestin | 61 | 63 | 5.8 | |
| Menstrual characteristics | ||||
| Regular periods | ||||
| No | 14 | 224 | 4.2 | 0.09 |
| Yes | 79 | 848 | 7.5 | |
| Periods lasting >1 week | ||||
| No | 44 | 726 | 3.8 | <0.001 |
| Yes | 49 | 346 | 12.2 | |
| Dysmenorrhea | ||||
| No | 62 | 908 | 4.3 | <0.001 |
| Yes | 31 | 164 | 18.1 | |
| Menorrhagia | ||||
| No | 62 | 848 | 5.6 | 0.03 |
| Yes | 31 | 224 | 11.4 | |
Copper IUD/barrier/traditional, or no method.
Mirena IUD, progestin-only pill, implant.
Discussion
This study sheds new light on women's experience with PMS by estimating the reported syndrome prevalence and identifying determinants of the syndrome among a nationally representative sample of reproductive-age women in France. This study also assesses the stability of PMS symptom severity over time.
Our results show that 4.1% of women qualified for severe PMS (six symptoms) and 8.1% qualified for moderate PMS (one to five symptoms), resulting in a combined population of 12.2% who reported cyclic symptoms that significantly disturbed their daily lives. Such estimates are consistent with the existing literature despite the fact that the lack of uniformity among PMS studies presents a challenge for comparison. As is true for other large population-based surveys, our measurement of PMS may have suffered from weaknesses intrinsic to retrospective, large-scale studies, including the need to collect data over the telephone. We lack variables requiring daily collection (exact timing of luteal and follicular phases, difference in symptom severity scores), and our data, drawn from a previously identified cohort of women, are limited to the variables elicited for the original research purpose. Data on psychiatric comorbidities, prior PMS treatment, and use of psychotropics, for example, were unavailable. In addition, the COCON cohort aged from the period between the survey's initiation in 2000 to the addition of PMS questions in 2003, making the youngest members of our study population slightly older than the standard population of women of reproductive age.
Nonetheless, the predominant focus of our study was not to establish a clinically irrefutable PMS diagnosis but rather to assess the burden that women attribute to their cyclic premenstrual symptoms. PMS is a concern for women, as it has been shown to decrease productivity in the workplace, increase healthcare utilization, and increase work absenteeism.3 In this study, we sought to identify women who experience significant distress from their PMS symptoms by establishing PMS symptom severity categories, rather than a binary PMS definition. Our PMS categories include women who meet subthreshold criteria for PMDD and, therefore, would have been considered syndrome free in other studies, although they are negatively impacted by their symptoms.2 Using a PMS spectrum also revealed a previously underrecognized fluctuation of the syndrome over time.
Our findings suggest that among the general population, risk factors for PMS fall into three categories: hormonal, psychosocial, and physiological. Life stressors and exogenous hormonal exposure, in particular, had an important impact on premenstrual syndrome. Hormonal fluctuation during the natural menstrual cycle has been shown to impact both target tissues and the central nervous system, producing a pattern of physical and emotional symptoms.11 As seen in our study, the use of combined estrogen/progestin contraceptive methods reduces premenstrual symptoms, likely by producing low, constant hormone levels. This finding is confirmed by prior cohort studies assessing PMS risk factors and by clinical trials that show relief of symptoms with hormonal contraceptive use.14,19,20 Women using combined oral contraceptive pills (COCs) are often excluded from PMS studies because of their presumptive anovulatory status. However, these women may in fact experience a period of estrogen rebound during the week of placebo pills, creating the potential for PMS symptoms.21 Studies show that women using COCs experience premenstrual and menstrual changes in physical and emotional symptoms.22 Our study corroborated this finding, with 29% of women with PMS reporting COC use.
The protective effect of hormonal contraception diminishes when controlling for menstrual disorders. Our hypothesis is that for some women, both PMS and menstrual symptoms are triggered by hormonal fluctuations. Stabilizing these fluctuations with hormonal contraception thus provides relief from both types of symptoms. Nevertheless, we continue to find a significant protective effect due to progestin-only methods in this model. Further work is needed to characterize the role of progestin-only methods in reduction of PMS symptoms.
Although controlling for menstrual disorders reduced the protective effect of hormonal contraception, we continued to find a strongly significant association between PMS and menstrual disorders when controlling for contraceptive use. This finding is consistent with previous studies, which have found a significant increase in PMS among women with menses lasting longer than 6 days.19 One possible explanation is that women who experience difficult menses may be apprehensive about their periods and thus experience emotional and somatic symptoms in the time leading up to menses.
Physiological variables had little impact on PMS status in this study. Only low BMI demonstrated a significant impact on PMS, a finding consistent with previous work that found a tendency for women with higher BMI to exhibit increased PMS risk.19 This association may reflect lower levels of estrogen in women with low body fat.
