Abstract
The aim of the present study is to test a hypothesis that 5-HT1A and 5-HT2C receptors in the amygdala play an important role in the regulation of anxiety behaviors. We examined alterations in anxiety-like behaviors after manipulation of the expression of 5-HT1A and 5-HT2C receptors in the amygdala using recombinant adenovirus approaches. Recombinant adenoviruses containing a 5-HT1A promoter-controlled 5-HT1A antisense sequence or a 5-HT2C promoter-controlled 5-HT2C sense sequence were injected into the amygdala. Elevated plus maze (EPM) and open field tests were conducted to determine anxiety-like behavior and locomotor activity. Reductions in the expression of 5-HT1A receptors in the amygdala significantly attenuated the time spent in the open arms of EPM and time spent in the center of an open field. Reduction in the percent of time spent in the open arms of EPM is negatively correlated with the density of 5-HT1A receptors in the central amygdala. On the other hand, increased expression of 5-HT2C receptors reduced the time spent in the open arms of EPM and time spent in the center of an open field. The reductions in the time spent and distance traveled in the open arms of EPM were correlated to the density of 5-HT2C receptors in the basolateral nucleus of amygdala. These data suggest that amygdaloid 5-HT1A receptors produce anxiolytic and 5-HT2C receptors produce anxiogenic effects. Together, the present results demonstrate the important role of the amygdaloid 5-HT1A and 5-HT2C receptors in the regulation of anxiety-like behaviors.
Keywords: 5-HT1A receptors, 5-HT2C receptors, amygdala, anxiety-like behaviors, recombinant adenovirus
1. Introduction
Serotonin (5-HT) is known to regulate anxiety behaviors. Among the fifteen 5-HT receptors, 5-HT1A and 5-HT2C receptors have gained particular attention. 5-HT1A receptor agonists produce anxiolytic effects (De Vry, 1995; Lacivita et al., 2008). Mice with a genetic deficit in 5-HT1A receptors showed an increase in anxiety-like behaviors (Gross et al., 2000; Heisler et al., 1998; Olivier et al., 2001; Ramboz et al., 1998). On the other hand, a 5-HT agonist with a relatively high affinity for 5-HT2C receptors, m-chlorophenylpiperazine (mCPP), induces anxiogenic effects (Gibson et al., 1994), which can be blocked by 5-HT2C receptor antagonists (Bagdy et al., 2001; Hackler et al., 2007). 5-HT2C receptor knockout mice displayed decreases in anxiety-like behavior (Heisler et al., 2007). These data suggest that activation of 5-HT1A receptors may play anxiolytic effects, whereas stimulation of 5-HT2C receptors produces an anxiogenic effect. However, the mechanisms and neurocircuitries mediating these effects of 5-HT1A and 5-HT2C receptors are still unclear.
The amygdala is known as a fear center. It is involved in the regulation of emotions and fear learning and memory (LeDoux, 2000). The amygdala can be divided into two subregions, the basolateral complex (including the lateral, basolateral and basomedial nuclei) and the centromedial subdivision (containing the central nucleus, medial nucleus and part of the bed nucleus of stria terminalis)(LeDoux, 2000; Sah et al., 2003). Evidence suggests that the basolateral complex receives sensory inputs of fear stimuli and conveys the information to the central nucleus, which further sends signals to other brain regions to express fear behaviors (LeDoux, 2000). The basolateral complex is vital for the acquisition of fear memory, whereas the central nucleus may be related to the expression of the fear memory. 5-HT1A and 5-HT2C receptors are relatively abundant in the amygdala.. The 5-HT1A receptors are mainly located in the central nucleus, whereas 5-HT2C receptors are rich in the basolateral nucleus (Li et al., 1997; Li et al., 2000; Li et al., 2003). Our previous study demonstrated that 5-HT1A receptors are decreased and 5-HT2C receptors are increased in the amygdala of mice lacking 5-HT transporters (Li et al., 2000; Li et al., 2003). Consistent with the results, the 5-HT transporter knockout mice showed an increase in anxiety-like behaviors (Holmes et al., 2003b). Furthermore, stress-induced increases in c-fos expression in the amygdala are blunted in 5-HT2C receptor knockout mice (Heisler et al., 2007). These data suggest that the amygdaloid 5-HT1A and 5-HT2C receptors may be involved in the regulation of anxiety-like behaviors.
Several studies have been reported on the role of amygdaloid 5-HT1A (Gonzalez et al., 1996; Graeff et al., 1993; Zangrossi and Graeff, 1994) and 5-HT2C receptors (Campbell and Merchant, 2003; Christianson et al., 2010; de Mello Cruz et al., 2005) in the regulation of anxiety-like behaviors in rats using pharmacological approaches. The results from these studies were not consistent, especially those concerning the effects of amygdaloid 5-HT1A receptors on anxiety-like behaviors. For example, Graeff et al reported an anxiogenic effect of activation of 5-HT1A receptor in the amygdala (Graeff et al., 1993). On the other hand, Gonzalez et al. and Zangrossi and Gaeff showed that activation of 5-HT1A receptors in the amygdala did not alter anxiety-like behaviors tested by the EPM (Gonzalez et al., 1996; Graeff et al., 1993; Zangrossi and Graeff, 1994). Furthermore, these data were contradictory with the data observed in 5-HT1A knockout mice and serotonin transporter knockout mice. In these mice, a lack or reduction of 5-HT1A receptors in the amygdala increased anxiety-like behaviors (Gross et al., 2000; Heisler et al., 1998; Holmes et al., 2003a; Li et al., 2000; Olivier et al., 2001; Ramboz et al., 1998). In contrast, the effects of 5-HT2C receptors in the amygdala were more consistent. Two studies reported that activation of 5-HT2C receptors in the basolateral amygdala produced an anxiogenic effects in EPM test (Campbell and Merchant, 2003; de Mello Cruz et al., 2005), although locomotor activity was also reduced by the 5-HT2C receptor agonists. Furthermore, Christianson et al reported recently that 5-HT2C receptors in the BLA are related to uncontrollable traumatic stress-induced anxiety-like behavior in rats (Christianson et al., 2010). To date, no study has reported using molecular approaches to investigate the effects of 5-HT1A and 5-HT2C receptors in the amygdala on the anxiety-like behaviors.
The aim of the present studies was to test the hypothesis that amygdaloid 5-HT1A and 5-HT2C receptors are related to anxiolytic and anxiogenic effects, respectively. Recombinant adenoviruses containing a 5-HT1A promoter-controlled 5-HT1A antisense sequence (P1A-5HT1A-AS-Ad) or a 5-HT2C promoter-controlled 5-HT2C sense sequence (P2C-5-HT2C-S-Ad) was used to manipulate the expression of 5-HT1A receptors and 5-HT2C receptors in the amygdala. Since expression of 5-HT1A antisense and 5-HT2C sense sequences was controlled by 5-HT1A and 5-HT2C promoter, respectively, the alterations in the expression of 5-HT1A and 5-HT2C receptors selectively occurred in the 5-HT1A and 5-HT2C receptor-positive cells, respectively. The EPM, a typical test for anxiety-like behavior, was used to examine the impact of amygdaloid 5-HT1A and 5-HT2C receptor expression. Another behavioral test, the open field test, was used to exclude possible changes in the locomotor activity and was also used as an indicator for anxiety-like behavior in mice by measuring time spent in the center of the open field. These studies provided direct evidence concerning the involvement of amygdaloid 5-HT1A and 5-HT2C receptors in the regulation of anxiety behaviors.
