Background: Yra1 is an mRNA export factor, but how it is recruited to nascent RNA is not known.
Results: We identified a phospho-CTD-interacting domain in Yra1 that binds best to Ser-2,5 doubly phosphorylated CTD repeats as found on elongating RNAPII; truncation of this domain leads to reduced amounts of Yra1 on active genes.
Conclusion: The PCTD-interacting domain is involved in recruiting Yra1 to active genes.
Significance: A step forward in understanding recruitment of the multicomponent mRNA packaging and export machineries.
Keywords: Phosphorylation, RNA Polymerase II, RNA Transport, RNA-binding Proteins, Transcription, CTD, CTD Kinase I, PCID, Hyperphosphorylated, Phospho-CTD Binding
Abstract
The unique C-terminal domain (CTD) of RNA polymerase II, composed of tandem heptad repeats of the consensus sequence YSPTSPS, is subject to differential phosphorylation throughout the transcription cycle. Several RNA processing factors have been shown to bind the phosphorylated CTD and use it to localize to nascent pre-mRNA during transcription. In Saccharomyces cerevisiae, the mRNA export protein Yra1 (ALY/RNA export factor in metazoa) cotranscriptionally associates with mRNA and delivers it to the nuclear pore complex for export to the cytoplasm. Here we report that Yra1 directly binds in vitro the hyperphosphorylated form of the CTD characteristic of elongating RNA polymerase II and contains a phospho-CTD-interacting domain within amino acids 18–184, which also include an “RNA recognition motif” (RRM) (residues 77–184). Using UV cross-linking, we showed that the RRM alone binds RNA, although a larger segment extending to the C terminus (amino acids 77–226) displayed stronger RNA binding activity. Although the RRM is implicated in both RNA and CTD binding, RRM point mutations separated these two functions. Both functions are important in vivo as RNA binding-defective or CTD binding-defective versions of Yra1 engendered growth and mRNA export defects. We also report the construction and characterization of a useful new temperature-sensitive YRA1 allele (R107A/F126A). Using ChIP, we demonstrated that removing the N-terminal 76 amino acids of Yra1 (all of the phospho-CTD-interacting domain up to the RRM) results in a 10-fold decrease in Yra1 recruitment to genes during elongation. These results indicate that the phospho-CTD is likely involved directly in the cotranscriptional recruitment of Yra1.
Introduction
The C-terminal domain (CTD)2 of RNA polymerase II (Pol II) is a conserved unstructured repeat domain that extends from the largest subunit of Pol II, Rpb1. The consensus heptapeptide sequence of the CTD, YSPTSPS, is conserved in eukaryotes, although the number of heptapeptide repeats varies (from 26 repeats in budding yeast up to 52 repeats in mammals). The CTD is essential for viability; however, the essential function has been shown to be independent of the catalytic activity of Pol II (1, 2). The CTD acts as a binding platform for factors involved in mRNA transcription-associated processes, including, but not limited to, chromatin remodeling and mRNA capping, splicing, and 3′-end processing (for reviews, see Refs. 3–7).
The CTD is subject to reversible post-translational modification, and these modifications have been shown to change during the course of transcription of protein-coding genes. The CTD is hyperphosphorylated (referred to as the II0 form) during the elongation phase of transcription with serines 2 (Ser-2) and 5 (Ser-5) of each repeat the primary phosphoacceptors. In addition to Ser-2 and Ser-5 phosphorylation, recent studies have also demonstrated that Ser-7 phosphorylation is present on transcribing Pol II in yeast and mammals (8, 9). chromatin immunoprecipitation (ChIP) and ChIP-chip experiments with CTD phosphospecific antibodies have shown that CTD phosphorylation varies across different genes: Ser-5 phosphorylation peaks at the 5′-end of genes, and the peak of Ser-2 phosphorylation follows and persists to the 3′-end of genes (10–13).
Four kinases and three phosphatases collaborate to modulate CTD phosphorylation in yeast. Ser-5 of the CTD is phosphorylated by Kin28 (CDK7) of transcription factor IIH and Srb10 (CDK8) of Mediator during transcription initiation (14). More recently, Kin28 (CDK7) was also shown to be responsible for serine 7 phosphorylation in yeast and mammals (8, 15). The other two kinases, Ctk1 (CDK12) of CTD kinase I (CTDK-I) and Bur1 (CDK9), have been genetically and functionally linked to transcription elongation. Ctk1 genetically interacts with multiple elongation factors (16, 17) and increases transcription elongation efficiency in vitro (18). In addition, aberrant CTD phosphorylation has been found in ctk1 mutants (19). CTDK-I can phosphorylate Ser-2 and Ser-5 in vitro and prefers a substrate that is already phosphorylated on one serine (20). Bur1/2 was recently found to be a CTD-binding protein as well as a CTD kinase. Bur1/2 binds to Ser(P)-5 CTD as produced by Kin28 and phosphorylates Ser-2 near promoters (21). In addition, Bur1/2 has been shown to replace Ser-7 phosphates during elongation (12). Finally, the yeast phosphatases Fcp1, Ssu72, and Rtr1 have been shown to remove phosphates from CTD substrates (22).
The synergistic action of these enzymes produces distinct binding surfaces on the CTD that recruit factors during the stage of transcription at which they are required. Although interactions between the CTD and many mRNA processing factors have been characterized, CTD-mediated association of mRNA packaging and export factors has not been demonstrated. It is likely that packaging and export are also coupled to transcription through CTD interactions as defects in transcription elongation, splicing, and 3′-end processing have been shown to affect export (23). In a screen of yeast proteins that potentially bind to CTDK-I-hyperphosphorylated CTD fusion protein, only one known mRNA export factor was identified, Yra1 (24).
Yra1 (RNA export factor (REF)/ALY in mammals) has been described as a general mRNA export factor because it genetically and physically interacts with the mRNA export receptor Mex67/Mtr2 (TAP/NXF1 in mammals), and depletion of Yra1 protein or the use of a temperature-sensitive allele induces nuclear retention of polyadenylated RNA (25–27). Although only a few targets of Yra1-mediated mRNA export are known, it is clear that Yra1 is not recruited to all loci and is not required for export of all mRNAs (28–30). However, there is evidence that distinct subpopulations of functionally similar transcripts are exported by a given export adaptor (29, 31) and that a given mRNA likely has binding sites for many adaptors (31).
Yra1 is thought to exist as part of a conserved complex referred to as the transcription export (TREX) complex, which is made up of the THO elongation complex, the Sub2 RNA helicase, Yra1, and Tex1 (32). The TREX complex members cotranscriptionally associate with some transcribed genes during elongation, but the mechanism that directs recruitment is unclear (28, 33). Yra1 and ALY have also been shown to interact with other transcription factors, including Iws1/Spn1 (34) and Pcf11 (35), and these proteins have also been hypothesized to recruit the export factor.
In previous work from our laboratory, Yra1 was identified as a potential phospho-CTD-associating protein using a systematic proteomics-based screen (24). To test the idea that Yra1 binds the phospho-CTD (PCTD) directly and to examine its poorly characterized RNA binding properties, we purified and analyzed recombinant constructs of Yra1 that represent either the complete protein or defined segments. We identified both a minimal PCTD-binding segment and an overlapping but distinct RNA-binding segment. Mutations in the overlapping region reduced RNA binding activity but did not affect PCTD binding. In addition, a hyperphosphorylated CTD peptide was unable to compete with RNA for binding to Yra1, indicating that the RNA and PCTD interactions are distinct. Using in vivo approaches, we showed that yeast strains expressing Yra1 mutants that affect RNA binding and CTD binding exhibited growth and mRNA export defects. Truncation of the phospho-CTD interaction domain of Yra1 resulted in a 10-fold decrease in Yra1 occupancy at genes as assayed by ChIP. Our results provide strong support for the idea that the PCTD is directly involved in the cotranscriptional recruitment of Yra1 to active genes.