In contrast to the physiological variables tested, subjectively reported life stressors were found to exhibit a significant effect on PMS status in both multivariate models. The hypothesis that PMS may be triggered by stressful life events is based on the finding that PMS exhibits substantial overlap with other mood disorders.23 The association between stressful events—loss of a loved one, recent breakup, work or financial difficulties, and illness—and PMS may, therefore, parallel the effect of stressful events on those who are vulnerable to episodes of major depression. This theory is supported by previous research showing a correlation between self-reported stress and PMS.14,19
In addition to studying risk factors associated with PMS, we were able to assess the stability of the premenstrual symptoms. We found that PMS appears to be an inconsistent syndrome over time. Although our PMS category only includes women who reported at least two consecutive cycles worth of symptoms in a given year, three of four women who met PMS criteria in at least 1 year did not qualify for PMS in both years. This high level of intraindividual variation in PMS status contradicts previous cohort studies, but prior studies did not focus on the fluctuation of symptom impact on daily life.7,13 Our findings are consistent with the qualitative study among women in the U.K., which documented women's perception of varying menstrual experiences from 1 month to the next.25 Documented fluctuation in reported PMS impact on daily life casts doubt on the criteria imposed by the standard clinical definition of PMS, which requires consistency of PMS symptoms over time.
Much of the variation in PMS status in our study was attributable to the women's assessment of the severity of their PMS symptoms, as the average number of symptoms reported did not differ between 2003 and 2004. A closer look at the variation between years shows that women who qualified for PMS in only 1 of the 2 years were most often inconsistent in their answer to the question of whether PMS symptoms significantly impact their lives. This subjective reassessment of PMS symptoms is perhaps affected by external influences, such as life stressors. Although we attempted to study the correlation between change in PMS status and changes in external stimuli, we were unable to draw conclusions because of the small sample size. In addition, there was considerably more variation in PMS status among women qualifying for moderate PMS than among those qualifying for severe PMS. This finding suggests that the more severe PMS definition may have a stronger biological basis than the moderate syndrome. More research is needed to describe the factors that influence variation in PMS status.
Conclusions
In the ongoing debate about whether PMS exists as a definable clinical entity, our study contributes by extending the boundaries of the traditional PMS diagnosis, establishing a spectrum of syndrome severity in order to better characterize the subset of women in the general population who perceive a diminished quality of life as a result of their PMS symptoms. Our results suggest that a greater proportion of women than previously identified may experience a negative impact of PMS symptoms on their daily lives. However, these women appear to vary greatly in their assessment of the burden of their PMS symptoms over time. These findings have implications for both women and medical providers, who should be aware that PMS symptoms are prevalent and often distressing, yet also understand that the severity of symptoms may remit over time. Our findings call for close monitoring of women for PMS symptoms, frequent reassessment of women who report distressing PMS symptoms, and careful consideration before initiation of long-term treatment.
Acknowledgments
This work was funded by the Institut National de la Santé et de la Recherche Médical (INSERM), the National Institute for Demographic Studies (INED), and the National Health Insurance Agency (CNAMTS). Funding for data collection and salary for the field coordinator of the study were provided by Wyeth Lederlé laboratories.
We thank all women who participated in the COCON survey. We also acknowledge the COCON Group, the research team responsible for designing, implementing, and analyzing the COCON survey. The COCON group includes Nathalie Bajos, Beatrice Ducot, Michäle Ferrand, Danielle Hassoun, Nadine Job-Spira, Monique Kaminski, Nathalie Lelong, Henri Leridon, Nicolas Razafindratsima, Clementine Rossier, and Josiane Warszawski.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Ramcharan S. Love EJ. Fick GH. Goldfin A. The epidemiology of premenstrual symptoms in a population-based sample of 2650 urban women: Attributable risk and risk factors. J Clin Epidemiol. 1992;45:377–392. doi: 10.1016/0895-4356(92)90039-p. [DOI] [PubMed] [Google Scholar]
