Background: Acidocalcisomes are acidic calcium and polyphosphate storage organelles found in diverse organisms.
Results: Knockdown of adaptor protein-3 (AP-3) complex subunits in Trypanosoma brucei affects the biogenesis of acidocalcisomes and their growth and virulence.
Conclusion: AP-3 is essential for the biogenesis of acidocalcisomes and the growth and virulence of T. brucei.
Significance: Learning the biogenesis mechanism of acidocalcisomes is important for understanding their roles.
Keywords: Adaptor Proteins, Calcium, Parasitology, Trypanosoma brucei, Vacuolar Acidification, Acidocalcisome, Osmoregulation, Polyphosphate
Abstract
Acidocalcisomes are acidic calcium and polyphosphate storage organelles found in a diverse range of organisms. Here we present evidence that the biogenesis of acidocalcisomes in Trypanosoma brucei is linked to the expression of adaptor protein-3 (AP-3) complex. Localization studies in cell lines expressing β3 and δ subunits of AP-3 fused to epitope tags revealed their partial co-localization with the vacuolar proton pyrophosphatase, a marker of acidocalcisomes, with the Golgi marker Golgi reassembly and stacking protein, and with antibodies against the small GTPase Rab11. Ablation of the β3 subunit by RNA interference (RNAi) resulted in disappearance of acidocalcisomes from both procyclic and bloodstream form trypanosomes, as revealed by immmunofluorescence and electron microscopy assays, with no alterations in trafficking of different markers to lysosomes. Knockdown of the β3 subunit resulted in lower acidic calcium, pyrophosphate, and polyphosphate content as well as defects in growth in culture, resistance to osmotic stress, and virulence in mice. Similar results were obtained by knocking down the expression of the δ subunit of AP-3. These results indicate that AP-3 is essential for the biogenesis of acidocalcisomes and for growth and virulence of T. brucei.
Introduction
Acidocalcisomes are acidic organelles rich in calcium and polyphosphate (polyP)4 that were first described as such in Trypanosoma brucei (1) and Trypanosoma cruzi (2), the etiologic agents of African sleeping sickness and Chagas disease, respectively. Later work found that acidocalcisomes are widely distributed (3). As the characteristic features of acidocalcisomes were revealed, it became apparent that they were morphologically and chemically similar to the “granules” described more than 100 years ago as “metachromatic granules” (4) or “volutin granules” (5, 6) and later called “polyP granules” (7) in different microorganisms. These granules were known to contain large amounts of calcium and polyP (8), although it was not clear whether they had a membrane. The finding of enzymes and transporters in the surrounding membrane of these organelles was fundamental in understanding their potential function and origin, and these studies started after their description in trypanosomatid and Apicomplexan parasites (3, 9, 10). The discovery of acidocalcisome-like organelles in bacteria (11, 12) and human platelets (13) suggested that the acidocalcisome is an organelle that either evolved before bacterial and eukaryotic lineages diverged (3) or appeared independently by convergent evolution.
Recent work has shown that acidocalcisomes have similarities to lysosome-related organelles (LROs). LROs are cell type-specific modifications of the post-Golgi endomembrane system that have a variety of functions and share some characteristics with lysosomes (14). Organelles belonging to this group include melanosomes, lytic granules, major histocompatibility complex (MHC) class II compartments, platelet-dense granules, basophil granules, and neutrophil azurophil granules (15). Acidocalcisomes resemble LROs in many of their properties. Platelet-dense granules, which are LROs, have several features in common with acidocalcisomes (13). They share a similar size, acidic properties, and composition because both contain pyrophosphate (PPi), polyP, and calcium (3).
Endocytic tracers, such as transferrin (16), horseradish peroxidase (17), and FM4-64 (18), do not accumulate in acidocalcisomes. However, acidocalcisomes do accumulate endocytic markers if the biosynthesis of sterols is inhibited (19), suggesting an association of acidocalcisomes with the endosomal/lysosomal pathway. A Leishmania major sphingolipid-deficient mutant is defective in the biogenesis of both multivesicular bodies (or late endosomes) and acidocalcisomes, suggesting a common origin for both compartments (20). In addition, Besteiro et al. (21) showed that a L. major mutant deficient in the δ subunit of the adaptor protein-3 (AP-3δ) complex, which is involved in trafficking of proteins to LROs (22), had less acidic acidocalcisomes that contained less polyP and lacked a membrane-bound proton pump (vacuolar proton pyrophosphatase) but contained a soluble pyrophosphatase (21). Surprisingly, in contrast to the results reported in mammals, Drosophila, Caenorhabditis elegans, and yeast (22–24), where alterations of AP-3 provoked alterations in the biogenesis of LRO, the AP-3 complex was apparently not essential for the biogenesis of acidocalcisomes in L. major (21).
Adaptor protein (AP) complexes are important mediators for vesicular transport of membrane proteins between cellular compartments, such as Golgi complex, endosomes, lysosomes, and plasma membrane (22). Four basic AP complexes have been described: AP-1, AP-2, AP-3, and AP-4. Each of these complexes is composed of two large subunits or adaptins (one each of γ/α/δ/ϵ and β1–4, respectively, 90–130 kDa), one medium adaptin (μ1–4, ∼50 kDa), and one small adaptin (σ1–4, ∼20 kDa). With some exceptions, the subunits of different AP complexes are not interchangeable. AP-2 participates in the formation of endocytic vesicles at the cell surface. AP-1 is involved in post-Golgi sorting to the endosomal compartments. AP-4 appears to have a role in sorting to the endosomal-lysosomal system, whereas AP-3 is involved in sorting of proteins to lysosomes and lysosome-related organelles from the Golgi (25) or from endosomes (26, 27).
T. brucei appears to have a simpler adaptor complex complement than yeast, mammals (28), or L. major (21). Specific secondary loss of the AP-2 complex from T. brucei has been reported (29, 30), together with the absence of other proteins involved in trafficking, such as Dab2, GGAs, PACS-1, and stonins (28, 30).
To study whether biogenesis of acidocalcisomes in T. brucei is linked to the expression of AP-3 function, we investigated the effects of ablation of its β and δ subunits (Tbβ3 and Tbδ) by RNAi. Knockdown of these subunits led to disappearance of acidocalcisomes from procyclic form (PCF) and bloodstream form (BSF) trypanosomes. This treatment also led to defects in growth and virulence in mice, indicating that AP-3 is essential for the biogenesis of acidocalcisomes and for growth and virulence in T. brucei.
EXPERIMENTAL PROCEDURES
Culture Methods
Cultivation of PCF and BSF of T. brucei Lister strain 427 was carried out as described previously (31). PCF 29-13 (T7RNAP NEO TETR HYG) co-expressing T7 RNA polymerase and Tet repressor were a gift from Dr. George A. M. Cross (Rockefeller University, New York) and were grown in SDM-79 medium (32) supplemented with hemin (7.5 μg/ml) and 10% heat-inactivated fetal calf serum and at 27 °C in the presence of G418 (15 μg/ml) and hygromycin (50 μg/ml) to maintain the integrated genes for T7 RNA polymerase and tetracycline repressor, respectively (33). BSF trypanosomes (single marker strain) were a gift from Dr. G. A. M. Cross and were grown at 37 °C in HMI-9 medium (34) supplemented with 10% fetal bovine serum (FBS), 10% serum plus (JRH Biosciences, Inc.), and 2.5 μg/ml G418.