2. Material and methods
2.1. Generation of recombinant adenovirus containing 5-HT2C promoter-controlled 5-HT2C sense sequence (P2C-5-HT2C-S-Ad)
Recombinant adenovirus containing 5-HT1A promoter-controlled 5-HT1A antisense sequence (P1A-5-HT1A-AS-Ad) was generated and evaluated, as described previously (Li et al., 2004). To generate P2C-5-HT2C-S-Ad, a fragment of 5-HT2C promoter sequence (Forward primer: 5′-AGTTGCAGCCATCCTTTCTG-3′ and reverse primer: 5′-GCAAGTCGACCTCCTGTGG-3′ encoding 28–1822 bp, Access No. S62283) and the 5-HT2C sense sequence (Forward primer: 5′-GGAGGTCGACTTGCCGGC-3′ and reverse primer: 5′-CTTTTGTCACACAGCAGTATTTAC-3′, encoding 364–2200 bp, Access No. NM_008312) was amplified by PCR. The promoter fragment contains a proximal promoter (28–1275bp, Access No. S62283) and part of the 5′-untranslated mRNA region (5′UTR) (1276–1822 bp, Access No. S62283). The 5′UTR overlaps with the PCR product of 5-HT2C sense sequence, which allowed the promoter and 5-HT2C sense fragments to be ligated using a Sal I site. The PCR products were inserted into a TOPO pCRII vector (Invitrogen Co, Carlsbad, CA). After verification of the constructs by restriction enzyme digestion followed by sequencing, the 5-HT2C promoter was inserted into Kpn I and Sal I sites of an Ad-track shuttle vector (He et al., 1998). Then the 5-HT2C sense sequence was inserted into Sal I and Xho I sites of the 5-HT2C promoter- containing Ad-track. Thus, the construct contains 1247 base pairs upstream of the 5-HT2C promoter region and 5-HT2C mRNA sequence (Ad-track-P2C-5-HT2C-S) (Fig. 1A). We included 5′-untranslated coding region of 5-HT2C mRNA in the construct because we found that this region is necessary for in vivo expression of 5-HT2C receptors. The Ad-track-P2C-5-HT2C-S was then recombined with an adenoviral vector (Ad-Easy-1) and high titer virus (1010–12 active viral particle/ml) was prepared as previously described (He et al., 1998; Li et al., 2004).
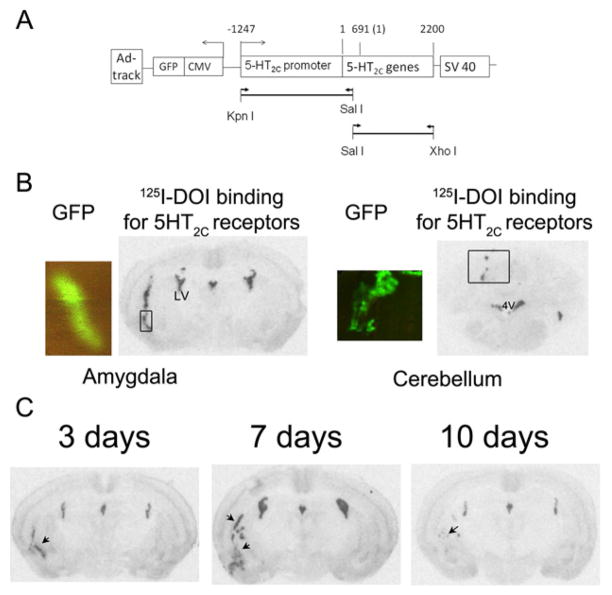
Figure 1.
Generation and evaluation of recombinant adenovirus containing 5-HT2C receptor sense sequence (P2C-5HT2C-S-Ad). A: Scheme for the 5-HT2C sense construct in P2C-5HT2C-S-Ad. The construct includes a proximal 5-HT2C promoter region (1247 bp upstream of the start site of 5-HT2C mRNA (= 1) and a 2200 bp 5-HT2C cDNA sequence, including 5′ UTR (1–690) and coding region (691–2070, Access No. NM_008312). The arrow heads indicate the direction of gene expression. The arrow lines indicate the primers used to amplify 5-HT2C promoter and mRNA regions. The lines represent the PCR products generated from the primers. The promoter fragment was inserted into Kpn I and Sal I sites and then 5-HT2C receptor sequence was ligated into Sal I and Xho I sites. B: Test for 5-HT2C promoter-controlled expression of 5-HT2C receptors. Autoradiography of 125I-DOI binding shows 5-HT2C receptor expression after injection of P2C-5HT2C-S-Ad into the amygdala and cerebellum. The viral infection was indicated by the expression of green fluorescent protein (GFP) in an adjacent section, as indicated by the box. C: Time course of P2C-5HT2C-S-Ad-induced expression of 5-HT2C receptors. Autoradiography of 125I-DOI binding shows the expression of 5-HT2C receptor 3, 7 and 10 days after injection of P2C-5HT2C-S-Ad (indicated by arrows).
2.2. Animal studies
2.2.1. Animals
Female C57BL/6 mice were purchased from Taconic Farm Inc. (Hudson, NY). Use of female mice was to be comparable to serotonin transporter knockout mice that showed more anxiety in female than male mice. The animals were 8 weeks old with body weights of 20–25g. The mice were housed in groups of four to five per cage in a light- (12 hour light/dark cycle, lights on at 6 a.m.), humidity- and temperature-controlled room. Food and water were available ad lib. The mice were single housed after the stereotaxic injection of recombinant adenovirus. All animal procedures were approved by the University of Texas Medical Branch Animal Care and Use Committee.
2.2.2. Amygdaloid injection of recombinant adenovirus
The high titer recombinant adenoviruses (1010–12 active viral particle/ml), P1A-5HT1A-AS-Ad and P2C-5-HT2C-S-Ad were injected into the amygdala using stereotaxic techniques. Mice were anesthetized with Avertin (375 mg/kg, ip) and placed on a stereotaxic apparatus. To prevent tissue damage induced by the high salt viral-storage solution, the high titer adenoviruses (about 30 μl) were dialyzed with saline (about 1L) for at least 30 min at 4°C followed by 1:1 dilution with saline prior the injection. The dialyzed viral solution was kept in ice until injected. The injection was performed using an internal injector (33 gage, C315I, Plastics One Inc, Roanoke, Va) that was connected to a 25 μl Hamilton syringe with PE50 tubing. The syringe was placed on an injection pump (WPI) to control the injection rate. The injector was placed into a 26 gage guide cannula with a tubing length of 2mm below the pedestal, which was then mounted on the stereotaxic device (Plastics One Inc, Roanoke, Va). For bilateral injections into the amygdala, two injectors were held by a double guide cannula (26 gage, C/C distance = 6.4mm, tubing length 2mm below the pedestal, Plastics One Inc, Roanoke, Va). Mice were placed on a stareotaxic device with a mouse adaptor. After an incision, one of the injectors was aligned with bregma and all of the coordinates were re-zeroed. The injector was then moved to the anterior/posterior (e.g −0.7mm) and medial/lateral (e.g ±3.2) coordinates. Two holes aligned with the coordinates were drilled. After being filled with the dialyzed viral solution, the injectors (not guide cannulas) were inserted into the brain at the dorsal/ventral coordinate (e.g −5.7mm). The dialyzed adenovirus was then bilaterally injected into the amygdala (1 μl/site at the coordinates of AP = −0.7 mm, ML = ±3.2 mm and DV = −5.7 mm from Bregma) (Paxinos and Franklin, 2001) with a rate of 0.5 μl/min. The needle was left in place for additional 20 min after the injection to allow the viral solution time to penetrate the surrounding tissue and reduce the amount of solution entering needle track. The injectors were then removed and the incision was closed. The sites of injection were verified by location of GFP expression in the amygdala sections. The GFP expression indicates the viral infection. Mice were excluded from the study if no GFP was observed or the GFP was not expressed in either side of the amygdala. The exclusion rate was less 10% of mice injected. No significant tissue damage was observed in sites injected with virus.