EXPERIMENTAL PROCEDURES
Reagents
The biotinylated CTD peptides were synthesized by Anaspec. All enzymes used for cloning were purchased from New England Biolabs. All Gateway cloning kits were purchased from Invitrogen. Precast gels and nitrocellulose were from Bio-Rad. Chemicals, including protease inhibitor mixtures, were purchased from Sigma-Aldrich.
Yeast Strains and Growth
The FY84 strain of Saccharomyces cerevisiae (MATa his3Δ200 leu2Δ1 ura3–52 lys2–128δ) was used to produce the yra1 mutant strains. To create a YRA1 plasmid shuffle strain, the chromosomal copy of YRA1 was deleted by homologous recombination with the kanMX cassette (36) with a covering plasmid, which consisted of a V5-tagged full-length YRA1 fusion in the pAG416GPD vector (37).
To compare growth of the mutants with that of wild type (WT), overnight yeast cultures were diluted to an A600 of 1 and used to make four 10-fold serial dilutions. Each dilution was spotted onto a YPD plate (3 μl/spot), and the plates were incubated at 16 °C for 7 days, 30 °C for 2 days, or 37 °C for 3 days. Expression of Yra1 mutant proteins was verified in whole cell extracts made in ethanol as described (19) by Western blot with an anti-V5 epitope antibody (data not shown).
Cloning
For expression of full-length Yra1 and truncations, the YRA1 cDNA was expressed in bacteria as maltose-binding protein fusions using the pMal vector (New England Biolabs). All plasmids were sequenced before transformation into BL21(DE3)RIL cells (Promega) for expression. To overcome issues with expression and solubility, the amino acid sequence of S. cerevisiae Yra1 was obtained from the Saccharomyces Genome Database and used to design a synthetically codon-optimized gene for expression in Escherichia coli (Integrated DNA Technologies). The synthetic gene was cloned into the pET15b vector (Novagen) and expressed as His tag fusion proteins in BL21(DE3) cells (Promega). Point mutations were created using the QuikChange site-directed mutagenesis system (Stratagene) according to the manufacturer's instructions. Mutants were verified by sequencing and transformed into BL21(DE3) cells (Promega) for protein expression.
For expression of mutant proteins in yeast, the full-length YRA1 gene was PCR-amplified from FY84 genomic DNA, tagged with the V5 epitope, and inserted into the pAG416GPD and pAG415GPD plasmids using the Gateway method (37) (Invitrogen). The F126A, R107A/F126A, and Y112A/F126A point mutants and the 77–226 truncation (−PCID) mutant were produced by PCR and inserted into the pAG415GPD plasmid. After sequencing, the plasmids were transformed into the YRA1 plasmid shuffle strain by the lithium acetate method (38). The YRA1-covering plasmid was counterselected using 5-fluoroorotic acid-containing plates.
Protein Expression and Purification
Recombinant GST-tagged yeast CTD (GSTyCTD) was expressed in E. coli and purified as described previously (39). Yra1-maltose-binding protein fusions were expressed in BL21(DE3)RIL cells and purified using amylose resin (New England Biolabs) according to the manufacturer's protocol.
His-tagged WT Yra1, truncations, and mutants were expressed in and purified from BL21(DE3) cells. Bacteria were grown in Luria-Bertani (LB) medium with antibiotics, and protein expression was induced with a final concentration of isopropyl 1-thio-β-d-galactopyranoside of 0.5 mm for 4 h. Cells were lysed in 50 mm Tris-HCl, pH 8.0 and 500 mm NaCl buffer with protease inhibitor mixture for general use (Sigma) by two passages though a cell cracker (Microfluidics), and the cell debris was pelleted by centrifugation. Imidazole (20 mm) was added to the soluble extract to decrease background binding, and the extract was incubated in batch with Ni-Sepharose Fast Flow resin (GE Healthcare) for 30 min at 4 °C with rotation. The resin was collected and washed with >10 column volumes of high salt wash buffer (50 mm Tris-HCl, pH 8.0, 500 mm NaCl, and 20 mm imidazole). The protein was eluted with 50 mm Tris-HCl, pH 8.0, 300 mm NaCl, 250 mm imidazole, and 8% glycerol. Elutions from the Ni-Sepharose were pooled and applied to an ÄKTA FPLC system equipped with a Mono S 5/5 HR column (GE Healthcare) at a flow rate of 1 ml/min with 50 mm Tris-HCl, pH 8.0, 8% glycerol, and 0.1 mm EDTA as Buffer A and 50 mm Tris-HCl, pH 8.0, 8% glycerol, 0.1 mm EDTA, and 1 m NaCl as Buffer B. A 40-column volume gradient from 0.35 to 1.0 m NaCl was used to elute proteins, and fractions containing Yra1 were identified by SDS-PAGE and pooled. The purified protein was exchanged into storage buffer (50 mm Tris-HCl, pH 8.0, 300 mm NaCl, and 8% glycerol) and concentrated to ∼1–2 μg/ml with microcentrifuge concentrators; aliquots of the purified protein were stored at −80 °C until use.
CTDK-I was expressed in baculovirus-infected Spodoptera frugiperda (Sf9) cells at a multiplicity of infection of 1 for 72 h at 27 °C. Cells were resuspended in cytoplasmic lysis buffer (10 mm HEPES, pH 8.0, 320 mm sucrose, 3 mm calcium chloride, 2 mm magnesium acetate, and 0.5% Nonidet P-40) with protease inhibitor mixture for tissue extracts (P8340, Sigma), and the solution was centrifuged 1500 × g for 15 min to pellet the nuclei. Because CTDK-I was predominantly nuclearly localized, the pellet was resuspended in nuclear resuspension buffer (20 mm HEPES, pH 8.0, 1.5 mm MgCl2, 20 mm KCl, 25% glycerol, and protease inhibitor mixture for tissue extracts). Nuclei were lysed by the addition of 12 ml of nuclear extraction buffer (80 mm HEPES, pH 7.6, 1.2 m NaCl, 20% glycerol, 0.06% Triton X-100, and protease inhibitor mixture for tissue extracts). One volume of PEG solution (18% PEG 8000, 1 m NaCl, and protease inhibitor mixture) was then added, and the solution was incubated at 4 °C for 45 min with rotation. Chromatin and debris were removed by centrifugation at 20,000 × g for 30 min. The soluble lysate containing the protein of interest was then diluted to adjust the salt concentration to around 300 mm, and imidazole was added to a final concentration of 10 mm. His-tagged recombinant CTDK-I was purified by nickel affinity and ion exchange chromatography similarly to Yra1 except under lower salt conditions (300 mm NaCl in lysis and wash buffers and 100 mm NaCl in elution buffer). CTDK-I was eluted from the Mono S column with 60% Buffer B and was confirmed by kinase assays. Fractions with kinase activity were stored at −80 °C until use.
Far Western Assay
Far Western assays were performed as described previously (40). Briefly, duplicate samples of purified proteins were separated on a 4–20% precast SDS gel. One-half of the gel was stained with Coomassie Brilliant Blue, and the second half was transferred to nitrocellulose membrane at 0.75 A for 2 h at 4 °C. The membrane was incubated overnight at 4 °C in blocking/renaturation buffer containing 1× PBS, 3% nonfat dry milk, 0.2% Tween 20, 0.1% PMSF, and 5 mm NaF with 2 mm dithiothreitol. The nitrocellulose was then probed with 300,000 cpm 32P-labeled GSTyCTD fusion protein that had been hyperphosphorylated with recombinant CTDK-I for 4 h at 4 °C. After extensive washing, the nitrocellulose was air-dried and exposed to x-ray film (Eastman Kodak Co.) for 1–2 days and developed. The stained gel was scanned with a LI-COR Odyssey scanner (LI-COR Biosciences), and the film was scanned with a document scanner.