Chemicals and Reagents
TRIzol reagent, Taq DNA polymerase, Superscript II reverse transcriptase, pCR 2.1-TOPO cloning kit, protein MagicMarker, and Alexa-conjugated secondary antibodies were purchased from Invitrogen. [α-32P]dCTP (3,000 Ci/mmol), EN3HANCE, and [35S]Met/Cys were from PerkinElmer Life Sciences. Rabbit antibody against T. brucei vacuolar H+-pyrophosphatase (TbVP1) (35) was a gift from Dr. Norbert Bakalara (Ecole Nationale Supérieure de Chimie de Montpellier, Montpellier, France). Antibody against GRASP (36) was a gift from Dr. Graham Warren (Max F. Perutz Laboratories, Vienna, Austria), and antibodies against p67 and TbCATL (37) were a gift from Dr. James Bangs (University of Wisconsin, Madison, WI). Purified HA.11 clone 16B12 monoclonal antibody against HA was purchased from Covance Inc. (Princeton, NJ). Rabbit anti-TbRab11 antibody (38) was kindly provided by Dr. Mark Fields (University of Cambridge). The pMOTag4H vector (39) was a gift from Dr. Thomas Seebeck (University of Bern). The p2T7Ti vector (40) was a gift from Dr. John Donelson (University of Iowa). The enhanced chemiluminescence (ECL) detection kit was from GE Healthcare. Pierce ECL Western blotting substrate was from Thermo Fisher Scientific Inc. (Rockford, IL). The protein assay reagent, Zeta-Probe GT Genomic Tested blotting membranes were from Bio-Rad. The AMAXA Human T-cell Nucleofector kit was purchased from Lonza (Cologne, Germany). The Prime-a-Gene Labeling System was from Promega (Madison, WI). The QIAquick gel extraction kit and MinElute PCR purification kit were from Qiagen (Valencia, CA). The primers were purchased from Integrated DNA Technologies (Coralville, IA). All other reagents of analytical grade were from Sigma.
Generation of Tbβ3 and Tbδ RNAi Constructs and Epitope-tagging Cassettes
To knock down the expression of the Tbβ3 and Tbδ genes (GenBankTM accession numbers XM_824036 and XM_839938, respectively) by double-stranded RNA expression, the inducible T7 RNA polymerase-based protein expression system and the p2T7Ti vector with dual inducible T7 promoters were employed. A 1.4-kb cDNA fragment of Tbβ3 (targeted to the 5′-end of the open reading frame (ORF)) was amplified using the forward primer 5′-TTCTAGATGAACCGCGCTATCATTGCAGAG-3′ and the reverse primer 5′-TCTCGAGACCACATGCTCCACGTGATA-3′ (where the underlined nucleotides indicate the introduced XbaI and XhoI sites, respectively), digested with XbaI and XhoI, and then cloned into the enzyme-cut p2T7Ti vector to generate p2T7(Tbβ3). Similarly, a 407-bp cDNA fragment of Tbδ targeted to nucleotides 1959–2365 of the ORF was amplified using the forward primer 5′-CGGGATCCCCGGAAGGATTGAATCTTGA-3′ and the reverse primer 5′-CCCAAGCTTCGAAACTTCTTCGTTGCCTC-3′ (where the underlined nucleotides indicate the introduced BamHI and HindIII sites, respectively) and cloned into the BamHI and HindIII sites of p2T7Ti to create p2T7(Tbδ). The recombinant constructs p2T7(Tbβ3) and p2T7(Tbδ) were confirmed by sequencing at the DNA Analysis Facility at Yale University (New Heaven, CT), NotI-linearized, and then purified with the Qiagen DNA purification kit for cell transfections.
We followed the one-step epitope tagging protocol reported by Oberholzer et al. (39) to produce the C-terminal HA-tagging cassette for PCR-mediated transfection of T. brucei PCF trypanosomes. In brief, the PCR forward and reverse primers were designed to contain the last 100–120 nucleotides of Tbβ3 or Tbδ ORF before their stop codons and the reverse complement of the first 100–120 nucleotides of the 3′-UTR of these target genes, respectively, followed in frame by the 21–26 nucleotides of the backbone sequences of pMOTag vector series (39). The HA-tagging cassettes containing a hygromycin-resistant gene as selection marker were generated for cell transfections by PCR using pMOTag4H (39) as template with the corresponding PCR primers of the Tbβ3 or Tbδ gene.
Cell Transfections
Mid-log phase PCF (∼5 × 106 cells/ml) were harvested by centrifugation at 1,000 × g for 7 min, washed with Cytomix buffer (2 mm EGTA, 3 mm MgCl2, 120 mm KCl, 0.5% glucose, 0.15 mm CaCl2, 0.1 mg/ml BSA, 10 mm K2HPO4/KH2PO4, 1 mm hypoxanthine, 25 mm Hepes, pH 7.6), and resuspended in 0.45 ml of the same buffer at a cell density of 2.5 × 107 cells/ml. The washed cells were mixed with 50 μl of NotI-linearized plasmid DNA or PCR product (10 μg) in a 0.4-cm electroporation cuvette and subjected to two pulses from a Bio-Rad Gene Pulser electroporator set at 1.5 kV and 25 microfarads. The stable transformants were obtained in SDM-79 medium supplemented with 15% FBS plus the appropriate antibiotic (5 μg/ml phleomycin, 15 μg/ml G418, and/or 50 μg/ml hygromycin).
For the BSF, 10 μg of NotI-linearized plasmid DNA or purified PCR product (<10 μl) was used per 4 × 107 mid-log phase cells in 100 μl of AMAXA Human T-cell Nucleofector solution. Electroporation was performed using 2-mm gap cuvettes with program X-001 of the AMAXA Nucleofector. Following each transfection, soluble transformants were selected and cloned by limiting dilution in HMI-9 medium containing 10% FBS and 10% serum plus with appropriate antibiotics (2.5 μg/ml phleomycin, 2.5 μg/ml G418) in 24-well plates.
Antibiotic-resistant clones were further characterized as described below. RNAi was induced with 1 μg/ml fresh tetracycline when the cells were at a density of 2 × 106 PCF or 1 × 105 BSF cells/ml. The correct epitope tagging of the target genes was confirmed by PCR followed by sequencing and Western blot analyses.
Northern Blot Analysis
Total RNA was isolated with TRIzol reagent and then treated with DNA-free following the manufacturers' instructions. RNA samples (10 μg/lane) were fractionated on 1% agarose/formaldehyde gels, transferred to Zeta-Probe nylon membranes by capillary action, and then fixed onto the membranes by baking at 80 °C for 1 h. The Tbβ3 and Tbδ probes were generated from p2T7(Tbβ3) and p2T7(Tbδ), respectively, by PCR using the same primers described above and labeled with [α-32P]dCTP using the Prime-a-Gene Labeling System according to the manufacturer's protocol. The [α-32P]dCTP-labeled probe of the Tb-β-tubulin gene (GeneDB Tb927.1.2396) was generated from T. brucei genomic DNA by PCR using the gene-specific primers (5′-ATGCGCGAAAATCGTCTGCGTTCAGG-3′ and 5′-AGTGCAGACGCGGGAATGGGACAAG-3′). The RNA-bound membranes were hybridized with the 32P-labeled Tbβ3, Tbδ, or Tb-β-tubulin probe in 0.5 m Na2HPO4, pH 7.4, and 7% SDS at 65 °C overnight with agitation. After hybridization, the membranes were washed twice for 10 min each at 68 °C with 1× SSC and 0.1% SDS and the twice for 30 min at 65 °C with 0.1× SSC and 0.1% SDS. Northern blots were visualized by autoradiography.
Immunofluorescence Microscopy
T. brucei cells were harvested, washed with PBS, and fixed with 4% formaldehyde in PBS for 1 h. After washing with PBS, parasites were allowed to adhere to poly-l-lysine-coated coverslips, permeabilized with 0.3% Triton X-100 in PBS for 3 min for PCF, and then blocked with PBS containing 3% bovine serum albumin, 1% fish gelatin, 50 mm NH4Cl, and 5% goat serum for 1 h. Cells were stained with the polyclonal rabbit antibody against TbVP1 (1:300), the purified HA.11 clone 16B12 monoclonal antibody against HA (1:500), the rabbit anti-TbRab11 antibody (1:400), and the rabbit anti-GRASP antibody (1:200) for 1 h. Cells were thoroughly washed with PBS and incubated with Alexa 488-conjugated goat anti-mouse antibody and/or Alexa 546-conjugated goat anti-rabbit antibody at 1:1,000 for 1 h. The cells were counterstained with DAPI before mounting with Gold ProLong Gold antifade reagent (Molecular Probes). Differential interference contrast and fluorescent optical images were captured using an Olympus IX-71 inverted fluorescence microscope with a Photometrix CoolSnapHQ CCD camera driven by DeltaVision software (Applied Precision, Seattle, WA). Images were deconvolved for 15 cycles using Softwarx deconvolution software. Pearson's correlation coefficients were calculated using the Softwarx software by measuring the whole cell images.