Ad-track-Ad, a recombinant adenovirus containing shuttle vector alone, was used as a control. Because P1A-5-HT1A-AS-Ad and P2C-5-HT2C-S-Ad were generated with full cDNA sequence of 5-HT1A and 5-HT2C receptors, respectively, we could not design mismatch or scrambled sequences for these receptors.
2.2.3. Behavioral tests
2.2.3.1. Elevated plus-maze test (EPM)
was performed as described by Holmes et al. (Holmes et al., 2000). The EPM apparatus (San Diego Instruments, San Diego, CA) was comprised of two open arms (30 × 5 × 0.3 cm) and two enclosed arms (30 × 5 × 15 cm) that extend from a common central platform (5 × 5 cm). A small raised lip (0.5 cm) around the perimeter of the open arms prevented the mouse from falling. The apparatus was constructed from polypropylene and plexiglas, with a white floor and clear walls, and elevated to a height of 38 cm above floor level. The apparatus was evenly illuminated by white overhead fluorescent lighting. Briefly, a mouse was placed on the center of the EPM facing one of the open arms. The mouse was allowed to freely explore the maze for 5 min. Mouse movements were recorded by a video camera. The distance traveled, the time spent and the entries in opened and closed arms were analyzed using a computer-based tracking program (Smart, San Diego Instruments, San Diego, CA). The total distance traveled, total entries and entries in closed arms were used as an index of the locomotor activity. The percent of time spent, the distance traveled and entries in the open arms were used as measurements of anxiety-like behavior.
2.2.3.2. Open field test
was conducted as described by Holmes et al (Holmes et al., 2003a). The open field was a square area (40 × 40 × 35 cm) with clear plexiglas walls and floor, evenly illuminated by white overhead fluorescent lighting. Mice were individually placed in the center of the open field and left to freely explore for 15 min. Activity was measured by a computer-based tracking program (Smart, San Diego Instruments, San Diego, CA). Distance traveled and time spent in the peripheral and the center areas (20 × 20 cm in the center of the box) of the open field were analyzed. The total distance traveled and the percent of time spent in the center were used to indicate the locomotor activity and anxiety-like behavior, respectively.
2.2.4. Experimental procedures
2.2.4.1. The effect of amygdaloid 5-HT1A receptors on anxiety-like behaviors
5-HT1A receptors in the amygdala were reduced by injection of P1A-5HT1A-AS-Ad into the amygdala. The high titer P1A-5HT1A-AS-Ad (1010 active viral particle/ml) was injected into the amygdala with stereotaxic technique as described above. Another group of mice received a control adenovirus, Ad-track-Ad (1010 active viral particle/ml), that contains viral vector alone. Five days after the injection, an EPM test was conducted. On the next day (day 6 after the injection) mice were tested in the open field apparatus. Twenty-four hours after the open-field test (day 7 after the injection), mice were decapitated and the brains were collected for autoradiography of 125I-MPPI binding to determine the reduction of 5-HT1A receptors. The test schedule was based on our previous data that the peak of adenovirus infection and knockdown of 5-HT1A receptors was 5–10 days (Li et al., 2004).
2.2.4.2. Evaluation of P2C-5HT2C-S-Ad
To determine the selectivity of P2C-5HT2C-S-Ad-induced expression of 5-HT2C receptors, we injected P2C-5HT2C-S-Ad into the amygdala and cerebellum and compared the expression of 5-HT2C receptors in these regions. As described in section 2.2.2, P2C-5HT2C-S-Ad was unilaterally injected into the amygdala or cerebellum (coordinates: AP= −6.3mm, ML= 0.5mm and DV= −2mm) with a rate of 0.5μl/min followed by the injector staying in place for an additional 20 min. Seven days after the injection, the mice were sacrificed and brains were collected. The brains were sectioned into 16 μm sections for autoradiography of 125I-DOI binding to determine the over-expression of 5-HT2C receptors in the amygdala and cerebellum. The GFP expression was observed to estimate viral expression.
To determine time-course of P2C-5HT2C-S-Ad-induced over expression of 5-HT2C receptors, we injected P2C-5HT2C-S-Ad into the amygdala as described in 2.2.2. Three, seven and ten days after the injection, the mice were sacrificed and the brains were sectioned for autoradiography of 125I-DOI binding assay to determine the over-expression of 5-HT2C receptors as described in 2.3.
2.2.4.3. The effect of amygdaloid 5-HT2C receptors on anxiety-like behaviors
To overexpress 5-HT2C receptors in the amygdala, high titer P2C-5HT2C-S-Ad (1011 active viral particle/ml) or Ad-track-Ad were bilaterally injected into the amygdala of mice as described above. Five days after the injection, mice were tested in the EPM.. Then, the open field test was conducted in these mice on the following day (day 6 after the injection). On day 7, the mice were sacrificed and brains were collected.. The brains were collected for autoradiography of 125I-DOI binding to determine the density of 5-HT2C receptors in the amygdala.
2.3. Autoradiography of receptor ligand binding
Autoradiographies of 125I-MPPI, a 5-HT1A antagonist, binding and 125I-DOI, a 5-HT2A/2C agonist, binding were conduced to measure the density of 5-HT1A and 5-HT2C receptors, respectively. Coronal brain sections (16 μm) containing amygdala (bregma −0.7 to ~ −1.94 mm) were cut using a cryostat and mounted on gelatin-coated slides. Each slide contained 8–12 sections from 4 levels of rostral-caudal amygdala. For each receptor binding assay, two slides were used for total binding and one slide was used for non-specific binding.
Autoradiography of 125I-MPPI binding for the density of 5-HT1A receptors was performed using a procedure modified from Kung et al (Kung et al., 1995) as previously described (Li et al., 2000). Briefly, the sections were pre-incubated in the assay buffer (50 mM Tris-HCl, pH 7.4, containing 200 nM MgCl2) for 30 min and then incubated with 0.14 nM 125I-MPPI (Kd=0.36 nM) (Zhuang et al., 1994) in assay buffer for 2 h at room temperature. Nonspecific binding was defined in the presence of 10−5 M 5-HT. Slides were then washed twice with assay buffer at 4°C for 15 min and rinsed with cold ddH2O. After being blow-dried with air, the slides were exposed to Kodak Biomax MR film at −80°C for 3–5 days. A set of 125I microscales (Amersham Biosciences, Piscataway, NJ) was exposed with the slides for calibration of the films.
Autoradiography of 125I-DOI binding for 5-HT2C receptors was conducted as previously described (Li et al., 2003). Because the affinity of DOI for 5-HT2C receptor is similar to that for 5-HT2A receptors (Ki= 4 and 0.2 nM for 5-HT2C and 5-HT2A receptors, respectively), we used the 5-HT2A receptor antagonist, spiperone (Ki = 2nM and 2.5 μM for 5-HT2A and 5- HT2C receptors, respectively) to block 5-HT2A receptor binding sites. Briefly, the brain sections were pre-incubated with assay buffer containing 50 mM Tris-HCl (pH 7.4), 0.5mM EDTA, 10 mM MgSO4, 0.1% ascorbic acid, 0.1% BSA and 10 μM pargyline for 30 min. Then, the sections were incubated with 0.2nM 125I-DOI in the presence of 100nM spiperone for 60 min at room temperature. Nonspecific binding was defined by in the presence of 10 μM RS 102221, a 5-HT2C antagonist (Ki = 4nM and 1 μM for 5-HT2C and 5-HT2A receptors, respectively). After washing two times with assay buffer at 4°C for 10 min, then rinsing with cold H2O and blow drying with air, the slides were exposed to Kodak Biomax MR film at −80°C for 3–5 days. A set of 125I microscales (Amersham Biosciences, Piscataway, NJ) was exposed with the slides for calibration of the films.