- 2.Robinson RL. Swindle RW. Premenstrual symptom severity: Impact on social functioning and treatment-seeking behaviors. J Womens Health Gend Based Med. 2000;9:757–768. doi: 10.1089/15246090050147736. [DOI] [PubMed] [Google Scholar]
- 3.Chawla A. Swindle R. Long S. Kennedy S. Sternfeld B. Premenstrual dysphoric disorder: Is there an economic burden of illness? Med Care. 2002;40:1101–1112. doi: 10.1097/01.MLR.0000032191.26152.90. [DOI] [PubMed] [Google Scholar]
- 4.Borenstein JE. Dean BB. Leifke E. Korner P. Yonkers K. Differences in symptom scores and health outcomes in premenstrual syndrome. J Womens Health. 2007;8:1139–1144. doi: 10.1089/jwh.2006.0230. [DOI] [PubMed] [Google Scholar]
- 5.Diagnostic, statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Association; 1994. pp. 717–718. [Google Scholar]
- 6.Halbreich U. Borenstein J. Pearlstein T. Kahn L. The prevalence, impairment, impact, and burden of premenstral dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;28:1–23. doi: 10.1016/s0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- 7.Wittchen HU. Becker E. Lieb R. Krause P. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol Med. 2002;32:119–132. doi: 10.1017/s0033291701004925. [DOI] [PubMed] [Google Scholar]
- 8.Campbell EM. Peterkin D. O'Grady K. Sanson-Fisher R. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637–646. [PubMed] [Google Scholar]
- 9.McHichi AK. Tahiri SM. Moussaoui D. Kadri N. Assessment of premenstrual dysphoric disorder symptoms: Population of women in Casablanca. Encephale. 2002;28:525–530. [PubMed] [Google Scholar]
- 10.Takeda T. Tasaka K. Sakata M. Murata Y. Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in Japanese women. Arch Womens Ment Health. 2006;4:209–212. doi: 10.1007/s00737-006-0137-9. [DOI] [PubMed] [Google Scholar]
- 11.Steiner M. Premenstrual syndromes. Annu Rev Med. 1997;48:447–455. doi: 10.1146/annurev.med.48.1.447. [DOI] [PubMed] [Google Scholar]
- 12.Cohen LS. Soares CN. Otto MW. Sweeney BH. Liberman RF. Harlow BL. Prevalence and predictors of premenopausal dysphoric disorder (PMDD) in older premenopausal women: The Harvard Study of Moods and Cycles. J Affect Disord. 2002;70:125–132. doi: 10.1016/s0165-0327(01)00458-x. [DOI] [PubMed] [Google Scholar]
- 13.Bloch M. Schmidt PJ. Rubinow DR. Premenstrual syndrome: Evidence for symptom stability across cycles. Am J Psychiatry. 1997;154:1741–1746. doi: 10.1176/ajp.154.12.1741. [DOI] [PubMed] [Google Scholar]
- 14.Sternfeld B. Swindle R. Chawla A. Long S. Kennedy S. Severity of premenstrual symptoms in a health maintenance organization population. Am J Obstet Gynecol. 2002;99:1014–1024. doi: 10.1016/s0029-7844(02)01958-0. [DOI] [PubMed] [Google Scholar]
- 15.Bancroft J. The premenstrual syndrome—A reappraisal of the concept and the evidence. Psychol Med Cambridge, MA: Cambridge University Press; 1993. [DOI] [PubMed] [Google Scholar]
- 16.Bajos N. Leridon H. Job Spira N. Contraception and abortion in France in the 2000s. The COCON survey. Population. 2004;59:347–356. [Google Scholar]
- 17.Razafindratsima N. Kishimba N. Attrition in the COCON cohort between 2000 and 2002. Population. 2004;59:419–448. [Google Scholar]
- 18.Dickerson LM. Mazyck PJ. Hunter M. Premenstrual syndrome. Am Fam Physician. 2003;67:1743–1752. [PubMed] [Google Scholar]
- 19.Deuster P. Adera T. South-Paul J. Biological, social, and behavioral factors associated with premenstrual syndrome. Arch Fam Med. 1999;8:122–128. doi: 10.1001/archfami.8.2.122. [DOI] [PubMed] [Google Scholar]
- 20.Yonkers KA. Brown C. Pearlstein TB. Foegh M. Sampson-Landers C. Rapkin A. Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Am J Obstet Gynecol. 2005;106:492–500. doi: 10.1097/01.AOG.0000175834.77215.2e. [DOI] [PubMed] [Google Scholar]
- 21.Coffee AL. Kuehl TJ. Willis S. Sulak P. Oral contraceptives and premenstrual symptoms: Comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311–1319. doi: 10.1016/j.ajog.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Abraham S. Luscombe G. Soo I. Oral contraception and cyclic changes in premenstrual and menstrual experiences. J Psychosom Obstet Gynaecol. 2003;24:185–193. doi: 10.3109/01674820309039672. [DOI] [PubMed] [Google Scholar]
- 23.Alexander JL. Dennerstein L. Kotz K. Richardson G. Women, anxiety and mood: A review of nomenclature, comorbidity and epidemiology. Expert Rev Neurotherapeutics. 2007;7:S45–S58. doi: 10.1586/14737175.7.11s.S45. [DOI] [PubMed] [Google Scholar]
- 24.Reilly J. Kremer J. A qualitative investigation of women's perceptions of premenstrual syndrome: Implications for general practitioners. Br J Gen Pract. 1999;49:783–786. [PMC free article] [PubMed] [Google Scholar]