Immobilized CTD Peptide Binding Assay
Synthetic biotinylated peptides were dissolved in PBS and incubated with ∼300 μl of TetraLink tetrameric avidin resin (Invitrogen) for 45 min at room temperature. The peptide in the column onput, flow-through, and washes was monitored by absorbance at 280 nm, and these values were used to approximate the amount of peptide on the column. Generally, 30–50 μg of biotinylated peptide was conjugated to the 300-μl column. The peptide columns were stored in PBS at 4 °C and were stable for at least 2 months.
About 20 μg of purified Yra1 and 5 μg of bovine serum albumin (BSA) were dissolved in PBS for a final volume of 500 μl as the onput; 450 μl of the onput was applied to the peptide resin and incubated for 20 min at RT with mixing every 5 min. The flow-through and two washes with half-column volumes of PBS were collected. The resin was then extensively washed with 5 ml of buffer containing 25 mm HEPES, pH 7.6, 8% glycerol, 150 mm NaCl, and 0.1 mm EDTA (HGNE150). The bound protein was then eluted with 4 half-column volumes of HGNE300 and 4 half-column volumes of HGNE1000. The resin was regenerated with 5 ml of HGNE1000 and 5 ml of PBS.
UV Cross-linking
The UV cross-linking assay was similar to that described previously (41). A synthetic oligonucleotide RNA (CAGUCGCAUAGUGCA; Integrated DNA Technologies) was end-labeled with [γ-32P]ATP and used for binding experiments. The labeled RNA was mixed with 1 μg of protein in cross-linking buffer (15 mm HEPES, pH 7.8, 75 mm NaCl, 0.1 mm KCl, 0.2 mm EDTA, 5 mm MgCl2, 0.05% Tween 20, and 10% glycerol). Because of the salt in the protein solution (300 mm), the final NaCl concentration in the binding reaction was 90 mm. The reaction mixture was exposed to 254-nm light for 5 min on ice in a Stratalinker and stopped by the addition of SDS-PAGE loading dye and boiling. The samples were run on a 4–20% precast gel, stained with Coomassie Blue, and dried. The dried gel was exposed to a PhosphorImager (GE Healthcare) and scanned.
Fluorescence in Situ Hybridization and Immunostaining
The method of Tokunaga and Tani (42) was used for fluorescence in situ hybridization (FISH) with some modifications. Briefly, the S. cerevisiae strains were grown in YPD to midlog phase at 30 °C. The cultures were then split to produce two identical cultures; one culture was incubated at the non-heat shock temperature (30 °C), and the other culture was incubated at the heat shock temperature (37 °C) for 30 min. After heat shock, the cells from 1.5 ml of each culture were harvested by centrifugation (3000 × g for 2 min at 4 °C) and fixed for 1 h at room temperature in 4% formaldehyde. The fixed cells were permeabilized with 40 μg of Zymolyase for 20 min at 37 °C. The spheroplasts were resuspended in 1.2 m sorbitol in 0.1 m sodium phosphate buffer, pH 6.0; the suspension was allowed to settle onto poly-l-lysine-coated glass slides for 30 min at room temperature. The slides were then washed with 70% ethanol; dehydrated with 70, 90, and 100% ethanol; and dried. After incubating the slides for 30 min in prehybridization solution (4× SSC, 5× Denhardt solution, and 1 mg/ml bakers' yeast tRNA), the slides were hybridized overnight at 42 °C with hybridization solution (prehybridization solution containing 1 ng/ml digoxigenin-labeled dT50 probe). The hybridized slides were stained with FITC-labeled anti-digoxigenin antibody (1:200 dilution; Roche Applied Science) and DAPI (0.1 μg/ml; Pierce). A coverslip was mounted using glycerol containing 1 mg/ml p-phenylenediamine and 0.075× PBS, and the slides were imaged on a Zeiss Axio Observer microscope with the 100× oil objective.
For immunostaining of V5-tagged Yra1, cells were grown, fixed, and permeabilized as described above. After allowing the cells to settle on poly-l-lysine-coated glass slides, the samples were blocked with 5% BSA in PBS-Tween 20. The slides were then incubated with a 1:200 dilution of anti-V5-FITC antibody (AbD Serotec) in 5% BSA in PBS-Tween 20 overnight at 4 °C. At this dilution, minimal background fluorescence was detected in an untagged strain (data not shown). After washing with 5% BSA in PBS-Tween 20, the cells were counterstained with 0.1 μg/ml DAPI, mounted, and imaged as described above.
Chromatin Immunoprecipitation
The method described in Rusché and Rine (43) was used with some modifications. Biological duplicate yeast cultures were grown in YPD at 30 °C to an A600 of 1.0. Approximately 50 A600s of cells were fixed with 1% formaldehyde for 30 min at room temperature. The cells were harvested by centrifugation at 3000 rpm for 5 min, resuspended in 400 μl of lysis buffer (50 mm HEPES, pH 7.6, 1 mm EDTA, 140 mm NaCl, 1% Triton X-100, 0.1% sodium deoxycholate, 1 mm PMSF, 5 μg/ml chymostatin, 2 μg/ml pepstatin A, 1 mm benzamidine, and a 1:100 dilution of protease inhibitor mixture for fungal extracts (P8215, Sigma-Aldrich)) and lysed by beat beading with 0.5-mm zirconia/silica beads (BioSpec). The lysate was then collected by pipetting and pooled with 400 μl of lysis buffer used to wash the beads. The pooled lysate was sonicated four times for 10 s to shear the DNA. The cell debris was removed by centrifugation for 10 min at 4 °C, and 160 μl of the soluble lysate was diluted to 400 μl with lysis buffer. After preclearing with sheared salmon sperm DNA and BSA-blocked Protein G Dynabeads (Invitrogen), the lysate was incubated overnight with 2.5 μg of mouse monoclonal antibody (Yra1: anti-V5 antibody, MCA1360, AbD Serotec; Rpb3: anti-Rpb3 antibody, WP012, Neoclone; mock: anti-β-galactosidase antibody, Z378A, Promega). The bound complexes were precipitated with blocked Protein G Dynabeads, washed, and eluted with 1% SDS in 0.1 m NaHCO3. The cross-links were reversed, and then DNA was isolated using standard techniques (44). The samples were used for quantitative real time PCR analysis with SYBR Green (Bio-Rad) and an Eppendorf Mastercycler realplex. Data were quantified relative to the standard curve of input DNA and are presented as the mean and S.D. of independent immunoprecipitations from biological replicates. The error bars represent the S.D. The primers used for quantitative PCR are detailed in supplemental Table S1.
RESULTS
Yra1 Interacts Directly with Hyperphosphorylated Yeast CTD
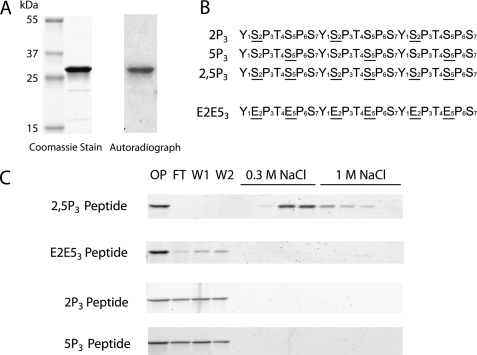
To verify that the previously observed interaction of Yra1 with the hyperphosphorylated CTD was direct (24), we expressed the YRA1 cDNA as a recombinant protein in E. coli, purified it under high salt conditions, and tested its ability to bind to the PCTD by a “far Western” assay. Specifically, the purified Yra1 protein was subjected to SDS-PAGE, transferred to nitrocellulose, and probed with a hyperphosphorylated [32P]CTD fusion protein generated by CTDK-I (“Experimental Procedures”). The radioactive PCTD fusion protein (retained on the nitrocellulose by virtue of binding to the immobilized protein) was visualized by autoradiography, and a discrete band at the same mobility as the purified Yra1 protein was observed (Fig. 1A).