To analyze autoradiographic images, brain images were digitized and analyzed using AIS image software (Imaging Research Inc., Ontario, Canada). The gray scale density readings were calibrated to nCi/mg of tissue equivalent using the 125I microscale. The density of nuclei was measured by outlining the nucleus according to the mouse brain atlas (Paxinos and Franklin, 2001). The receptors in the basolateral (including lateral and basolateral nuclei, BLA), basomedial nucleus (BMA) and central nucleus (CeA) of amygdala were measured (Fig. 2 and Fig. 5). Due to over-expression of 5-HT2C receptors in the caudate putamen region dorsal to the amygdala observed in some mice, we also examined the density of 5-HT2C receptors in the region of the caudate putamen (CP, Fig 5). Specific 125I-MPPI or 125I-DOI binding in each nuclei of the amygdala was determined by subtracting the nonspecific binding sites from the total binding sites in each nucleus. Data for the density of each nucleus of individual subjects were the mean of 4–6 adjacent sections.
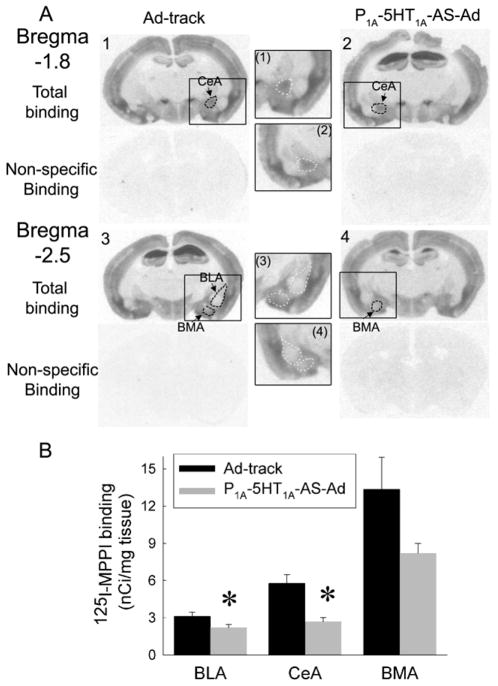
Figure 2.
The amygdaloid injection of P1A-5-HT1A-AS-Ad reduced the density of 5-HT1A receptors in the nuclei of the amygdala. A: An example of autoradiography of 125I-MPPI binding for the density of 5-HT1A receptors. Sections 1 & 3 are from a mouse injected with Ad-track and sections 2 & 4 are from a P1A-5HT1A-AS-Ad injected mouse. (1), (2), (3) and (4) show the inserts in the section 1, 2, 3 and 4, respectively, as indicated by rectangular boxes. Dashed circles indicate outlines of the area measured for each nucleus. The outline in sections 2 and 4 indicate the reduced density of 5-HT1A receptors induced by P1A-5HT1A-AS-Ad (also see white outline in the inserts). B. The density of 5-HT1A receptors was reduced in the central (CeA) and basolateral nucleus (BLA), but not in the basomedial nucleus (BMA). The data were represented as mean ± SEM (n = 8–10 mice). * Significantly different from AD-track injected mice, P<0.05.
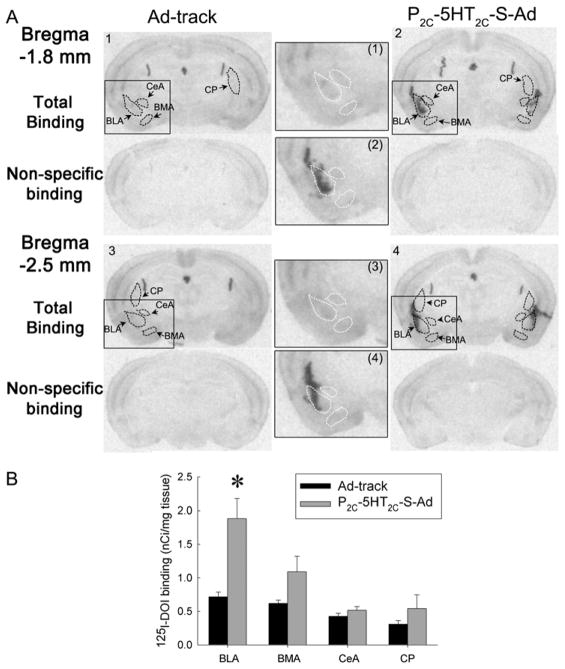
Figure 5.
Amygdaloidal injection of P2C-5HT2C-S-Ad produces an over-expression of 5-HT2C receptors in the BLA. The density of 5-HT2C receptors was measured by autoradiography of 125 antagonist, spiperone I-DOI binding in the presence of a 5-HT2A (see methods for details). A: an example of autoradiography of 125I-DOI binding. Sections 1 & 3 are from a mouse injected with Ad-track and sections 2 & 4 are from a P2C-5HT2C-S-Ad injected mouse. (1), (2), (3) and (4) show the inserts in the sections 1, 2, 3 and 4, respectively, as indicated by rectangular boxes. Dashed circles represent the outlines of the area measured for each nucleus (also see white outline in the inserts). Top sections present the total binding, while bottom sections are non-specific binding (in the presence of RS 102221, a 5-HT2C antagonist). The abbreviations are same as those in Figure 2. CP: Caudoputamen. B: The effect of P2C-5HT2C-S-Ad on the density of 5-HT2C receptors in the amygdaloidal nuclei. Data are presented as the mean ± SEM (n=10–12).
2.4. Data analysis and statistics
All of the data were analyzed by one-way or two-way analysis of variance (ANOVA). If a significant difference was detected, a student-Newman-Keuls post-hoc test was used to evaluate differences between individual groups. A computer program, StatView (Abacus Concepts Inc, Berkeley, CA) was used in all statistical analysis. The data were presented as group means ± SEM of 12–14 mice, unless otherwise noted.
3. Results
3.1. Evaluation of P2C-5HT2C-S-Ad
To manipulate the expression of 5-HT2C receptors, we generated a recombinant adenovirus containing 5-HT2C receptor sense sequence that is controlled by the 5-HT2C promoter. In the process of the generation of P2C-5HT2C-S-Ad, we found that the 5′-UTR of the 5-HT2C receptor mRNA is required for the in vivo expression of 5-HT2C receptors. Similar to the recombinant adenovirus containing 5-HT1A receptor sequences shown in previous publication (Li et al., 2004), the P2C-5HT2C-S-Ad only spreads in a limited area in the brain after injection. Thus, it is useful to study the function of 5-HT2C receptors in specific brain regions.
To determine the 5-HT2C promoter-controlled expression of 5-HT2C receptors, we compared the expression of 5-HT2C receptors when P2C-5HT2C-S-Ad was injected into the amygdala and cerebellum. The cerebellum normally has low expression levels of 5-HT2C receptors. If the 5-HT2C promoter sequence in the P2C-5HT2C-S-Ad is able to limit the expression of 5-HT2C receptors to only the cells that normally express 5-HT2C receptors, the P2C-5HT2C-S-Ad-induced expression of 5-HT2C receptors should be less in the cerebellum than that in the amygdala. As Fig 1B shows, the expression of 5-HT2C receptors in the cerebellum was much less than that in the amygdala, although viral expression, indicated by GFP expression, was similar in both regions. These data suggest that the 5-HT2C promoter sequence is able to control 5-HT2C receptor expression, so that it is mainly expressed in 5-HT2C receptor positive cells.
To determine the optimal period for viral-mediated 5-HT2C receptor expression, we conducted a time-course study on P2C-5HT2C-S-Ad-induced 5-HT2C receptor expression. The results showed that a considerable amount of 5-HT2C receptors was expressed even 3 days after the viral injection. The expression was highest on 7 days and was reduced 10 days after the injection (Figure 1C). Based on this data, we conducted further studies on 5–7 days after the viral injection.