Extraction and Determination of PPi, Long-chain, and Short-chain Polyphosphate
Cells were harvested and washed with Buffer A (116 mm NaCl, 5.4 mm KCl, 0.8 mm MgSO4, 50 mm Hepes, pH 7.2, 5.5 mm glucose) twice. For PPi, and short-chain polyP extraction, the cell pellet was resuspended in ice-cold 0.5 m perchloric acid (HClO4) and incubated on ice for 30 min. After centrifugation at 3,000 × g for 5 min, the supernatant was neutralized with the addition of 0.72 m KOH, 0.6 m KHCO3. The precipitated KClO4 was removed by centrifugation at 12,000 × g for 1 min, and the supernatant was transferred to a new tube for polyP determination.
Long-chain polyP was extracted with glassmilk as described by Ault-Riché et al. (41). Briefly, the cell pellet was resuspended with 500 μl of GITC-lysis buffer (4 m guanidine isothiocyanate, 50 mm Tris-HCl, pH 7.0) prewarmed at 95 °C by vortexing for a few s. The cell mixture was incubated in a heat block at 95 °C for 2–5 min and sonicated briefly. Thirty μl of 10% SDS, 500 μl of ethanol, and 5 μl of glassmilk (Qbiogene, Irvine, CA) were added to each tube. After incubating for 5 min with brief vortexing, the tube was centrifuged at 14,000 × g for 20 s, and the pelleted glassmilk was washed with 0.5 ml of cold freshly prepared washing buffer (5 mm Tris-HCl, 50 mm NaCl, 5 mm EDTA, 50% ethanol, pH 7.5) three times. The washed pellet was resuspended with 50 μl of 50 mm Tris-HCl, 10 mm MgCl2, pH 7.5, containing 20 μg/ml DNase and 20 μg/ml RNase. After incubation in a water bath at 37 °C for 10 min, the pellet was washed once with 150 μl of GITC-lysis buffer and 150 μl of ethanol and then twice with washing buffer. Finally, polyP was eluted with 50 mm Tris-HCl (pH 8.0) at 95 °C and collected by centrifugation at 14,000 × g for 20 s. All extracted samples were stored at −80 °C until use.
PPi level was determined by the amount of Pi released upon treatment with an excess of Saccharomyces cerevisiae inorganic pyrophosphatase (catalog no. I-1891, Sigma). The free Pi (released) amount was determined by using a standard curve. Briefly, the enzymatic reaction was performed on 96-well plates with 50 mm Tris-HCl (pH 7.4), 6 mm MgCl2, inorganic pyrophosphatase, and extracted PPi samples at a final volume of 100 μl. After incubation at 30 °C for 10 min, the reaction was immediately stopped by the addition of an equal amount of the fresh mixture of 3 parts of 0.045% malachite green with 1 part of 4.2% ammonium molybdate (Sigma), which was filtered prior to use as described (42). The absorbance at 660 nm was read using a SpectraMax M2e plate reader (Molecular Devices, Sunnyvale, CA).
Short-chain and long-chain polyP levels were determined by the amount of Pi released upon treatment with an excess of the purified recombinant exopolyphosphatase of S. cerevisiae (rScPPX1) from E. coli strain CA38pTrcPPX1 as described previously (31, 43). The concentration of all phosphorus compound was calculated based on the intracellular cell volume of 0.48 ± 0.02 μl/107 T. brucei procyclic forms.
Electron Microscopy
For imaging whole procyclic forms or bloodstream forms, cells were washed with filtered buffer A twice and directly applied to Formvar-coated copper grids, allowed to adhere for 10 min, carefully blotted dry, and observed in an energy-filtering Zeiss EM 902 electron microscope operating at 80 kV. Electron spectroscopic images were recorded at a range of energy loss of 60–80 eV, unless otherwise indicated, using a spectrometer slit width of 20 eV, and the number of acidocalcisomes per cell was counted in 50 cells from at least two different preparations.
For conventional electron microscopy, trypanosomes were harvested and washed twice with cold PBS. The parasites were fixed with freshly prepared 2.5% glutaraldehyde, 4% paraformaldehyde, and 0.1 m sodium cacodylate buffer (pH 7.3) at room temperature for 50 min and then embedded in epoxy resin, sectioned, and stained using standard methods.
Intracellular Calcium Analysis
Calcium stores were assessed after loading cells with Fura 2/AM as described previously (35).
Regulatory Volume Changes
The cells were collected and washed twice with isotonic chloride buffer (137 mm NaCl, 4 mm KCl, 1.5 mm KH2PO4, 8.5 mm Na2HPO4, 20 mm Hepes, 11 mm glucose, 1 mm CaCl2, 0.8 mm MgSO4, pH 7.4). The buffer osmolarity was 300 ± 5 mosm (“isosmotic”), as verified by an Advanced Instruments 3D3 Osmometer (Norwood, MA). The washed cells were resuspended in isotonic chloride buffer to a cell density of 1 × 108/ml. The cells were distributed in 96-well plates with 100 μl/well in triplicates. Hyposmotic stress was induced by the addition of 100 μl of sterile deionized water using a multichannel pipettor (Rainin, Woburn, MA), resulting in a final osmolarity of 150 mosm. Hyperosmotic stress was induced by the addition of 100 μl of 1,300 mosm sorbitol, which resulted in a final osmolarity of 800 mosm. The absorbance changes at 550 nm were measured every 10 s for 10 min using a SpectraMax M2e plate reader (Molecular Devices) (31). A decrease in absorbance corresponds to an increase in cell volume.
Metabolic Labeling and Immunoprecipitation
Pulse-chase metabolic radiolabeling with [35S]Met/Cys and immunoprecipitation of PCF trypanosomes were performed as described previously (37) with some modifications. Briefly, PCF trypanosomes grown in the presence or absence of 1 μg/ml tetracycline were harvested, washed once in PBS, and resuspended at 5 × 107/ml in prewarmed (27 °C) Met/Cys-deficient TMP with 10% dialyzed FBS. Cells were labeled by the addition of [35S]Met/Cys to 200 μCi/ml (pulse times were 15 min (p67) or 10 min (TbCATL) and chased by 1:10 dilution in prewarmed complete TMP medium. Aliquots of cells (107, 2.0 ml) were collected at appropriate time points, placed on ice, and then washed once with ice-cold PBS. Labeled cells were lysed in radioimmunoprecipitation assay buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1.0% Nonidet P-40, 0.5% deoxycholate, and 0.1% SDS) containing complete protease inhibitor mixture (Roche Applied Science) for 30 min. Lysates were cleared of debris by centrifugation at 4 °C for 5 min at 12,000 rpm and immunoprecipitated overnight at 4 °C with either anti-p67 or anti-TbCATL antibody bound to protein A-agarose (Roche Applied Science) with gentle rotation. Precipitates were washed three times in radioimmunoprecipitation assay buffer and once in TEN (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, and 5 mm EDTA). Final precipitates were resuspended in 25 μl of SDS-PAGE loading buffer and fractionated by 12% SDS-polyacrylamide gel electrophoresis. Fixed gels were treated with EN3HANCE, dried with a Gel Dryer (Bio-Rad), and exposed to x-ray film (Hyblot CL). Metabolically labeled protein bands were quantified by measuring the total integrated density of a band after background subtraction using ImageJ (National Institutes of Health, Bethesda, MD) (available on the World Wide Web).