FIGURE 1.
Yra1 directly and specifically interacts with hyperphosphorylated yeast CTD. A, the Coomassie-stained gel (left) shows purified recombinant His-tagged Yra1 (∼28 kDa). The purified protein was subjected to a far Western assay probed with GSTyCTD fusion protein hyperphosphorylated (32P-labeled) with CTDK-I. The autoradiograph (right) shows that the GSTyCTD probe was retained at the same mobility as Yra1. B, synthetic biotinylated three-repeat consensus CTD peptides used for the subsequent binding assays. An underlined S indicates a phosphoserine. C, the CTD peptides shown in B were immobilized on avidin resin. Yra1 was applied to the columns and onput (OP), flow-through (FT), washes (W1 and W2), and salt elution fractions (0.3 m NaCl and 1 m NaCl) were analyzed on 4–20% SDS-polyacrylamide gels stained with Coomassie Blue.
Because phosphorylation by CTDK-I likely produces a combination of CTD phosphoepitopes on the fusion protein, synthetic biotinylated three-repeat consensus CTD peptides were used to characterize the specificity of the Yra1 interaction (Fig. 1B). The synthetic peptides were immobilized on tetrameric avidin resin (24), and purified Yra1 was bound and eluted with two salt steps (Fig. 1C). Under these conditions (150 mm NaCl), Yra1 bound to the hyperphosphorylated 2,5P3 peptide, did not wash off the peptide with excess buffer (5 ml of PBS), and eluted with 300 mm NaCl. Yra1 did not bind to a CTD charge control in which both Ser-2 and -5 were substituted with glutamates (E2E53). Likewise, peptides that contained phosphoserines only at position 2 or 5 of the heptad (2P3 or 5P3, respectively) or lacked phosphoserines altogether were unable to bind Yra1 (Fig. 1C and data not shown). These results demonstrate that Yra1 binds directly to a hyperphosphorylated CTD fusion protein and specifically to synthetic heptad repeats with phosphates on both Ser-2 and -5.
The Phospho-CTD-interacting Domain of Yra1 Spans the N-variable and RRM Regions
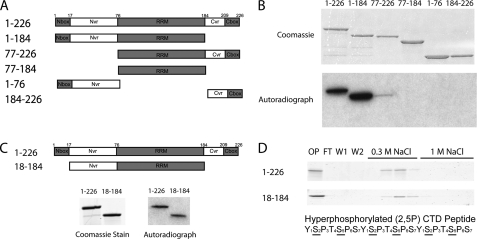
Based on homology with other REF family members, the architecture of Yra1 has been defined as shown in Fig. 2A (25, 26). Amino acids 77–184 contain an RNA recognition motif (RRM) in the center of Yra1, and two regions that vary in sequence and length among the REF proteins, called N-variable and C-variable regions, flank the RRM. The termini are more conserved within the family and are designated the N-box and C-box regions. Based on this primary structure model, we generated truncated versions of Yra1 and used them to identify the PCTD-interacting domain (PCID) (Fig. 2A). Using PCR, Yra1 segments representing amino acids 1–226, 1–184, 77–226, 77–184, 1–76, and 184–226 were subcloned, and the resulting proteins were expressed and purified as maltose-binding protein fusions (“Experimental Procedures”). As for full-length Yra1 (Fig. 1C), the purified proteins were tested for binding to the [32P]CTD fusion protein generated with CTDK-I (Fig. 2B). Both full-length Yra1 (1–226) and the C-terminal truncation (1–184) bound the PCTD probe; the latter interaction was verified using the immobilized hyperphosphorylated 2,5P3 peptide column (supplemental Fig. S1). Although there was some signal observed for the 77–226 construct (Fig. 2B), which lacks the region N-terminal to the RRM, this protein did not bind well to the 2,5P3 peptide column (supplemental Fig. S1). Finally, the RRM region alone (77–184) did not interact with the PCTD probe in the far Western assay (Fig. 2B) nor did a larger RRM construct (70–190) that included more flanking sequence, which would presumably improve the folding of the RRM (supplemental Fig. S1).
FIGURE 2.
PCID of Yra1 lies within N-variable plus RRM regions (residues 18–184). A, Yra1 architecture based on homology among the REF family of proteins (26). Gray regions, including the N-box, RRM, and C-box, have higher conservation, whereas the white regions, including the N-variable (Nvr) and C-variable regions (Cvr), have variable sequences and lengths in the REF proteins. The Yra1 truncated proteins shown were constructed. B, top, Coomassie-stained gel of Yra1 truncation proteins subjected to far Western assay. Bottom, autoradiograph of the far Western assay of Yra1 truncation proteins probed with 32P-labeled hyperphosphorylated GSTyCTD fusion protein. C, the N-terminal and RRM region (residues 18–184), which lacks the N-box and the entire C terminus (top), was cloned, purified, and subjected to a far Western assay probed with 32P-labeled hyperphosphorylated GSTyCTD fusion protein (stained gel and autoradiograph below the protein diagrams). D, Coomassie-stained gels of fractions (onput (OP), flow-through (FT), washes (W1 and W2), and elutions in two salt steps) from an immobilized peptide binding assay (2,5P3 peptide) of full-length Yra1 (1–226) (top) and the truncated variant 18–184 (bottom). An underlined S indicates a phosphoserine.
To subdivide the 1–184 amino acid fragment, we truncated the N terminus to eliminate the N-box region, yielding a protein fragment composed of residues 18–184. The 18–184 fragment retained PCTD binding activity as assessed by both far Western and immobilized peptide binding assays (Fig. 2, C and D). Expression and stability problems precluded studying further truncations of this region of Yra1. Amino acids 18–184 were therefore designated the minimum stable PCID-containing fragment of Yra1.
RNA and CTD Binding Domains Overlap but Involve Distinct Residues
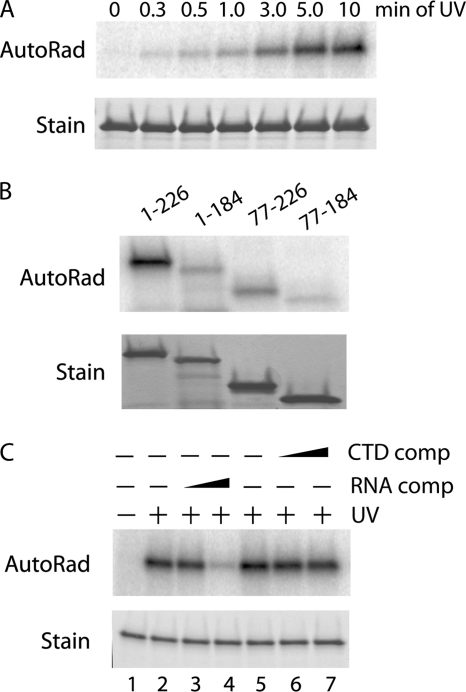
It has been reported previously that the RRM of Yra1 is unable to bind RNA, whereas the N- and C-terminal regions of the protein can bind RNA (26). To test RNA binding with a different method and to determine whether the PCID was also involved in RNA binding, we used a UV cross-linking assay with purified Yra1 and a 32P end-labeled synthetic 15-base RNA oligonucleotide (5′-CAGUCGCAUAGUGCA-3′). This probe was utilized because the RNA sequence specificity of Yra1 is currently not known and because this sequence has been shown to bind to the metazoan homologue of Yra1, ALY (41). Similarly to ALY, full-length Yra1 cross-linked to the [32P]RNA probe after exposure to UV radiation (Fig. 3A).
FIGURE 3.