3.2. Effect of 5-HT1A receptors in the amygdala on anxiety-like behaviors
5-HT1A receptors are distributed in the central nucleus (CeA) and basomedial nucleus (BMA) of amygdala. The density of 5-HT1A receptors in the basolateral nucleus of amygdala (BLA) is relatively low. Since we hypothesized that 5-HT1A receptors in the CeA may be related to the regulation of anxiety-like behaviors, the injection of adenovirus was targeted to the dorsal region of the amygdala, including CeA and BLA. Injection of P1A-5HT1A-AS-Ad into the amygdala significantly reduced the density of 5-HT1A receptors in the CeA and BLA nuclei (One way ANOVA for CeA: F(1,28) = 13.33, P< 0.01; For BLA: F(1,24) = 5.1, P< 0.05). In the BMA, 5-HT1A receptor binding sites were not significantly reduced by P1A-5HT1A-AS-Ad, although they were lower than in Ad-track treated mice (Fig. 2). This could be due to an inconsistent infection of P1A-5HT1A-AS-Ad.
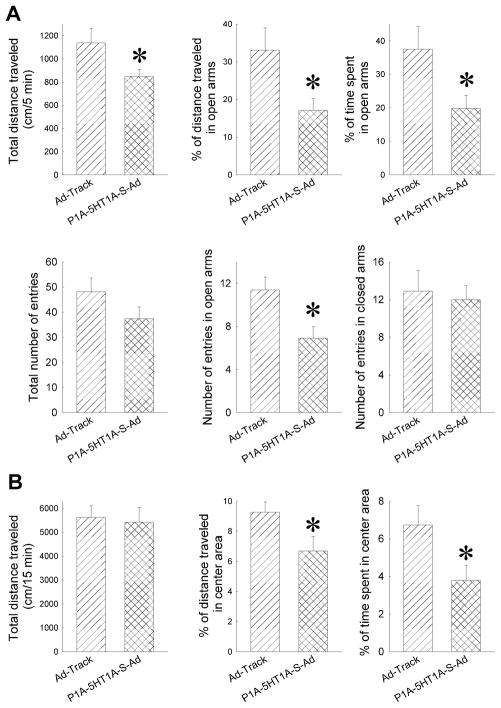
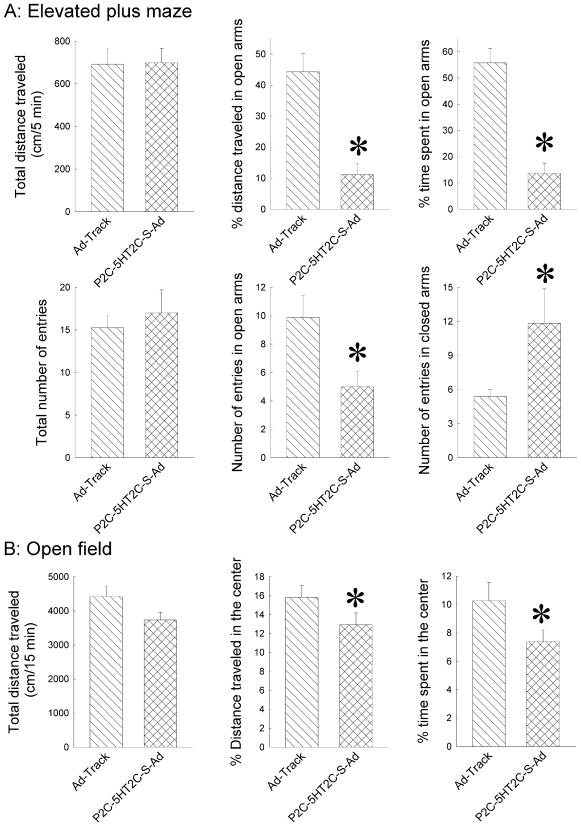
Anxiety-like behavior of mice was indicated by the time spent, distance traveled and number of entries in the open arms of the EPM. The longer the mouse stays in the open arms, the less anxious the mouse is. On the other hand, the total distance, total number of entries and the number of entries into the closed arms can be used as an index for locomotor activity. The results from the EPM test showed that the percent of distance traveled (%D), the percent of time spent (%T) and the number of entries (open NE) in the open arms were significantly reduced in P1A-5HT1A-AS-Ad treated mice relative to the mice that received Ad-track-Ad (Fig 3A) (One-Way ANOVA for %D: F(1,24) = 6.18, P< 0.05, for %T: F(1,24) = 5.38, P< 0.05, for open NE: F(1,25)=7.74, P<0.05). Although the total distance traveled was reduced in P1A-5HT1A-AS-Ad treated mice relative to that in Ad-track-Ad treated mice (One-way ANOVA: F(1,24) = 6.18, P< 0.05), the total number of entries and number of entries in closed arm were not changed (Fig. 3A).
Figure 3.
Amygdaloid injection with P1A-5-HT1A-AS-Ad significantly increases anxiety-like behavior as measured by EPM (A) and open field (B). The data are presented as the mean ± SEM (n = 13–14 mice), *: Significantly different from AD-track injected mice, P<0.05.
An open field test was performed to further evaluate the locomotor activity of these mice. The total distance traveled in the open field test was not significantly changed in the P1A-5HT1A-AS-Ad-treated mice, suggesting that the locomotor activity is not altered in P1A-5HT1A-AS-Ad-treated mice (Fig 3B). Consistent with the lowered anxiety-like behavior in the EPM, the open field test showed that the percent of distance traveled and time spent in the center were significantly reduced in P1A-5HT1A-AS-Ad mice (One-way ANOVA for (% center distance: F(1,14)=4.966, P<0.05; and for (% center time: F(1,13)=4.878, P<0.05) (Fig. 3B).
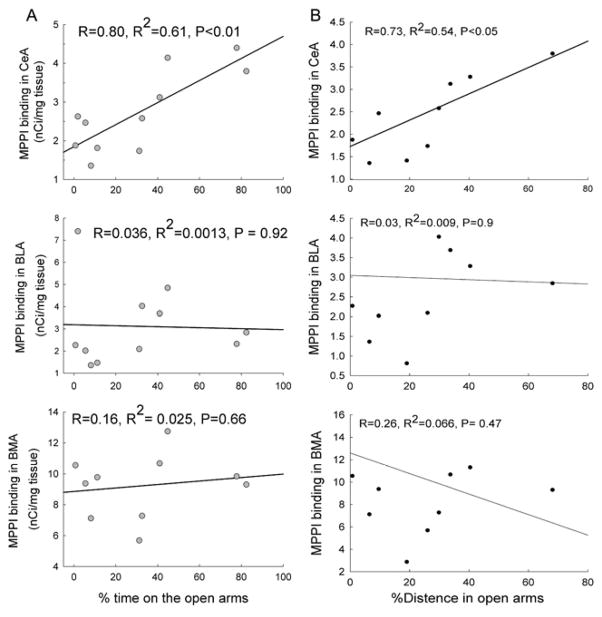
To determine in which sub-region of the amygdala 5-HT1A receptors may be related to anxiety-like behavior, the regression coefficients between the density of 5-HT1A receptors in each sub-region of the amygdala and the percent of time spent and distance traveled in open arms of EPM were calculated. The results showed that the density of 5-HT1A receptors in the central nucleus of amygdala was correlated with the % of time spent and distance traveled in open arms (R = 0.8, P< 0.01 and R = 0.735, P<0.05 for percent of time spent and distance traveled in open arms, respectively) (Fig. 4). This correlation was not observed in the basomedial or basolateral nuclei (Fig. 4).
Figure 4.
Correlation between the density of 5-HT1A receptors in the amygdaloidal nuclei and the % of time spent or distance traveled in the open arms of the EPM. Non-linear regression analysis was conducted to compare the 125I-MPPI binding sites in the CeA, BLA and BMA to the % of time spent or % of distance traveled in the open arms of the EPM (n = 10). R: Correlation coefficient; R2: R squared.