Western Blot Analyses
The cells were harvested, washed twice in PBS, and lysed with radioimmunoprecipitation assay buffer containing protease inhibitor mixture (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 1 mm phenylmethylsulfonyl fluoride) in ice for 1 h. The protein concentration was determined by using the Pierce BCA protein assay kit with a SpectraMax M2e microplate reader (Sunnyvale, CA). The total cell lysates were mixed with 2× Laemmli sample buffer (Bio-Rad) at a 1:1 (v/v) ratio and directly loaded. The separated proteins were transferred onto nitrocellulose membranes using a Bio-Rad transblot apparatus. The membranes were blocked with 10% nonfat milk in PBS containing 0.5% Tween 20 (PBS-T) at 4 °C overnight. The blots were incubated with the antibodies against HA (1:5,000) for 1 h. After five washes with PBS-T, the blots were incubated with horseradish peroxidase-conjugated anti-mouse IgG (H+L) antibody at a dilution of 1:20,000 for 1 h. After washing five times with PBS-T, the immunoblots were visualized using Pierce ECL Western blotting substrate according to the manufacturer's instructions.
In Vivo Studies
Exponentially growing cell lines (wild type and RNAi) were washed once in HMI-9 medium without selectable drugs and suspended in the same medium. Eight-week-old BALB/c mice (5–6 per group) were infected with a single intraperitoneal injection of 2 × 104 BSF trypanosomes in 0.2 ml of HMI-9 medium. For the RNAi cells, a single inoculum of non-induced trypanosomes was used in two sets of mice with one group being given 200 μg/ml doxycycline in 5% sucrose in their drinking water and the other group being supplied with drinking water containing 5% sucrose only. The drinking water with or without doxycycline was provided 3 days before infection and exchanged every 2–3 days, continuing throughout the 20-day period. Animals were fed ad libitum on standard chow. Parasitemia levels were monitored at different days postinfection (44). This study was carried out in strict accordance with the recommendations in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the Institutional Animal Care and Use Committee (IUCAC) of the University of Georgia.
RESULTS
Subcellular Localization of Tbβ3 and Tbδ
To investigate the localization of T. brucei AP-3, the C termini of the β3 and δ adaptins were tagged in PCF trypanosomes with an HA tag, using homologous recombination with the endogenous gene locus. Western blot analysis confirmed expression of proteins of the expected size (supplemental Fig. S1). Both proteins partially localized to acidocalcisomes and the Golgi complex in PCF trypanosomes, as demonstrated by co-localization with antibodies against T. brucei acidocalcisomal marker vacuolar proton pyrophosphatase (VP1) (35) and Golgi marker Golgi reassembly and stacking protein (GRASP) (36), respectively. An additional punctuated staining that did not co-localize with TbVP1 was also detected (Fig. 1) that could correspond to either trafficking vesicles or endosomal compartments, as it has been reported for the localization of AP-3 in mammalian cells (26). Partial co-localization of the β3 and δ adaptins with antibodies against Rab11, a marker for recycling endosomes in T. brucei (38), confirmed this hypothesis (Fig. 2). The partial co-localization of β3 and δ subunits in these compartments reflects the dynamic behavior of the AP-3 complex that is trafficking through different parts of the cell.
FIGURE 1.
Localization of Tbβ3 and Tbδ in PCF trypanosomes. Tbβ3 and Tbδ partially co-localized with GRASP (α-GRASP) in the Golgi apparatus (A and C, respectively) (Pearson's correlation coefficient of 0.919 and 0.873 for A and C, respectively) and with TbVP1 (VP1) in acidocalcisomes (B and D, respectively) (Pearson's correlation coefficient of 0.855 and 0.533 for B and D, respectively) as shown by immunofluorescence microscopy analysis. The yellow arrows in merge images show the co-localization. Scale bars, 10 μm. DIC, differential interference contrast.
FIGURE 2.
Co-localization of Tbβ3 and Tbδ with Rab11 in PCF trypanosomes. Tbβ3 and Tbδ partially co-localized with Rab11 in endosomes of PCF trypanosomes (A and B, respectively) (Pearson's correlation coefficient of 0.620 and 0.514 for A and B, respectively), as shown by immunofluorescence microscopy analysis. The yellow arrows in merge images show the co-localization. Scale bars, 10 μm.
Tbβ3 and Tbδ Are Essential for Growth in Vitro
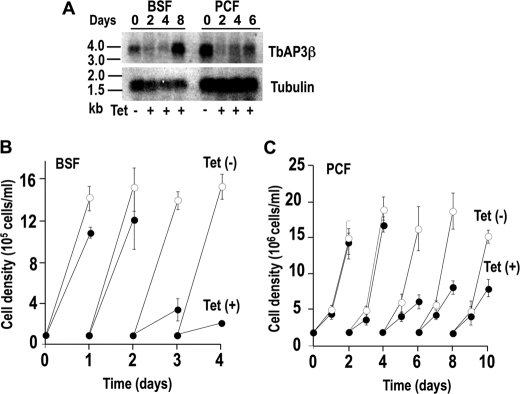
Knockdown of either β3 or δ adaptin by induction of double-stranded RNA resulted in growth defects in both PCF and BSF trypanosomes, the effects being more pronounced with BSF trypanosomes, with up to 85–90% reduction in the number of cells (Fig. 3 and supplemental Fig. S2). Northern blot analysis showed that the mRNA was down-regulated after 2 days of tetracycline addition to BSF trypanosomes (Fig. 3A and supplemental Fig. S2A), when cell growth was also inhibited (Fig. 3B and supplemental Fig. S2B). Similar results were observed after RNAi of PCF trypanosomes (Fig. 3C and supplemental Fig. S2C). In the case of β3 adaptin RNAi, the inhibition of growth was maximal at 4 days after tetracycline addition, and the PCF trypanosomes then recovered in agreement with the recovery of the mRNA signal (Fig. 3, A and C). In the case of δ adaptin RNAi, both inhibition of growth (supplemental Fig. S2C) and down-regulation of mRNA levels (supplemental Fig. S2A) continued for at least 8 days. All further phenotypic analyses were performed on day 4 and day 2 of RNAi induction for PCF and BSF trypanosomes, respectively, unless indicated otherwise.
FIGURE 3.
Effect of inhibition of Tbβ3 expression by tetracycline-inducible RNAi on cell growth. A, Northern blot analysis of Tbβ3 RNAi of BSF and PCF trypanosomes grown in the absence or presence of tetracycline (Tet). Total RNA was subjected to gel electrophoreses before transfer to a nylon membrane and then hybridized with the 32P-labeled probe corresponding to the Tbβ3 coding sequence (top). Tubulin is shown as a loading control (bottom). The RNA ladder is shown on the left (values in kb). The transcript of Tbβ3, including the 5′- and 3′-UTR was ∼3.7 kb in length. B and C show the growth of BSF and PCF trypanosomes in the absence (white circles) or presence (black circles) of 1 μg/ml tetracycline. Values are means ± S.D. (error bars) (n = 3).
Changes in Acidocalcisome Number after AP-3 Depletion
Acidocalcisomes are very electron-dense and are easily observed and counted if cells are allowed to dry onto carbon- and Formvar-coated grids and then observed by direct transmission electron microscopy without fixation or staining (3). This is especially possible if the microscope is equipped with an energy filter, so that electron spectroscopic images (contrast-tuned images) can be obtained (3). After transfection of the PCF trypanosomes with a Tbβ3 RNAi vector, and beginning on day 4 after tetracycline induction, there was a significant decrease in the number of acidocalcisomes in Tbβ3 RNAi transgenic lines, as detected by energy-filtering transmission electron microscopy (Fig. 4C), with >45% of the cells completely devoid of acidocalcisomes (Fig. 4E, inset) and >90% of the cells with only 0–5 acidocalcisomes (Fig. 4E), whereas >90% of the control cells without tetracycline induction possessed >10 and up to >40 acidocalcisomes/cell (Fig. 4E). When present in low numbers, acidocalcisomes were larger (supplemental Fig. S3A (arrows) and Table S1). X-ray microanalysis of these large acidocalcisomes showed that they contained less phosphorus and calcium relative to other elements than uninduced cells (supplemental Fig. S3B). Similar results were observed after RNAi of BSF trypanosomes after 2 days of RNAi (Fig. 4D). More than 90% of the BSF had fewer than 5 acidocalcisomes/cell, with 40% of the cells completely devoid of acidocalcisomes (Fig. 4F, inset), whereas about 90% of the control cells without tetracycline had more than 10 acidocalcisomes/cell (Fig. 4F).