CTD and RNA binding by PCID fragment involves different amino acids. A, UV cross-linking of full-length Yra1 to a 32P-labeled 15-mer RNA probe (5′-CAGUCGCAUAGUGCA-3′) with increasing exposure times to UV light. Top, phosphorimage of cross-linked RNA. Bottom, Coomassie-stained gel of proteins. B, UV cross-linking of full-length (1–226) and truncated versions (1–184, 77–226, and 77–184) of Yra1 to the 15-mer RNA probe. Top, phosphorimage of cross-linked RNA. Bottom, Coomassie-stained gel of proteins. C, UV cross-linking of 15-mer RNA to full-length Yra1 with no competitor (comp) (lanes 1, 2, and 5) or a 25-fold (lanes 3 and 6) and 100-fold (lanes 4 and 7) molar excess of cold RNA or 2,5P3 CTD peptide competitors, respectively. Top, phosphorimage of cross-linked RNA. Bottom, Coomassie-stained gel of proteins. AutoRad, autoradiograph.
UV cross-linking was then used to characterize the RNA binding of the truncated Yra1 proteins (1–184, 77–226, and 77–184). We found that all of the truncated proteins, including the RRM (77–184), cross-linked to the RNA probe (Fig. 3B). The full length (1–226) and N-terminal deletion (77–226) bound better than the C-terminal deletion (1–184) and the RRM (77–184). These data indicate that the RRM (77–184), although not sufficient for maximal RNA binding, is indeed able to bind RNA and that there is potential overlap between the PCID and the RNA binding region.
Because the minimal PCID fragment contains the RRM, which binds RNA, the ability to block RNA binding of full-length Yra1 with excess 2,5P3 CTD peptide was analyzed. When a 100-fold molar excess of unlabeled RNA was preincubated with Yra1 before exposure to UV, cross-linking to the [32P]RNA probe was almost completely blocked. In contrast, preincubation with a 100-fold molar excess of 2,5P3 CTD peptide had no effect on cross-linking to the RNA probe (Fig. 3C), indicating that the CTD does not compete with RNA for binding.
Interestingly, we found that Yra1 protein expressed in baculovirus-infected S. frugiperda (Sf9) cells would not bind RNA in the UV cross-linking assay (supplemental Fig. S2A); using the same conditions and protein concentrations, Yra1 expressed in E. coli showed robust UV cross-linking (as in Fig. 3A; see supplemental Fig. S2A). On the other hand, both versions of Yra1 bound to the doubly phosphorylated CTD peptide (supplemental Fig. S2B). Although the cause of the difference between the two versions of Yra1 is unclear, we hypothesize that, like ALY (41), post-translational modifications on Yra1 expressed in Sf9 cells block RNA binding but not CTD binding. In any case, these results further demonstrate that CTD binding and RNA binding are distinct events.
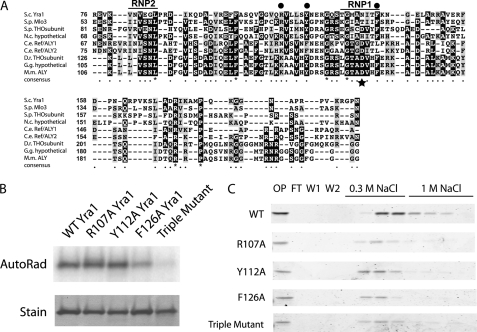
To further probe the involvement of the RRM in RNA and CTD binding, we first identified conserved amino acids in the RRM sequences of budding yeast Yra1 and eight Yra1 homologues from other eukaryotes (Fig. 4A); these include one positively charged residue (Arg-107; leftmost dot) and two aromatic residues (Tyr-112 and Phe-126, middle and right dots). These two aromatic residues are extremely well conserved in evolution except in Caenorhabditis elegans. Another well conserved residue in most RRMs of proteins that bind RNA (other than Yra1 homologues) is an aromatic residue in the middle of the RNP1 motif; its position is indicated with a star in Fig. 4A. In Yra1 and its homologues, this residue is an aspartic acid or asparagine, the presence of which has been proposed to account for the inability of these RRMs to bind RNA. In the Yra1-like proteins, however, there is a nearby aromatic amino acid, Phe-126, that we thought might play a role in RNA binding.
FIGURE 4.
A, amino acid alignment of the RRM sequences of Yra1 and homologous proteins. The RNP1 and RNP2 motifs are labeled. Three conserved residues indicated with dots were identified (Arg-107, Tyr-112, and Phe-126 in Yra1) and mutated to alanines. The star below the alignment (Asn-123 in Yra1) indicates the position of a conserved phenylalanine in most (non-Yra1) RRM sequences. BLAST was used to identify homologues of Yra1, and ClustalW and BoxShade were used to make the alignment. S.c., S. cerevisiae Yra1 (NP_010669.1); S.p., Schizosaccharomyces pombe Mlo3 (NP_595715.1) and THO subunit (NP_595161.1); N.c., Neurospora crassa hypothetical protein NCU01793 (NCBI Protein database XP_956471.1); C.e., C. elegans ALY1 (NCBI Protein database NP_501588.1) and ALY2 (NCBI Protein database NP_501594.1); D.r., Danio rerio THO subunit 4 (NCBI Protein database NP_001098578.1); G.g., Gallus gallus hypothetical protein (NCBI Protein database XP_001232393.1); M.m., Mus musculus ALY (NCBI Protein database NP_062357.3). B, UV cross-linking of wild type and alanine mutants of Yra1 to a 32P-labeled 15-mer RNA probe. Top, phosphorimage of cross-linked RNA. Bottom, Coomassie-stained gel of proteins. C, Coomassie-stained gels of fractions from the immobilized 2,5P3 peptide column binding assays of Yra1 R107A, Y112A, F126A, and R107A/Y112A/F126A (Triple) mutants. All mutants displayed binding to the immobilized 2,5P3 peptide and eluted with 0.3 m NaCl. Note the slight differences in the amounts of protein in the onput (OP) for the RRM point mutants. AutoRad, autoradiograph; FL, flow-through; W1 and W2, washes.
To test whether the conserved residues Arg-107, Tyr-112, and Phe-126 are involved in RNA binding, we performed an alanine scan and then analyzed the purified mutant proteins by UV cross-linking (Fig. 4B). The R107A and Y112A mutations did not seem to significantly affect RNA binding in vitro; however, the F126A mutant protein showed a decrease in RNA binding. In contrast, all of these mutant proteins, including the R107A/Y112A/F126A triple mutant, retained the ability to bind to the immobilized CTD 2,5P3 peptide (Fig. 4C) (slight apparent differences in column elution behavior are within the range of experimental variation; e.g. see Fig. 2D and supplemental Fig. S2). These results demonstrate that the RRM as an isolated protein segment binds RNA very weakly, whereas the same RRM in the context of the full-length protein is required for optimal RNA binding. Together, the RNA binding and PCTD binding results indicate that although the RNA and PCTD binding activities share the same protein segment the two activities involve distinct amino acids and possibly distinct binding surfaces.