3.3. Effect of 5-HT2C receptors in the amygdala on anxiety-like behaviors
To determine the effect of amygdaloid 5-HT2C receptors, we injected P2C-5HT2C-S-Ad into the amygdala, especially targeting the BLA, because the BLA contains a relatively high density of 5-HT2C receptors. Injection of P2C-5HT2C-S-Ad significantly increased the expression of 5-HT2C receptors in the BLA (One-way ANOVA: F(1,20)=10.9, P<0.01, Fig. 5). The density of 5-HT2C receptors in other nuclei in the amygdala was not significantly altered (Fig. 5). To determine whether injection of P2C-5HT2C-S-Ad affected the expression of 5-HT2C receptors in the adjacent brain regions, we also examined the density of 5-HT2C receptors in the caudoputamen region (CP) located dorsal to the BLA. The density of 5-HT2C receptors in the CP was not significantly altered in P2C-5HT2C-S-Ad treated mice. The unchanged density of 5-HT2C receptor could be due to inconsistent viral infection.
In EPM test, mice injected with P2C-5HT2C-S-Ad showed a significant reduction in the distance traveled (%D), time spent (%T) and number of entries (NE open) in open arms relative to the control-virus injected mice (One-way ANOVA for %D: F(1,13)=21.75, P<0.01; for %T: F(1,13)=37.05, P<0.01, and for NE open: F(1,12)=5.86, P<0.05). On the other hand, total distance traveled and the number of total entries were not significantly changed in P2C-5HT2C-S-Ad-injected mice, whereas the number of entries in closed arms was significantly increased in P2C-5HT2C-S-Ad-injected mice (One-way ANOVA for the number of entries in closed arms: F(1,12)=5.82, P<0.05, Fig. 6a). Unexpectedly, in Ad-track injected mice, the %T and %D in the open arms were higher in the present study than those in other studies, including the Ad-track injected mice in the 5-HT1A antisense study above. Thus, it is unlikely that the increased %T and %D in the open arms of the Ad-track injected mice is due to the viral injection. We do not have good explanation for the increase, and it could be due to random variation among mice.
Figure 6.
Amygdaloidal injections with P2C-5-HT2C-S-Ad significantly increase anxiety-like behavior as measured by EPM (A) and open field (B). The data are presented as the mean ± SEM (n = 7–8 mice). * Significantly different from AD-track injected mice, P<0.05.
Similarly, the distance traveled and time spent in the center of open field were also reduced in P2C-5HT2C-S-Ad injected mice relative to mice injected with Ad-track (One-way ANOVA for %D: F(1,16)=6.05, P<0.05; for %T: F(1,16)=8.3, P<0.01, Fig. 6b). On the other hand, the total distance traveled was not significantly altered in P2C-5HT2C-S- Ad-treated mice relative to Ad-track injected mice (Fig. 6b), suggesting that increases in the distance traveled and time spent in the open arms are unlikely due to alterations in locomotor activity. These data suggest that increase in the expression of 5-HT2C receptors in the amygdala increases anxiety-like behaviors in mice. Interestingly, the degree of P2C-5HT2C-S-Ad-induced reductions in the distance traveled and time spent in the center of open field was much less than those in the open arms of EPM, suggesting that P2C-5HT2C-S-Ad-induced fear of heights may be more extensive than their fear of novelty.
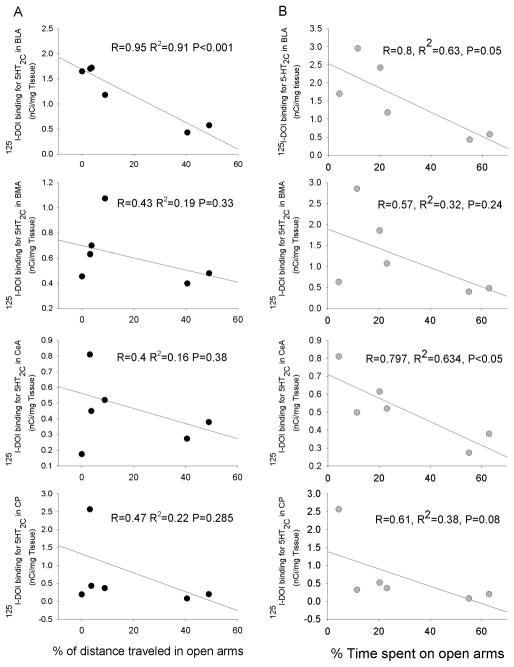
To determine the sub-region of the amygdala in which 5-HT2C receptors may be related to anxiety-like behavior, the regression coefficients between the density of 5-HT2C receptors in each sub-region of the amygdala and the percent of time spent and distance traveled in open arms of EPM were calculated. The results showed that the density of 5-HT2C receptors in the basolateral nucleus of amygdala (BLA) was correlated with the % of distance traveled on open arms (R = 0.95, P< 0.01). Although the % of time spent in open arms had strong trend to correlate with the density of 5-HT2C receptors in the BLA, it did not reach statistical significance(R=0.8, P = 0.058) (Fig. 7). Interestingly, the % of time spent in open arms was significantly correlated with the density of 5-HT2C receptors in the center nucleus of amygdala (CeA, R=0.797, P<0.05). This correlation was not observed in other sub-regions of amygdala and caudate putamen that is located dorsal to the amygdala (Fig 7). These data suggest that the 5-HT2C receptors in the BLA and probably CeA may involved in the regulation of anxiety-like behaviors in mice.
Figure 7.
Correlation between the density of 5-HT2C receptors in the amygdaloidal nuclei and the % of time spent or distance traveled in the open arms of the EPM. Nonlinear regression analysis was conducted to compare the 125I-DOI binding sites in the BLA, CeA, BMA and CP to the % of time spent or % of distance traveled in the open arms of the EPM (n = 8). R: Correlation coefficient; R2: R squared.
Discussion
Stimulation of 5-HT1A receptors and 5-HT2C receptors produce anxiolytic and anxiogenic effects, respectively (Holmes, 2008). However, the neuro-circuitries and brain regions mediating these effects are still unclear. In the present studies, we provide the first direct evidence, using molecular approaches, to demonstrate the involvement of amygdaloid 5-HT1A and 5-HT2C receptors in the regulation of anxiety-like behaviors. Reduced expression of 5-HT1A receptors in the central nucleus of the amygdala increased anxiety-like behaviors. On the other hand, increased expression of 5-HT2C receptors in the basolateral nucleus of the amygdala enhanced anxiety-like behaviors. Furthermore, these data suggest that 5-HT1A and 5-HT2C receptors in the central nucleus and basolateral nucleus of the amygdala, respectively, regulate anxiety-like behaviors in an opposite direction.
Two behavioral tests, an EPM and an open field test, were performed in the present studies. The EPM is a typical test for anxiety-like behaviors. Naïve EPM test is related to exploratory responses and anxiety produced by the open space and height of the apparatus. Measures of activity in the open arms of the EPM (time spent, entries and distance traveled) are used as indices of anxiety-like behaviors. On the other hand, the total distance traveled, the number of total entries and the number of entries into the closed arms can be used as an index of locomotor activity. The open field test measures locomotor activity by calculating the total distance traveled, whereas the percent of time spent and distance travelled in the center of the field can be used as an index of anxiety-like behaviors. In the present studies, a reduction of 5-HT1A receptors and an increase of 5-HT2C receptors in the amygdala significantly attenuated the time and movement in the open arms of EPM and the center of open field. The consistent results from these two tests strongly suggest that 5-HT1A receptors and 5-HT2C receptors in the amygdala are related to anxiety-like behaviors. Unlike the results for anxiety-like behaviors, the total distance traveled in the EPM and open field tests were not consistent in P1A-5HT1A-AS-Ad treated mice, although the number of total entries and entries into the closed arms in EPM were not altered in these mice relative to the Ad-track treated mice. The total distance traveled in the P1A-5HT1A-AS-Ad injected mice was reduced in EPM, but not changed in open field test. Since the open field test mainly measures locomotor activity whereas EPM determines anxiety-like behavior, the data from open field test are a more reliable index for the locomotor activity of the P1A-5HT1A-AS-Ad injected mice. Furthermore, the other two measures of locomotor activity in EPM, the number of total entries and number of entries in the closed arms, were not changed in P1A-5HT1A-AS-Ad treated mice, suggesting that the locomotor activity of these mice may be not altered. The reduced total distance traveled in P1A-5HT1A-AS-Ad treated mice could result from the increased anxiety-like behavior.