FIGURE 4.
Morphological changes after RNAi of Tbβ3 and numeric distribution of acidocalcisomes in T. brucei. A–D, energy-filtering transmission electron microscopy of whole PCF (A and C) or BSF trypanosomes (B and D) imaged with a range of electron energy loss of 60–80 eV. The dark granules are the acidocalcisomes. A and B show several acidocalcisomes in non-induced (Tet(−)) PCF and BSF trypanosomes, respectively. Note that the number of acidocalcisomes drastically disappear in Tet(+) PCF (C, 4 days after induction) and BSF (D, 2 days after induction) trypanosomes. Scale bars, 2 μm (A), 0.8 μm (B), 1 μm (C), and 1 μm (D). E and F, whole unfixed PCF (E) or BSF (F) trypanosomes were observed using a Zeiss EM 902 transmission electron microscope equipped with an energy filter, and the number of acidocalcisomes per cell in 50 random cells was counted. Insets show the percentage of PCF and BSF trypanosomes with 0–10 acidocalcisomes.
To confirm the absence of acidocalcisomes rather than simply the depletion of their content, we examined the samples at a range of energy loss of 20–100 eV (instead of the 60–80 eV used originally), but there was still no detection of acidocalcisomes (data not shown). These results were further confirmed by immunofluorescence and immunoelectron microscopy assays of control and induced cells after RNAi of the β3 adaptin (Fig. 5). Most of the PCF and BSF trypanosomes lost their staining with antibodies against the vacuolar proton pyrophosphatase (TbVP1) (Fig. 5, C and G), which is the marker for acidocalcisomes (9), and had a considerably reduced number or absence of acidocalcisomes, as detected by transmission electron microscopy (supplemental Fig. S4). Taken together, these results are different from the results observed after knock-out of the δ subunit of L. major AP-3, where no apparent decrease in the number of acidocalcisomes per cell was detected (21), but similar to observations in different cells where knock-out of AP-3 subunits led to depletion of lysosome-related organelles (22–24).
FIGURE 5.
Labeling of TbVP1 after RNAi of Tbβ3. PCF and BSF trypanosomes cultured in the absence (Tet(−)) or presence (Tet(+)) of 1 μg/ml tetracycline for 4 days (PCF) or 2 days (BSF), respectively, were collected, fixed, and probed with rabbit antibodies against T. brucei vacuolar H+-pyrophosphatase (TbVP1) (A–D and E–H, red). Scale bars, 10 μm. DAPI staining (blue) shows the kinetoplasts and nuclei. B, D, F, and H are merged images of differential interference contrast and fluorescence staining. A cell showing normal TbVP1 labeling is indicated by the arrow in C.
Functional Changes Associated with the Loss of Acidocalcisomes
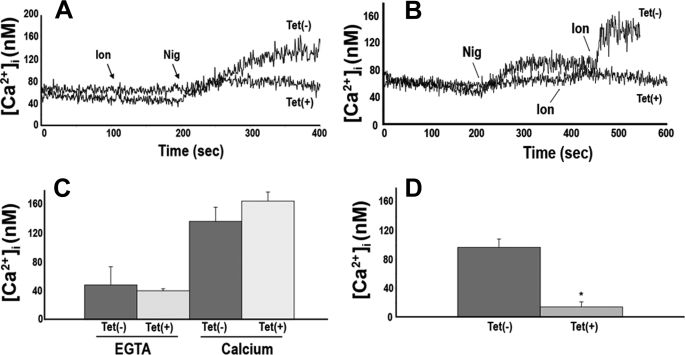
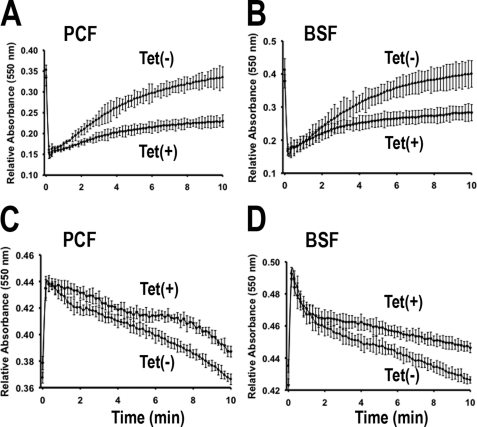
In agreement with the decrease in the number of acidocalcisomes per cell, when PCF trypanosomes were loaded with the Ca2+-sensitive fluorophore Fura 2, subcellular acidic Ca2+ stores, which can be specifically assessed by the sequential addition of the Ca2+ ionophore ionomycin and the K+/H+ exchanger nigericin, were greatly reduced in tetracycline-induced cells (Fig. 6, A, B, and D). However, the steady state levels of intracellular Ca2+ were not affected either in the absence (Fig. 6C, EGTA) or presence (Fig. 6C, Calcium) of extracellular Ca2+. These results suggest that trypanosomes can still maintain their normal Ca2+ homeostasis, despite the reduction in the number of acidocalcisomes. RNAi of Tbβ3 significantly reduced the levels of PPi and short-chain (<50 phosphate units) and long-chain (>50 up to 700–800 phosphate units) polyP compared with control cells (Table 1). When induced with tetracycline, PCF and BSF trypanosomes were less able to recover their volume after hyposmotic (Fig. 7, A and B) or hyperosmotic (Fig. 7, C and D) stress, in agreement with the postulated role of acidocalcisomes in regulatory volume changes (31).
FIGURE 6.
Effect of RNAi of Tbβ3 on calcium homeostasis and acidic calcium stores in PCF trypanosomes. A and B, acidic Ca2+ stores, assessed by the sequential addition of 1 μm ionomycin (Ion) and 1 μm nigericin (Nig) in Fura 2-loaded PCF trypanosomes. Tet(+) traces, cells grown 6 days in the presence of tetracycline; Tet(−) traces, control cells grown in the absence of tetracycline. Acidic stores were considerably reduced in tetracycline-induced cells as compared with controls grown in the absence of tetracycline. C, steady state intracellular Ca2+ concentration ([Ca2+]i) in Fura 2-loaded PCF. Cells were incubated with gentle agitation at room temperature for 20 min in the presence of 1 mm extracellular EGTA or calcium (CaCl2), as indicated, and [Ca2+]i was subsequently determined. There was no statistically significant difference in [Ca2+]i, indicating that intracellular Ca2+ homeostasis is maintained at normal levels. Values are mean ± S.E. (p < 0.05, Student's t test, n = 3). D, graphical representation of tracings from A and B, where the bars represent the difference in [Ca2+]i between the base-line [Ca2+]i and the [Ca2+]i after the addition of ionomycin plus nigericin. The values are mean ± S.E. (error bars). The difference in the [Ca2+]i was statistically significant (p < 0.05, Student's t test, n = 3).
TABLE 1.
Effect of Tbβ3 silencing on levels of PPi and short- and long-chain polyP in PCF trypanosomes
PCF trypanosomes were cultured in the absence (Tet (−)) or presence (Tet(+)) of 1 μg/ml tetracycline for 6 days. Extraction and determination of phosphorus compounds are described under “Experimental Procedures.” The concentration is shown in mm and is intended as an average value assuming distribution across the entire volume of the cell. The values shown are means ± S.D. (n = 3). Concentrations of short- and long-chain polyP are expressed as phosphate monomer.
| Tet(−) | Tet(+) | |
|---|---|---|
| mm | mm | |
| PPi | 5.54 ± 0.94 | 2.33 ± 0.68 |
| Short-chain polyP | 65.44 ± 12.93 | 19.16 ± 9.13 |
| Long-chain polyP | 13.08 ± 2.12 | 5.35 ± 0.93 |
FIGURE 7.