CTD and RNA Binding Mutations Affect Growth of S. cerevisiae
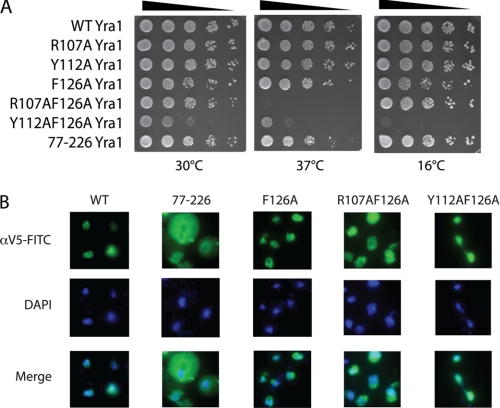
Based on the in vitro binding experiments, we used yra1 mutants that affect CTD binding (77–226 truncation) or RNA binding (F126A) to probe the requirements for these interactions in vivo; we also constructed double mutants (Y112A/F126A and R107A/F126A). In dilution plating experiments at 30 °C, the R107A and Y112A yra1 mutants displayed wild-type growth, whereas F126A, R107A/F126A, and 77–226 appeared to display a slight slow growth phenotype. In contrast, the Y112A/F126A strain was very slow growing at 30 °C (Fig. 5A, left panel). When the strains were tested for growth at high (37 °C; Fig. 5A, middle panel) and low (16 °C; Fig. 5A, right panel) temperatures, the R107A and Y112A strains behaved like WT, whereas the F126A and 77–226 strains showed slight growth defects at high but not at low temperature. In contrast, the double mutants showed marked but distinct temperature-sensitive phenotypes. The R107A/F126A strain was inviable at 37 °C but grew like wild type at 16 °C. The Y112A/F126A strain, which was slow growing at 30 °C, displayed slower growth at both higher and lower temperatures. Western blots for the V5-tagged proteins showed that all of the mutant Yra1 proteins were expressed at similar levels (data not shown). Thus, although these Yra1 mutant proteins are functional enough to support viability, the F126A, R107A/F126A, Y112A/F126A, and 77–226 strains manifest growth defects at one or more temperatures.
FIGURE 5.
Yra1 RRM mutations in S. cerevisiae cause growth defects. A, the RRM point mutations (R107A, Y112A, F126A, R107A/F126A, and Y112A/F126A) and the N-terminal truncated protein (77–226) were expressed in a YRA1-deleted yeast strain. Serial 10-fold dilutions of cells were spotted on rich medium (YPD plates) and incubated at 30 °C (left), 37 °C (middle), or 16 °C (right). B, in situ localization of Yra1 proteins in yeast. V5 epitope-tagged Yra1 constructs were detected by immunofluorescence using a FITC-labeled anti-V5 antibody (green); DAPI (blue) was used to stain the nuclei.
As deletion of the N-variable region has been shown to change the subcellular localization of Yra1 (26), we used immunostaining to verify that the point mutations did not alter nuclear localization. As shown in Fig. 5B, the full-length WT protein displayed nuclear and perinuclear staining as did the RRM mutant proteins F126A, R107A/F126A, and Y112A/F126A. Thus, the RRM point mutations do not alter Yra1 subcellular localization. In contrast, the 77–226 protein was diffusely localized in the nucleus and cytoplasm.
Defects in RNA and CTD Binding Produce mRNA Export Defects
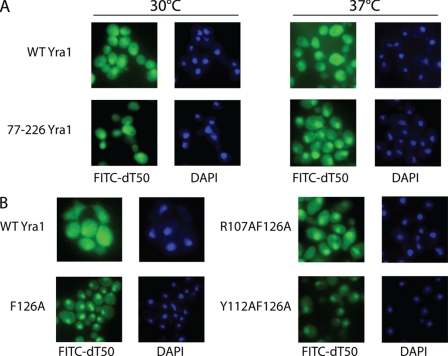
To determine whether the growth defects of the mutant strains resulted from defects in mRNA export, the distribution of poly(A) RNA was analyzed by FISH. In wild-type cells, poly(A) RNA, which was detected with a FITC-labeled oligo(dT)50 probe, was uniformly distributed throughout cells (Fig. 6A), and the poly(A) RNA distribution did not change at high temperature. In contrast, the CTD binding-deficient mutant (77–226) displayed significant nuclear accumulation of poly(A) RNA at 37 °C (Fig. 6A). In addition, at the permissive temperature, the F126A, R107A/F126A, and Y112A/F126A strains showed greater nuclear retention of poly(A) RNA compared with wild-type cells (Fig. 6B). Nuclear retention was particularly severe in the Y112A/F126A strain, which also displayed the most severe growth defect. These results are consistent with the growth assays and the hypothesis that the PCID of Yra1 is required for optimal cotranscriptional recruitment of Yra1 and its mRNA export activity.
FIGURE 6.
Yra1 RRM mutations and N-terminal truncation in S. cerevisiae cause general mRNA export defects. A, FISH of poly(A)+ RNA (green) in yeast cells expressing full-length (WT) or truncated (77–226) Yra1. DAPI (blue) was used to stain the nuclei. Non-heat shock (30 °C; left) and heat shock (37 °C; right) conditions were used. At 37 °C, greater nuclear accumulation of poly(A)+ RNA was observed in the truncated (77–226) Yra1 strain compared with WT. B, FISH of poly(A)+ RNA (green) in yeast expressing wild type and RRM point mutants of Yra1 at 30 °C. DAPI (blue) was used to stain the nuclei. The F126A and Y112A/F126A mutants display greater nuclear accumulation of poly(A)+ RNA compared with wild type, a result consistent with an mRNA export defect.
Defects in PCTD Binding Alter Cotranscriptional Recruitment of Yra1
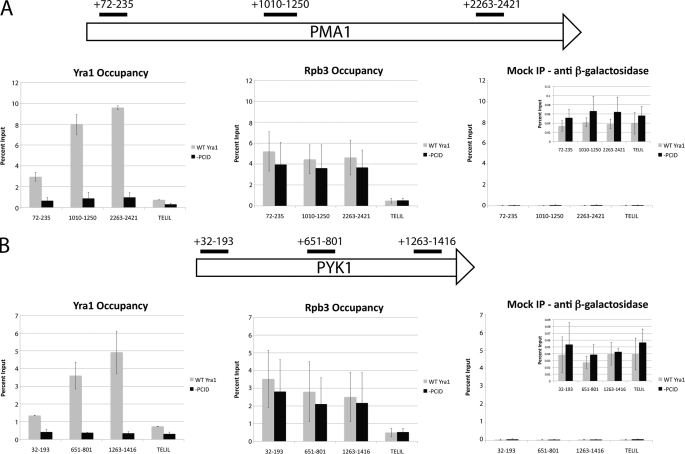
We hypothesized that the role of PCTD binding is to increase the efficiency of Yra1 recruitment to its nascent mRNA cargo. One measure of this recruitment can be obtained by ChIP, which provides an indication of protein occupancy along a gene. Using primer sets specific to the 5′-end, middle, and 3′-end of the PMA1 and PYK1 genes (supplemental Table S1 and Fig. 7), we used ChIP assays to measure occupancy levels of Yra1 and Rpb3 on these two genes (Fig. 7). Consistent with previous studies (28, 33), wild-type Yra1 displayed low occupancy at the 5′-ends of both genes, and the level of Yra1 occupancy increased along the length of the genes. An internal control, Pol II (as indicated by its Rpb3 subunit), showed essentially constant occupancy levels across the genes as seen previously (11–13). Interestingly, the level of the PCID truncated protein (amino acids 77–226; Fig. 7, “−PCID”) at either PMA1 or PYK1 was lower than the WT protein by up to 10-fold (mean values of 9.60 ± 0.18% input for WT versus 0.97 ± 0.46% input for −PCID with the 2263–2421 primer set of PMA1). Note that the PCID truncation of Yra1 did not affect levels of Pol II at either gene as shown by the Rpb3 occupancy values. Moreover, mock immunoprecipitations with an anti-β-galactosidase antibody showed that the background was consistently less than 0.1% input. A region on the left telomere of chromosome I was also used as a transcriptionally inactive control segment for ChIP. Thus, a version of Yra1 lacking part of the PCID was present on transcription units at much lower levels than the full-length protein.
FIGURE 7.
Chromatin immunoprecipitation of WT and truncated (−PCID) Yra1 at PMA1 (A) and PYK1 (B) genes. The schematic shows the primer set locations on the PMA1 and PYK1 genes. The TELIL primer set recognizes a region in the telomere of chromosome I that should be transcriptionally inactive based on the annotation of the yeast genome. The mock immunoprecipitation (IP) was performed with an anti-β-galactosidase mouse monoclonal antibody. All graphs for each gene are plotted on the same scale for comparison, and the inset for the mock immunoprecipitation is zoomed in on the bars. The data are shown as the percentage of DNA immunoprecipitated relative to the input DNA for each strain. For each sample, the mean of two independent immunoprecipitations from biological replicates is shown, and the error bars represent the S.D. of the biological replicates.