5-HT1A receptors are involved in the regulation of anxiety behaviors. The most convincing evidence for the effects of 5-HT1A receptors on anxiety behaviors is that mice lacking 5-HT1A receptors are more anxious (Groenink et al., 2003; Ramboz et al., 1998; Toth, 2003). 5-HT1A agonists, such as buspirone, have been used as anxiolytic drugs in humans. Studies suggest that 5-HT1A receptors in different brain regions may play different roles in the regulation of anxiety behaviors (Graeff and Zangrossi, 2010; Millan, 2003). Thus, it is important to find the brain regions that are related to the relatively high density of 5-HT1A receptors located in the central nucleus of amygdala, the data concerning whether 5-HT1A receptors in the amygdala are involved in regulation of anxiety behaviors were not consistent. Our present results demonstrated that 5-HT1A receptors in the amygdala, especially in the central nucleus, play an anxiolytic role in mice. This result is consistent with our previous findings in SERT knockout mice. The density of 5-HT1A receptors in the central nucleus of amygdala is reduced (Li et al., 2000) and, correspondingly, anxiety-like behaviors measured by exploratory tests are increased in the SERT knockout mice relative to their SERT normal littermates (Holmes et al., 2003a). However, our present results are not consistent with those studies using pharmacological approaches to determine the effects of amygdaloid 5-HT1A receptors on anxiety-like behaviors in rats. The results from the pharmacological studies showed that injection of 5-HT1A receptor agonists into the amygdala either produced an anxiogenic effect or had no effect on anxiety-like behavior tested by EPM (Gonzalez et al., 1996; Graeff et al., 1993; Zangrossi and Graeff, 1994). These differences could be due to the location of the injections of the 5-HT1A receptor agonist which was targeted to the BLM, a region with a low density of 5-HT1A receptors and is not significantly related to anxiety-like behaviors measured by EPM as the present data show. Another possible reason for the difference could be the duration of alterations in the activity of 5-HT1A receptors in the amygdala. The expression of 5-HT1A receptors was reduced for days in viral-injected mice or for an entire lifetime in knockout mice, whereas acute injection of a 5-HT1A agonist induces activation of 5-HT1A receptors for only minutes. Additionally, we could not rule out the possibility of species differences, since most pharmacological studies were conducted in rats but the viral study and knockout studies were in mice. On the other hand, the 5-HT1A receptors in the amygdala may not mediate other anxiety-like behaviors. Overstreet et al (Overstreet et al., 2006) reported that withdraw from repeated ethanol administration induces a reduction in social interaction which is considered to be a different type of anxiety-like behavior. The social interaction deficits can be reduced by activation of 5-HT1A receptors in the raphe nuclei but not in the amygdala. These data suggest that neurocircuitries mediating anxiety-related exploratory behaviors may be different from those mediating anxiety-related social interaction behaviors.
Evidence for the anxiogenic effect of 5-HT2C receptors was initially provided by studies using a non-selective 5-HT agonist, mCPP, which produces anxiogenic effects. The effect of mCPP can be blocked by 5-HT2C antagonists (Bagdy et al., 2001; Hackler et al., 2007). Recent studies demonstrated that anxiety-like behavior is reduced in 5-HT2C receptor knockout mice (Heisler et al., 2007). Furthermore, stress-induced c-fos expression in the amygdala is reduced in 5-HT2C knockout mice (Heisler et al., 2007), suggesting that the amygdala may mediate 5-HT2C receptor-induced anxiogenic effects. It has been reported that administration of mCPP into the amygdala reduced the time spent in the center of the open field and a 5-HT2C receptor antagonist reversed this action (Campbell and Merchant, 2003; Cornelio and Nunes-De-Souza, 2007). Furthermore, Christianson et al reported recently that 5-HT2C receptors in the BLA are related to uncontrollable traumatic stress-induced anxiety-like behavior in rats (Christianson et al., 2010). In the present study, we used a molecular approach to over-express 5-HT2C receptors in the amygdala, which avoids the limitations of pharmacological approaches. The consistency of the present results with those reports from pharmacological studies further support our hypothesis that an increase in the activity of 5-HT2C receptors in the amygdala, especially in the basolateral nucleus, produces an anxiogenic effect. Interestingly, our results also showed a correlation between the density of 5-HT2C receptors in the central nucleus of amygdala and the time spent in the open arms of EPM, even though 5-HT2C receptors were not significantly increased by viral infection. These data suggest that the physiological levels of 5-HT2C receptors in the central nucleus of amygdala may also be related to the regulation of anxiety-like behaviors.
As a fear center, the amygdala can be divided into at least two subgroups, the basolateral nuclei and the centromedial nuclei (LeDoux, 2003; Sah et al., 2003). The basolateral subdivision includes lateral (LA), basal lateral (BLA) and basal medial (BMA) nuclei. They receive sensory inputs from prefrontal cortex and other brain regions and then send the information to the centromedial subdivision. Additionally, neurons in the basolateral nuclei also project to other brain regions, such as the prefrontal cortex, striatum and nucleus accumbens. These innervations of the basolateral amygdala may be related to learning and memory (Abe et al., 2009; Duvarci and Nader, 2004; Ehrlich et al., 2009; Maren, 2008). The centromedial subdivision contains the central nucleus, medial nucleus and part of the bed nucleus of stria terminalis. These nuclei receive the inputs from basolateral subdivision and project to other brain regions, such as the hypothalamus, hippocampus and brainstem to express the fear and anxiety behaviors. Furthermore, the interaction between the amygdala and hippocampus is related to acquisition and extinction of fear learning and memory. Evidence indicates that the amygdaloid neurons involved in regulation of anxiety and fear conditioning are signaling through glutamatergic and GABAergic neurotransmitter systems (Roozendaal et al., 2009). The present results suggest that stimulation of 5-HT1A receptors in the central nucleus of amygdala reduces anxiety-like behaviors, suggesting that an inhibitory signaling pathway may be involved. On the other hand, activation of 5-HT2C receptors in the lateral nucleus and basolateral nucleus of amygdala enhances anxiety-like behaviors, suggesting excitatory signaling neuro-circuitries. It will be important to determine the types of neurons containing 5-HT1A receptors in the central nucleus and 5-HT2C receptors in the basolateral nucleus of amygdala. Although we did not find an association between 5-HT1A and 5-HT2C receptors in the basomedial nucleus of amygdala and alterations in anxiety-like behaviors in the present studies, we cannot rule out the role of these receptors in the BMA in regulation of anxiety-like behaviors because they were not significantly changed in the present studies.
Altogether, the present results provide information concerning the effects of serotonergic receptors in the amygdaloid nuclei on regulation of anxiety behaviors. This knowledge will have a significant impact on future studies of the neuro-circuitries that regulate anxiety behaviors.
Highlights.
We altered the expressions of 5-HT1A and 5-HT2C receptors in the amygdala using adenovirus technique.
Reduction of 5-HT1A receptors in the amygdala increased anxiety-like behaviors.
Increase of 5-HT2C receptors in the amygdala increased anxiety-like behaviors.
5-HT1A receptors in the CeA and 5-HT2C receptors in the BLA are important on regulation of anxiety.