Regulatory volume changes in PCF and BSF trypanosomes after induction of RNAi of Tbβ3. The same amount of non-induced (Tet(−)) and induced cells (Tet(+)) incubated with 1 μg/ml tetracycline for 4 days (PCF) or 2 days (BSF) were suspended in isotonic chloride buffer. The cells were diluted with water to a final osmolarity of 150 mosm (A and B) or with sorbitol to final osmolarity of 800 mosm (C and D), and relative changes in cell volume were followed by monitoring the absorbance at 550 nm, as described under “Experimental Procedures.” Note that the relative absorbance at time 0 was based on the absorbance of isosmotic controls because the initial decrease or increase in absorbance was too fast to be recorded. Values are means ± S.E. (error bars) of three independent experiments, each one in triplicate.
Trafficking to the Lysosome Is Unaffected in Tbβ3 and Tbδ RNAi Cell Lines
The AP-3 complex has also been reported to be involved in sorting events toward the lysosomes (45–47). To investigate whether this was also the case in T. brucei, we examined whether the localization of the membrane glycoprotein p67, a lysosomal marker (37), was affected in RNAi-induced cells. Immunofluorescence staining in uninduced cells revealed the typical pattern of endogenous p67, a single discrete vacuole in the postnuclear region (supplemental Fig. S5, B and C). Silenced cells did not have appreciable changes in this localization (supplemental Fig. S5, E and F). We also investigated the effect of Tbβ3 and Tbδ silencing on the trafficking of p67 in PCF trypanosomes by pulse-chase metabolic radiolabeling (Fig. 8A). In PCF trypanosomes, p67 is delivered to the lysosome, where proteolytic fragmentation generates discrete gp75, gp42, gp32, and gp28 glycoforms (37, 48). A similar pattern of processing was observed in Tbβ3- and Tbδ-silenced cells (Tet(+) gels is Fig. 8A) as compared with control cells (Tet(−) gels in Fig. 8A). Quantification of the relative levels of gp100 at different times (Fig. 8B) did not reveal significant differences.
FIGURE 8.
Effect of Tbβ3 and Tbδ silencing on p67 trafficking in PCF trypanosomes. A, p67 turnover. After culture without (Tet(−)) or with (Tet(+)) induction of Tbβ3 RNAi, PCF trypanosomes were subjected to pulse-chase radiolabeling, as described under “Experimental Procedures.” p67 polypeptides were specifically immunoprecipitated from cell extracts at the indicated times and analyzed by SDS-PAGE and exposure to x-ray film. The mobilities of intact p67 (gp100) and its derivative fragmentary glycoforms (gp75 to gp28) are indicated. All lanes contained 107 cell equivalents. Data are from a representative experiment of three done with similar results. B, p67 quantitation. The relative abundance of gp100 as a percentage of the total immunoreactive proteins detected was quantified. The data represent means ± S.E. (error bars) for three experiments.
We also explored whether silencing of Tbβ3 and Tbδ affected the trafficking of the luminal protein trypanopain, a cathepsin L orthologue (TbCATL) (37). As it has been previously described (37) in controls, TbCATL is synthesized as a cell-associated 53-kDa precursor that is converted to the mature 44-kDa lysosomal form by proteolytic removal of an N-terminal prodomain (supplemental Fig. S6A, left panels). An additional 50-kDa “precursor X” form is also detected, and it too is converted to the mature form (37). A similar pattern of processing was observed in both Tbβ3 and Tbδ-silenced cells (supplemental Fig. S6A, right panels), and quantification of levels of P + X bands with time (supplemental Fig. S5B) did not show significant differences.
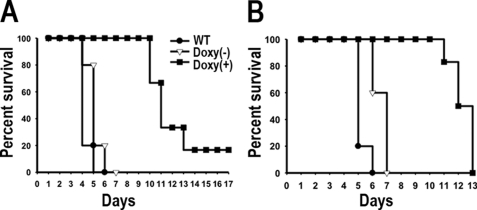
AP-3 Is Essential for Virulence of T. brucei in Mice
To investigate involvement of AP-3 and acidocalcisomes in mammalian infectivity, we inoculated groups of 5–6 mice with wild type and RNAi transgenic BSF trypanosomes for the β3 and δ subunits of AP-3 (Fig. 9). Induction of RNAi was obtained by feeding mice with water containing doxycycline. Mice infected with the wild type and non-induced cells died 5–7 days postinfection (Fig. 9, A and B). This was in contrast to the doxycycline-fed mice, where all mice survived more than 10 days (Fig. 9, A and B). By 13 days, most doxycycline-fed mice died except for one mouse inoculated with Tbβ3 RNAi knockdown cells (Fig. 9A). Our results suggest that a small number of parasites (RNAi escape mutants) are able to survive in the presence of doxycycline and subsequently outgrow and kill the mice. In agreement with this suggestion, tetracycline addition in vitro reduced but did not completely eliminate the expression of both β3 and δ subunits (Fig. 3 and supplemental Fig. S2).
FIGURE 9.
Effect of silencing of Tbβ3 and Tbδ expression on virulence in mice. Groups of five mice were infected with WT, or trypanosomes transfected with the constructs for RNAi of Tbβ3 (A) and Tbδ (B). Doxycycline (Doxy(+); 200 μg/ml) was given in the drinking water throughout a 20-day period.
DISCUSSION
Our work clearly establishes that AP-3 has a critical role in the biogenesis of acidocalcisomes in T. brucei. In contrast to the results reported in L. major, where knock-out of the δ subunit of AP-3 did not affect growth in culture or acidocalcisome biogenesis (21), knockdown of the β3 or δ subunits of the AP-3 complex affected growth in vitro and led to a decrease in the number of acidocalcisomes in both PCF and BSF trypanosomes. These phenotypic changes were revealed by immmunofluorescence and electron microscopy assays and by the decrease in their acidic calcium, PPi, and polyP content. However, as occurs with T. brucei mutants, L. major promastigotes mutants deficient in functional acidocalcisomes were also less virulent in vivo (21). Although the mechanism for the phenotypic differences between L. major and T. brucei in vitro is unknown, a possible explanation is the formation of partial adaptor complexes in L. major constituted by just two subunits, a phenomenon that has been described for mouse models deficient in the AP-3 δ or β3 chain (49, 50). For example, β3Aβ2 chimeras are able to functionally rescue defects in δ-deficient mouse cell lines (mocha) (49). The construction of L. major mutants deficient in additional adaptins could clarify this issue.
Localization of the majority of AP-3 in mammalian cells is in endosomal compartments. In human skin fibroblasts, AP-3 was found associated with peripheral cytoplasmic structures, many of which contained the endosomal marker transferrin receptor (51). Most of the AP-3 in HepG2 cells is found in endosomal tubular profiles (26), whereas in MNT-1 human melanoma cells, AP-3 is found in early endosomes (27). It was proposed that AP-3 functions in mammalian cells to rescue proteins from a default route toward late endosomal multivesicular bodies and to sort them toward lysosomes or LROs (27). However, it has been stated that it cannot be ruled out that AP-3 also functions at the trans-Golgi network (52). Our results using the C termini of β3 and δ subunits of AP-3 tagged with epitopes suggest similar predominant endosomal localization in T. brucei PCF trypanosomes. However, we also observed partial co-localization with markers of the trans-Golgi apparatus and with acidocalcisomes, suggesting that, as it was suggested for other LROs (53), acidocalcisomal integral membrane proteins can follow a pathway from the trans-Golgi network to endosomes rather than from the trans-Golgi network to the plasma membrane and then to endosomes.
In yeast, AP-3 does not appear to require clathrin association for its function (49, 54), but in mammals, AP-3 binds the clathrin heavy chain via its β3 subunit (55). A clathrin-binding consensus sequence, L(L/I)(D/E/N)(L/F)(D/E), has been identified in mammalian β3A and β3B adaptins, which can interact with clathrin in vitro, and similar sequences are also found in C. elegans and Drosophila melanogaster β3 adaptins (55, 56). However, these sequences are absent in yeast β3 adaptin (49) as well as in T. brucei β3 adaptin (ELM server), in agreement with the lack of alterations detected in acidocalcisomes after clathrin knockdown in these parasites (57). Only one other protein, with features similar to clathrin, has been characterized with selective function in AP-3 vesicle formation in yeast, Vps41p (58). Vps41p is a member of the homotypic vacuole fusion and vacuole protein sorting complex (59–61), which is involved in tethering vesicles to the vacuole and delivery of clathrin-dependent and AP-3-dependent cargo (62, 63). Interestingly, knockdown of the Vsp41 orthologue in PCF trypanosomes resulted in an increase in the number of intracellular acidocalcisome-like vesicles (64).