It is important to note that the localization defect caused by truncation of the N terminus of Yra1 (Fig. 5B) probably also contributes to the ChIP results as a lower amount of Yra1 in the nucleus would likely decrease the amount of protein on a given gene. However, altogether, these data indicate that Yra1 specifically binds to the hyperphosphorylated CTD of Pol II in vivo, and disruption of this interaction results in a defect in Yra1 recruitment.
DISCUSSION
The phospho-CTD of Pol II plays an important role in the cotranscriptional recruitment of elongation, chromatin remodeling, and mRNA processing factors; here we report the PCTD binding of an mRNA export factor, Yra1. Previously, using ChIP approaches, Yra1 was shown to be cotranscriptionally recruited to active genes during elongation (45). Although it was not clear whether Yra1 associated with the transcription machinery or the nascent mRNA during elongation, the ChIP association was shown to be significantly resistant to nuclease treatment (28), which is consistent with a protein-protein interaction. We showed here that Yra1 binds to the hyperphosphorylated form of the CTD and to CTD peptides phosphorylated on Ser-2 and -5, the phosphorylation pattern thought to predominate during transcription elongation. These in vitro data are consistent with the previous ChIP studies, and we propose that Yra1 may be recruited to genes through interaction with the PCTD.
Because Yra1 is a uniformly positively charged RNA-binding protein, we sought to demonstrate that the PCTD interaction is a bona fide binding event. To this end, we showed that Yra1 binds to the CTD phosphorylated in an elongation-specific pattern (Ser(P)-2, Ser(P)-5 but not Ser(P)-5 or Ser(P)-2). The interaction is specific for phosphoserines in the context of the CTD as a peptide with substitution of Ser-2 and -5 with the phosphomimetic amino acid glutamate was not bound by Yra1. Using truncations of Yra1, we further showed that CTD binding is confined to amino acids 18–184, whereas optimal RNA binding was obtained with amino acids 77–226. Although these regions both contain the RRM, preincubation of Yra1 with the Ser(P)-2, Ser(P)-5 CTD peptide did not interfere with RNA binding. Moreover, although Yra1 expressed in baculovirus-infected Sf9 cells was unable to bind RNA due to potential post-translational modifications, CTD peptide binding was not blocked. These results therefore demonstrate that Yra1 binds specifically to the PCTD in a manner that is distinct from RNA binding.
Previous studies indicated that all regions of Yra1 except the RRM are responsible for RNA binding (26). Using UV cross-linking, we were able to detect binding of the isolated RRM to an RNA probe; however, a construct that included the sequence C-terminal to the RRM displayed RNA binding that was the closest to that of the full length. Moreover, point mutations in the RRM of full-length Yra1 affected RNA binding but not CTD binding. These mutations also produced alleles of Yra1 with both growth and mRNA export defects, a result consistent with the in vitro RNA binding defects. This is the first demonstration that the RRM of Yra1 is directly involved in RNA binding. It is possible that, when expressed alone, the RRM does not fold well enough to bind RNA strongly and that increasing the amount of sequence around the RRM improves folding. In addition, the C terminus of Yra1 may contribute to RNA binding. In ALY/REF2-I, the RRM and N terminus of the export factor were shown to bind RNA in chemical shift perturbation studies (46). Similar studies on Yra1 would clarify the roles of the N-terminal and C-terminal regions in RNA binding.
It is not surprising that the RRM in Yra1 may play a role in a protein-protein interaction as many RRMs have been shown to be important for protein and not RNA interaction. For example, the spliceosomal components U2AF35 and U2AF65 both contain non-canonical RRMs that mediate interactions with U2AF65 and SF1, respectively (47). Moreover, a recent study indicated that the non-canonical RRM of U2AF65 may also mediate an interaction between the splicing factor and the CTD (48). The RRM thus may be another domain that proteins utilize to interact with the CTD of RNA polymerase II.
Using a truncation of the N terminus to abrogate CTD binding without affecting RNA binding, we analyzed the role of the CTD in Yra1 function. The 77–226 strain showed a slight slow growth phenotype and only a minor mRNA export phenotype during heat stress. However, the ChIP assay indicated that even at 30 °C recruitment of Yra1 to the PMA1 and PYK1 genes was severely compromised. The lack of growth or export phenotypes in the 77–226 truncation strain indicates that PCTD binding is not essential for export at the normal growth temperature. It is possible that at the higher temperature the cotranscriptional recruitment defect of the 77–226 protein is even more severe or that the mutant protein is recruited to a different subset of genes. In view of the phosphoepitope binding specificity of the PCID in Yra1, we also expect that abnormalities in recruitment would accompany alterations in CTD phosphorylation patterns; thus, we predict that inhibiting CTD kinase activities in vivo will also lead to aberrant Yra1 recruitment. These possibilities can be investigated in future studies.
It is currently unclear how the Yra1-PCTD interaction is involved in or affects the cotranscriptional associations of other Yra1-interacting proteins, such as the TREX complex (which in yeast contains the THO proteins Hpr1, Tho2, Mft1, and Thp2 along with Yra1 and Sub2) or Mex67/Mtr2. Although the proteins in TREX usually co-immunoprecipitate, Yra1 may associate weakly with the complex as the levels of Yra1 and Sub2 are often substoichiometric; in addition, a high salt wash has been shown to remove Yra1 from the complex completely (32). The implied interaction between Yra1 and TREX is supported by the observation that the ChIP enrichment of Yra1 at PMA1 is decreased ∼50% in an hpr1Δ strain (33); however, because hpr1Δ has been shown to affect transcription elongation, the decrease in Yra1 chromatin association may be due to differences in elongation rather than a direct effect of Hpr1 on the export protein (49). In addition, ChIP analysis of TREX complex members on multiple genes showed that the recruitment patterns of Yra1 and Hpr1 are not always the same (28). Thus, the TREX complex may not be important for Yra1 cotranscriptional association at all genes. Pcf11 has also been shown to bind to and potentially recruit Yra1 to mRNAs during transcription (35). It is possible that Yra1 uses different modes of recruitment at different genes or that the Yra1-Pcf11 interaction plays a distinct role in mRNA quality control prior to export. Interestingly, the PCID potentially overlaps with part of the Mex67 binding site on Yra1, which has been shown to include the N-variable region (50), whereas the Yra1-Sub2 interaction involves a different region. It will be informative to investigate the recruitment behavior of other export-related factors in a strain in which Yra1 recruitment via the CTD is defective. The mutants identified here may contribute to understanding the order of recruitment of multiple mRNA packaging and export factors. Genome-wide studies might be particularly interesting to investigate the suggestion that Yra1 and TREX are differentially recruited to subsets of genes.
In the course of our investigations, we generated a double point mutant version of Yra1 with novel and potentially useful properties. R107A/F126A displayed wild-type growth at 30 °C but was inviable at 37 °C. In contrast, the most commonly used Yra1 temperature-sensitive mutant yra1-1 (25) contains five point mutations and displayed defective growth at 30 °C. The normal growth of the R107A/F126A mutant at the permissive temperature makes it potentially more useful for future examination of Yra1 function and mRNA export in yeast.
It is currently not known whether Yra1 binds to specific RNA sequences. As a general mRNA export factor, it would be advantageous for Yra1 to bind to RNA in a sequence-independent manner. If sequence is not involved in Yra1 cargo recognition, protein-protein interactions may be responsible for directing Yra1 to nascent mRNAs for export. In this way, the hyperphosphorylated CTD may be involved in directing Yra1 to a given mRNA for export.
Supplementary Material
Acknowledgments
We thank Mariano Garcia-Blanco for discussions regarding RNA binding assays and Laura Rusché for advice regarding ChIP.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 GM040505.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and Table S1.