Acknowledgments
The authors thank Dr. Bert Vogelstin in The Johns Hopkins oncology center for kindly providing adenoviral vectors and Dr. Tong-Chuan He for his excellent technical advice in the generation of recombinant adenovirus. The authors thank Kate Davis and Yan Liu for their important technical assistance with the experiments. The authors also thank Dr. Nancy A Muma in the University of Kansas, who kindly provided feedback and proof-reading of the manuscript. The studies were supported by USPHS MH72938 (Q.L) and a NARSAD Young Investigator Award to Qian Li.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Fujimoto T, Akaishi T, Misawa M. Stimulation of basolateral amygdaloid serotonin 5-HT(2C) receptors promotes the induction of long-term potentiation in the dentate gyrus of anesthetized rats. Neurosci Lett. 2009;451:65–68. doi: 10.1016/j.neulet.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. International Journal of Neuropsychopharmacology. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Research. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry. 2010;67:339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelio AM, Nunes-De-Souza RL. Anxiogenic-like effects of mCPP microinfusions into the amygdala (but not dorsal or ventral hippocampus) in mice exposed to elevated plus-maze. Behavioural Brain Research. 2007;178:82–89. doi: 10.1016/j.bbr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- de Mello Cruz AP, Pinheiro G, Alves SH, Ferreira G, Mendes M, Faria L, Macedo CE, Motta V, Landeira-Fernandez J. Behavioral effects of systemically administered MK-212 are prevented by ritanserin microinfusion into the basolateral amygdala of rats exposed to the elevated plus-maze. Psychopharmacology (Berl) 2005;182:345–354. doi: 10.1007/s00213-005-0108-2. [DOI] [PubMed] [Google Scholar]
- De Vry J. 5-HT 1A receptor agonists: Recent developments and controversial issues. Psychopharmacology (Berl) 1995;121:1–26. doi: 10.1007/BF02245588. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K. Characterization of fear memory reconsolidation. Journal of Neuroscience. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Gibson EL, Barnfield AM, Curzon G. Evidence that mCPP-induced anxiety in the plus-maze is mediated by postsynaptic 5-HT2C receptors but not by sympathomimetic effects. Neuropharmacology. 1994;33:457–465. doi: 10.1016/0028-3908(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Andrews N, File SE. 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res. 1996;732:145–153. doi: 10.1016/0006-8993(96)00517-3. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Silveira MC, Nogueira RL, Audi EA, Oliveira RM. Role of the amygdala and periaqueductal gray in anxiety and panic. Behav Brain Res. 1993;58:123–131. doi: 10.1016/0166-4328(93)90097-a. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Zangrossi H., Jr The dual role of serotonin in defense and the mode of action of antidepressants on generalized anxiety and panic disorders. Cent Nerv Syst Agents Med Chem. 2010;10:207–217. doi: 10.2174/1871524911006030207. [DOI] [PubMed] [Google Scholar]
- Groenink L, van Bogaert MJV, Van der Gugten J, Oosting RS, Olivier B. 5-HT1A receptor and 5-HT1B receptor knockout mice in stress and anxiety paradigms. Behavioural Pharmacology. 2003;14:369–383. doi: 10.1097/01.fbp.0000087737.21047.75. [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang XX, Hen R. Altered fear circuits in 5-HT1A receptor KO mice. Biological Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Hackler EA, Turner GH, Gresch PJ, Sengupta S, Deutch AY, Avison MJ, Gore JC, Sanders-Bush E. 5-Hydroxytryptamine2C receptor contribution to m-chlorophenylpiperazine and N-methyl-beta-carboline-3-carboxamide-induced anxiety-like behavior and limbic brain activation. J Pharmacol Exp Ther. 2007;320:1023–1029. doi: 10.1124/jpet.106.113357. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou SB, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain and Behavior. 2007;6:491–496. doi: 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neuroscience And Biobehavioral Reviews. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Li Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behaviour in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain and Behavior. 2003a;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Parmigiani S, Ferrari PF, Palanza P, Rodgers RJ. Behavioral profile of wild mice in the elevated plus-maze test for anxiety. Physiology & Behavior. 2000;71:509–516. doi: 10.1016/s0031-9384(00)00373-5. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT1A receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003b;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Kung MP, Frederick D, Mu M, Zhuang ZP, Kung HF. 4-(2′-methoxy-phenyl)-1-[2′-(n-2″-pyridinyl)-p-iodobenzamido]-ethyl-piperazine ([125 I] p-MPPI) as a new selective radioligand of serotonin-1A sites in rat brain: In vitro binding and autoradiographic studies. Journal Of Pharmacology And Experimental Therapeutics. 1995;272:429–437. [PubMed] [Google Scholar]
- Lacivita E, Leopoldo M, Berardi F, Perrone R. 5-HT1A receptor, an old target for new therapeutic agents. Current Topics in Medicinal Chemistry. 2008;8:1024–1034. doi: 10.2174/156802608785161385. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular And Molecular Neurobiology. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review Of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Li Q, Battaglia G, Van de Kar LD. Autoradiographic evidence for differential G-protein coupling of 5-HT1A receptors in rat brain: lack of effect of repeated injections of fluoxetine. Brain Research. 1997;769:141–151. doi: 10.1016/s0006-8993(97)00693-8. [DOI] [PubMed] [Google Scholar]
- Li Q, Holmes A, Ma L, Van de Kar LD, Garcia F, Murphy DL. Medial hypothalamic 5-hydroxytryptamine (5-HT)1A receptors regulate neuroendocrine responses to stress and exploratory locomotor activity: application of recombinant adenovirus containing 5-HT1A sequences. Journal of Neuroscience. 2004;24:10868–10877. doi: 10.1523/JNEUROSCI.3223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch KP, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: Gender and brain region differences. Journal of Neuroscience. 2000;20:7888–7895. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems CH, Ma L, Van de Kar LD, Garcia F, Murphy DL. Brain region-specific alterations of 5-HT2A and 5-HT2C receptors in serotonin transporter knockout mice. Journal of Neurochemistry. 2003;84:1256–1265. doi: 10.1046/j.1471-4159.2003.01607.x. [DOI] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. European Journal Of Neuroscience. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Progress in Neurobiology. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Olivier B, Pattij T, Wood SJ, Oosting R, Sarnyai Z, Toth M. The 5-HT1A receptor knockout mouse and anxiety. Behavioural Pharmacology. 2001;12:439–450. doi: 10.1097/00008877-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Angel RA, Navarro M, Breese GR. Reduction in repeated ethanol-withdrawal-induced anxiety-like behavior by site-selective injections of 5-HT(1A) and 5-HT (2C) ligands. Psychopharmacology (Berl) 2006;187:1–12. doi: 10.1007/s00213-006-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press Inc; 2001. [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: An animal model of anxiety- related disorder. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De AM, Power J. The amygdaloid complex: anatomy and physiology. Physiological Reviews. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Toth M. 5-HT1A receptor knockout mouse as a genetic model of anxiety. European Journal of Pharmacology. 2003;463:177–184. doi: 10.1016/s0014-2999(03)01280-9. [DOI] [PubMed] [Google Scholar]
- Zangrossi JH, Graeff FG. Behavioral effects of intra-amygdala injections of GABA and 5-HT acting drugs in the elevated plus-maze. Braz J Med Biol Res. 1994;27:2453–2456. [PubMed] [Google Scholar]
- Zhuang ZP, Kung MP, Chumpradit S, Mu M, Kung HF. Derivatives of 4-(2′-methoxyphenyl)-1-[2′-(N-2″-pyridinyl- p-iodobenzamido)ethyl]piperazine (p-MPPI) as 5-HT 1A ligands. Journal of Medicinal Chemistry. 1994;37:4572–4575. doi: 10.1021/jm00052a018. [DOI] [PubMed] [Google Scholar]