Loss of acidocalcisomes was accompanied by decreased levels of acidic calcium and short- and long-chain polyP. Although acidocalcisome numbers declined more markedly as compared with the changes in acidocalcisome PPi and polyP, this discordance can be explained by the differences in cell volume of the remaining acidocalcisomes. These phenotypic changes did not lead to significant changes in intracellular Ca2+ levels, in agreement with the reported major role of the plasma membrane in the regulation of intracellular Ca2+ concentration under steady state conditions (65, 66). Loss of polyP is in agreement with the presence in acidocalcisomes of the vacuolar transporter chaperone complex (31), which is involved in its synthesis. Their higher susceptibility to hyposmotic and hyperosmotic stresses agrees well with the proposed role of polyP in osmoregulation in trypanosomatids (67). Although it is generally assumed that T. brucei BSF trypanosomes live under constant conditions in blood and are thus not exposed to osmotic stress, abrupt changes in extracellular osmolarity occur when they travel several times per day through the renal medulla, where osmolarities can reach up to 1,400 mosm (68), and then return to the isosmotic environment (300 mosm) at the general circulation. The lower resistance of Tbβ3 and Tbδ knockdown trypanosomes to osmotic stress could explain in part their lower survival in vivo. Resistance to osmotic stress would also be important during the course of their life cycle within the insect vector. The decreased virulence of Tbβ3 and Tbδ knockdown trypanosomes also agrees with previous studies revealing that acidocalcisomes are important for virulence of a number of protist parasites (20, 35, 69, 70).
Why AP-3 cargo is sorted to acidocalcisomes and not to lysosomes, as occurs in some mammalian cells (47–49), is not yet understood, but it may depend upon cell type-specific distribution of target endosomal SNARE proteins or their regulators. In conclusion, we show that Tbβ3 and Tbδ are essential for the biogenesis of acidocalcisomes and that disappearance of acidocalcisomes is associated with loss of cell viability, lower resistance to osmotic stress, and lower virulence in vivo, supporting an essential role for these organelles in T. brucei.
Supplementary Material
Acknowledgments
We thank George A. M. Cross for providing strains 29-13 (PCF) and single marker (BSF), John Donelson for the p2T7Ti vector, Norbert Bakalara for the anti-TbVP1 antibody, Thomas Seebeck for the pMOTag4H vector, Graham Warren for anti-TbGRASP antibody, Mark Field for anti-TbRab11 antibody, and James Bangs for antibodies against T. brucei p67 and TbCATL, for helpful discussions, and for advice concerning the pulse-chase experiments.
This work was supported, in whole or in part, by National Institutes of Health Grant AI-077538 (to R. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S6.
- polyP
- polyphosphate
- AP
- adaptor protein
- BSF
- bloodstream form
- GRASP
- Golgi marker Golgi reassembly and stacking protein
- LRO
- lysosome-related organelle
- PCF
- procyclic form
- VP1
- vacuolar proton pyrophosphatase.
REFERENCES
- 1. Vercesi A. E., Moreno S. N., Docampo R. (1994) Biochem. J. 304, 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Docampo R., Scott D. A., Vercesi A. E., Moreno S. N. (1995) Biochem. J. 310, 1005–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Docampo R., de Souza W., Miranda K., Rohloff P., Moreno S. N. (2005) Nat. Rev. Microbiol. 3, 251–261 [DOI] [PubMed] [Google Scholar]
- 4. Babes V. (1895) Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. 20, 412–420 [Google Scholar]
- 5. Grimme A. (1902) Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. 32, 161–166 [Google Scholar]
- 6. Meyer A. (1904) Bot. Zeit. 62, 113–152 [Google Scholar]
- 7. Wiame J. H. (1947) Biochim. Biophys. Acta 1, 234–255 [Google Scholar]
- 8. Kornberg A. (1995) J. Bacteriol. 177, 491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodrigues C. O., Scott D. A., Docampo R. (1999) Mol. Cell. Biol. 19, 7712–7723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Docampo R., Moreno S. N. (2001) Mol. Biochem. Parasitol. 114, 151–159 [DOI] [PubMed] [Google Scholar]
- 11. Seufferheld M., Vieira M. C., Ruiz F. A., Rodrigues C. O., Moreno S. N., Docampo R. (2003) J. Biol. Chem. 278, 29971–29978 [DOI] [PubMed] [Google Scholar]
- 12. Seufferheld M., Lea C. R., Vieira M., Oldfield E., Docampo R. (2004) J. Biol. Chem. 279, 51193–51202 [DOI] [PubMed] [Google Scholar]
- 13. Ruiz F. A., Lea C. R., Oldfield E., Docampo R. (2004) J. Biol. Chem. 279, 44250–44257 [DOI] [PubMed] [Google Scholar]
- 14. Cutler D. F. (2002) Semin. Cell Dev. Biol. 13, 261–262 [DOI] [PubMed] [Google Scholar]
- 15. Dell'Angelica E. C., Mullins C., Caplan S., Bonifacino J. S. (2000) FASEB J. 14, 1265–1278 [DOI] [PubMed] [Google Scholar]
- 16. Scott D. A., Docampo R., Dvorak J. A., Shi S., Leapman R. D. (1997) J. Biol. Chem. 272, 28020–28029 [DOI] [PubMed] [Google Scholar]
- 17. Coppens I., Baudhuin P., Opperdoes F. R., Courtoy P. J. (1993) Mol. Biochem. Parasitol. 58, 223–232 [DOI] [PubMed] [Google Scholar]
- 18. Mullin K. A., Foth B. J., Ilgoutz S. C., Callaghan J. M., Zawadzki J. L., McFadden G. I., McConville M. J. (2001) Mol. Biol. Cell 12, 2364–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vannier-Santos M. A., Martiny A., Lins U., Urbina J. A., Borges V. M., de Souza W. (1999) Microbiology 145, 3213–3220 [DOI] [PubMed] [Google Scholar]
- 20. Zhang K., Hsu F. F., Scott D. A., Docampo R., Turk J., Beverley S. M. (2005) Mol. Microbiol. 55, 1566–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Besteiro S., Tonn D., Tetley L., Coombs G. H., Mottram J. C. (2008) J. Cell Sci. 121, 561–570 [DOI] [PubMed] [Google Scholar]
- 22. Boehm M., Bonifacino J. S. (2002) Gene 286, 175–186 [DOI] [PubMed] [Google Scholar]
- 23. Hermann G. J., Schroeder L. K., Hieb C. A., Kershner A. M., Rabbitts B. M., Fonarev P., Grant B. D., Priess J. R. (2005) Mol. Biol. Cell 16, 3273–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ohno H. (2006) J. Biochem. 139, 943–948 [DOI] [PubMed] [Google Scholar]
- 25. Ihrke G., Kyttälä A., Russell M. R., Rous B. A., Luzio J. P. (2004) Traffic 5, 946–962 [DOI] [PubMed] [Google Scholar]
- 26. Peden A. A., Oorschot V., Hesser B. A., Austin C. D., Scheller R. H., Klumperman J. (2004) J. Cell Biol. 164, 1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Theos A. C., Tenza D., Martina J. A., Hurbain I., Peden A. A., Sviderskaya E. V., Stewart A., Robinson M. S., Bennett D. C., Cutler D. F., Bonifacino J. S., Marks M. S., Raposo G. (2005) Mol. Biol. Cell 16, 5356–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Allen C. L., Liao D., Chung W. L., Field M. C. (2007) Mol. Biochem. Parasitol. 156, 175–190 [DOI] [PubMed] [Google Scholar]