- CTD
- C-terminal domain
- Pol II
- RNA polymerase II
- CTDK-I
- CTD kinase I
- RRM
- RNA recognition motif
- PCTD
- phospho-CTD
- PCID
- PCTD-interacting domain
- TREX
- transcription export
- 2,5P3
- 3-repeat synthetic CTD peptide with phosphoserines in positions 2 and 5 of each repeat
- GSTyCTD
- GST-tagged yeast CTD
- REF
- RNA export factor.
REFERENCES
- 1. Nonet M., Sweetser D., Young R. A. (1987) Cell 50, 909–915 [DOI] [PubMed] [Google Scholar]
- 2. Zehring W. A., Lee J. M., Weeks J. R., Jokerst R. S., Greenleaf A. L. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 3698–3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phatnani H. P., Greenleaf A. L. (2006) Genes Dev. 20, 2922–2936 [DOI] [PubMed] [Google Scholar]
- 4. Buratowski S. (2009) Mol. Cell 36, 541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meinhart A., Kamenski T., Hoeppner S., Baumli S., Cramer P. (2005) Genes Dev. 19, 1401–1415 [DOI] [PubMed] [Google Scholar]
- 6. de Almeida S. F., Carmo-Fonseca M. (2008) FEBS Lett. 582, 1971–1976 [DOI] [PubMed] [Google Scholar]
- 7. Pandit S., Wang D., Fu X. D. (2008) Curr. Opin. Cell Biol. 20, 260–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim M., Suh H., Cho E. J., Buratowski S. (2009) J. Biol. Chem. 284, 26421–26426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chapman R. D., Heidemann M., Albert T. K., Mailhammer R., Flatley A., Meisterernst M., Kremmer E., Eick D. (2007) Science 318, 1780–1782 [DOI] [PubMed] [Google Scholar]
- 10. Komarnitsky P., Cho E. J., Buratowski S. (2000) Genes Dev. 14, 2452–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim H., Erickson B., Luo W., Seward D., Graber J. H., Pollock D. D., Megee P. C., Bentley D. L. (2010) Nat. Struct. Mol. Biol. 17, 1279–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tietjen J. R., Zhang D. W., Rodríguez-Molina J. B., White B. E., Akhtar M. S., Heidemann M., Li X., Chapman R. D., Shokat K., Keles S., Eick D., Ansari A. Z. (2010) Nat. Struct. Mol. Biol. 17, 1154–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayer A., Lidschreiber M., Siebert M., Leike K., Söding J., Cramer P. (2010) Nat. Struct. Mol. Biol. 17, 1272–1278 [DOI] [PubMed] [Google Scholar]
- 14. Hengartner C. J., Myer V. E., Liao S. M., Wilson C. J., Koh S. S., Young R. A. (1998) Mol. Cell 2, 43–53 [DOI] [PubMed] [Google Scholar]
- 15. Akhtar M. S., Heidemann M., Tietjen J. R., Zhang D. W., Chapman R. D., Eick D., Ansari A. Z. (2009) Mol. Cell 34, 387–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jona G., Wittschieben B. O., Svejstrup J. Q., Gileadi O. (2001) Gene 267, 31–36 [DOI] [PubMed] [Google Scholar]
- 17. Lindstrom D. L., Hartzog G. A. (2001) Genetics 159, 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee J. M., Greenleaf A. L. (1997) J. Biol. Chem. 272, 10990–10993 [DOI] [PubMed] [Google Scholar]
- 19. Lee J. M., Greenleaf A. L. (1991) Gene Expr. 1, 149–167 [PMC free article] [PubMed] [Google Scholar]
- 20. Jones J. C., Phatnani H. P., Haystead T. A., MacDonald J. A., Alam S. M., Greenleaf A. L. (2004) J. Biol. Chem. 279, 24957–24964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qiu H., Hu C., Hinnebusch A. G. (2009) Mol. Cell 33, 752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mosley A. L., Pattenden S. G., Carey M., Venkatesh S., Gilmore J. M., Florens L., Workman J. L., Washburn M. P. (2009) Mol. Cell 34, 168–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brodsky A. S., Silver P. A. (2000) RNA 6, 1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Phatnani H. P., Jones J. C., Greenleaf A. L. (2004) Biochemistry 43, 15702–15719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strässer K., Hurt E. (2000) EMBO J. 19, 410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zenklusen D., Vinciguerra P., Strahm Y., Stutz F. (2001) Mol. Cell. Biol. 21, 4219–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stutz F., Bachi A., Doerks T., Braun I. C., Séraphin B., Wilm M., Bork P., Izaurralde E. (2000) RNA 6, 638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abruzzi K. C., Lacadie S., Rosbash M. (2004) EMBO J. 23, 2620–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hieronymus H., Silver P. A. (2003) Nat. Genet. 33, 155–161 [DOI] [PubMed] [Google Scholar]
- 30. Rollenhagen C., Hodge C. A., Cole C. N. (2007) Eukaryot. Cell 6, 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hogan D. J., Riordan D. P., Gerber A. P., Herschlag D., Brown P. O. (2008) PLoS Biol. 6, e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strässer K., Masuda S., Mason P., Pfannstiel J., Oppizzi M., Rodriguez-Navarro S., Rondón A. G., Aguilera A., Struhl K., Reed R., Hurt E. (2002) Nature 417, 304–308 [DOI] [PubMed] [Google Scholar]
- 33. Zenklusen D., Vinciguerra P., Wyss J. C., Stutz F. (2002) Mol. Cell. Biol. 22, 8241–8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoh S. M., Cho H., Pickle L., Evans R. M., Jones K. A. (2007) Genes Dev. 21, 160–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson S. A., Cubberley G., Bentley D. L. (2009) Mol. Cell 33, 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wach A., Brachat A., Pöhlmann R., Philippsen P. (1994) Yeast 10, 1793–1808 [DOI] [PubMed] [Google Scholar]
- 37. Alberti S., Gitler A. D., Lindquist S. (2007) Yeast 24, 913–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gietz R. D., Woods R. A. (2002) Methods Enzymol. 350, 87–96 [DOI] [PubMed] [Google Scholar]
- 39. Morris D. P., Lee J. M., Sterner D. E., Brickey W. J., Greenleaf A. L. (1997) Methods 12, 264–275 [DOI] [PubMed] [Google Scholar]
- 40. Phatnani H. P., Greenleaf A. L. (2004) Methods Mol. Biol. 257, 17–28 [DOI] [PubMed] [Google Scholar]
- 41. Hung M. L., Hautbergue G. M., Snijders A. P., Dickman M. J., Wilson S. A. (2010) Nucleic Acids Res. 38, 3351–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tokunaga K., Tani T. (2008) Curr. Protoc. Cell Biol. Chapter 22, Unit 22.13 [DOI] [PubMed] [Google Scholar]
- 43. Rusché L. N., Rine J. (2001) Genes Dev. 15, 955–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moore D., Dowhan D. (2002) Curr. Protoc. Mol. Biol. Chapter 2, Unit 2.1A [DOI] [PubMed] [Google Scholar]
- 45. Lei E. P., Krebber H., Silver P. A. (2001) Genes Dev. 15, 1771–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Golovanov A. P., Hautbergue G. M., Tintaru A. M., Lian L. Y., Wilson S. A. (2006) RNA 12, 1933–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kielkopf C. L., Lücke S., Green M. R. (2004) Genes Dev. 18, 1513–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. David C. J., Boyne A. R., Millhouse S. R., Manley J. L. (2011) Genes Dev. 25, 972–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rondón A. G., Jimeno S., García-Rubio M., Aguilera A. (2003) J. Biol. Chem. 278, 39037–39043 [DOI] [PubMed] [Google Scholar]
- 50. Strässer K., Hurt E. (2001) Nature 413, 648–652 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.