- 29. Morgan G. W., Allen C. L., Jeffries T. R., Hollinshead M., Field M. C. (2001) J. Cell Sci. 114, 2605–2615 [DOI] [PubMed] [Google Scholar]
- 30. Berriman M., Ghedin E., Hertz-Fowler C., Blandin G., Renauld H., Bartholomeu D. C., Lennard N. J., Caler E., Hamlin N. E., Haas B., Böhme U., Hannick L., Aslett M. A., Shallom J., Marcello L., Hou L., Wickstead B., Alsmark U. C., Arrowsmith C., Atkin R. J., Barron A. J., Bringaud F., Brooks K., Carrington M., Cherevach I., Chillingworth T. J., Churcher C., Clark L. N., Corton C. H., Cronin A., Davies R. M., Doggett J., Djikeng A., Feldblyum T., Field M. C., Fraser A., Goodhead I., Hance Z., Harper D., Harris B. R., Hauser H., Hostetler J., Ivens A., Jagels K., Johnson D., Johnson J., Jones K., Kerhornou A. X., Koo H., Larke N., Landfear S., Larkin C., Leech V., Line A., Lord A., Macleod A., Mooney P. J., Moule S., Martin D. M., Morgan G. W., Mungall K., Norbertczak H., Ormond D., Pai G., Peacock C. S., Peterson J., Quail M. A., Rabbinowitsch E., Rajandream M. A., Reitter C., Salzberg S. L., Sanders M., Schobel S., Sharp S., Simmonds M., Simpson A. J., Tallon L., Turner C. M., Tait A., Tivey A. R., Van Aken S., Walker D., Wanless D., Wang S., White B., White O., Whitehead S., Woodward J., Wortman J., Adams M. D., Embley T. M., Gull K., Ullu E., Barry J. D., Fairlamb A. H., Opperdoes F., Barrell B. G., Donelson J. E., Hall N., Fraser C. M., Melville S. E., El-Sayed N. M. (2005) Science 309, 416–42216020726 [Google Scholar]
- 31. Fang J., Rohloff P., Miranda K., Docampo R. (2007) Biochem. J. 407, 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cunningham I. (1977) J. Protozool. 24, 325–329 [DOI] [PubMed] [Google Scholar]
- 33. Wirtz E., Leal S., Ochatt C., Cross G. A. (1999) Mol. Biochem. Parasitol. 99, 89–101 [DOI] [PubMed] [Google Scholar]
- 34. Hirumi H., Hirumi K. (1989) J. Parasitol. 75, 985–989 [PubMed] [Google Scholar]
- 35. Lemercier G., Dutoya S., Luo S., Ruiz F. A., Rodrigues C. O., Baltz T., Docampo R., Bakalara N. (2002) J. Biol. Chem. 277, 37369–37376 [DOI] [PubMed] [Google Scholar]
- 36. He C. Y., Ho H. H., Malsam J., Chalouni C., West C. M., Ullu E., Toomre D., Warren G. (2004) J. Cell Biol. 165, 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tazeh N. N., Silverman J. S., Schwartz K. J., Sevova E. S., Sutterwala S. S., Bangs J. D. (2009) Eukaryot. Cell 8, 1352–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jeffries T. R., Morgan G. W., Field M. C. (2001) J. Cell Sci. 114, 2617–2626 [DOI] [PubMed] [Google Scholar]
- 39. Oberholzer M., Morand S., Kunz S., Seebeck T. (2006) Mol. Biochem. Parasitol. 145, 117–120 [DOI] [PubMed] [Google Scholar]
- 40. LaCount D. J., Barrett B., Donelson J. E. (2002) J. Biol. Chem. 277, 17580–17588 [DOI] [PubMed] [Google Scholar]
- 41. Ault-Riché D., Kornberg A. (1999) Prog. Mol. Subcell. Biol. 23, 241–252 [DOI] [PubMed] [Google Scholar]
- 42. Shatton J. B., Ward C., Williams A., Weinhouse S. (1983) Anal. Biochem. 130, 114–119 [DOI] [PubMed] [Google Scholar]
- 43. Ramos I. B., Miranda K., Ulrich P., Ingram P., LeFurgey A., Machado E. A., de Souza W., Docampo R. (2010) Biol. Cell 102, 421–434 [DOI] [PubMed] [Google Scholar]
- 44. Herbert W. J., Lumsden W. H. (1976) Exp. Parasitol. 40, 427–431 [DOI] [PubMed] [Google Scholar]
- 45. Le Borgne R., Alconada A., Bauer U., Hoflack B. (1998) J. Biol. Chem. 273, 29451–29461 [DOI] [PubMed] [Google Scholar]
- 46. Baust T., Anitei M., Czupalla C., Parshyna I., Bourel L., Thiele C., Krause E., Hoflack B. (2008) Mol. Biol. Cell 19, 1942–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Braulke T., Bonifacino J. S. (2009) Biochim. Biophys. Acta 1793, 605–614 [DOI] [PubMed] [Google Scholar]
- 48. Alexander D. L., Schwartz K. J., Balber A. E., Bangs J. D. (2002) J. Cell Sci. 115, 3253–3263 [DOI] [PubMed] [Google Scholar]
- 49. Peden A. A., Rudge R. E., Lui W. W., Robinson M. S. (2002) J. Cell Biol. 156, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang W., Li C., Ward D. M., Kaplan J., Mansour S. L. (2000) J. Cell Sci. 113, 4077–4086 [DOI] [PubMed] [Google Scholar]
- 51. Dell'Angelica E. C., Shotelersuk V., Aguilar R. C., Gahl W. A., Bonifacino J. S. (1999) Mol. Cell 3, 11–21 [DOI] [PubMed] [Google Scholar]
- 52. Anitei M., Wassmer T., Stange C., Hoflack B. (2010) Mol. Membr. Biol. 27, 443–456 [DOI] [PubMed] [Google Scholar]
- 53. Mullins C., Bonifacino J. S. (2001) BioEssays 23, 333–343 [DOI] [PubMed] [Google Scholar]
- 54. Vowels J. J., Payne G. S. (1998) EMBO J. 17, 2482–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dell'Angelica E. C., Klumperman J., Stoorvogel W., Bonifacino J. S. (1998) Science 280, 431–434 [DOI] [PubMed] [Google Scholar]
- 56. ter Haar E., Harrison S. C., Kirchhausen T. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 1096–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Allen C. L., Goulding D., Field M. C. (2003) EMBO J. 22, 4991–5002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anand V. C., Daboussi L., Lorenz T. C., Payne G. S. (2009) Mol. Biol. Cell 20, 1592–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nakamura N., Hirata A., Ohsumi Y., Wada Y. (1997) J. Biol. Chem. 272, 11344–11349 [DOI] [PubMed] [Google Scholar]
- 60. Seals D. F., Eitzen G., Margolis N., Wickner W. T., Price A. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 9402–9407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wurmser A. E., Sato T. K., Emr S. D. (2000) J. Cell Biol. 151, 551–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bowers K., Stevens T. H. (2005) Biochim. Biophys. Acta 1744, 438–454 [DOI] [PubMed] [Google Scholar]
- 63. Ostrowicz C. W., Meiringer C. T., Ungermann C. (2008) Autophagy 4, 5–19 [DOI] [PubMed] [Google Scholar]
- 64. Lu S., Suzuki T., Iizuka N., Ohshima S., Yabu Y., Suzuki M., Wen L., Ohta N. (2007) Parasitology 134, 1639–1647 [DOI] [PubMed] [Google Scholar]
- 65. Ríos E. (2010) J. Physiol. Sci. 60, 81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Benaim G., Lopez-Estraño C., Docampo R., Moreno S. N. (1993) Biochem. J. 296, 759–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Docampo R., Ulrich P., Moreno S. N. (2010) Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lang F. (2007) J. Am. Coll. Nutr. 26, 613S–623S [DOI] [PubMed] [Google Scholar]
- 69. Lemercier G., Espiau B., Ruiz F. A., Vieira M., Luo S., Baltz T., Docampo R., Bakalara N. (2004) J. Biol. Chem. 279, 3420–3425 [DOI] [PubMed] [Google Scholar]
- 70. Luo S., Ruiz F. A., Moreno S. N. (2005) Mol. Microbiol. 55, 1034–1045 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.