Abstract
Septic shock results from bacterial infection and is associated with multi-organ failure, high mortality, and cardiac dysfunction. Sepsis causes both myocardial inflammation and energy depletion. We hypothesized that reduced cardiac energy production is a primary cause of ventricular dysfunction in sepsis. The JNK pathway is activated in sepsis and has also been implicated in impaired fatty acid oxidation in several tissues. Therefore, we tested whether JNK activation inhibits cardiac fatty acid oxidation and whether blocking JNK would restore fatty acid oxidation during LPS treatment. LPS treatment of C57BL/6 mice and adenovirus-mediated activation of the JNK pathway in cardiomyocytes inhibited peroxisome proliferator-activated receptor α expression and fatty acid oxidation. Surprisingly, none of the adaptive responses that have been described in other types of heart failure, such as increased glucose utilization, reduced αMHC:βMHC ratio or induction of certain microRNAs, occurred in LPS-treated mice. Treatment of C57BL/6 mice with a general JNK inhibitor (SP600125) increased fatty acid oxidation in mice and a cardiomyocyte-derived cell line. JNK inhibition also prevented LPS-mediated reduction in fatty acid oxidation and cardiac dysfunction. Inflammation was not alleviated in LPS-treated mice that received the JNK inhibitor. We conclude that activation of JNK signaling reduces fatty acid oxidation and prevents the peroxisome proliferator-activated receptor α down-regulation that occurs with LPS.
Keywords: Cardiac Metabolism, Fatty Acid Oxidation, Heart, Inflammation, Jun N-terminal Kinase (JNK), Lipopolysaccharide (LPS), Peroxisome Proliferator-activated Receptor (PPAR), Sepsis
Introduction
Septic shock is the most severe complication of sepsis and is associated with reduced cardiac contractility (1, 2). Hearts rely mostly (70%) on fatty acid (FA)3 oxidation for energy homeostasis (3), and in sepsis, cardiac FA oxidation is markedly reduced. Unlike heart failure (4), this reduction of FA oxidation in sepsis is not compensated by a simultaneous increase in glucose oxidation (5–7). Thus, sepsis leads to cardiac energy deficiency.
LPS is a bacterial cell wall component that induces many of the pathophysiological consequences of sepsis, including cardiac dysfunction. LPS associates with plasma LPS-binding protein and targets cluster of differentiation (CD)14 and toll-like receptor 4 (8). This binding leads to production of inflammatory cytokines, such as TNFα, IL-1, and IL-6, which might directly alter heart function (9–12). LPS also alters cardiac energy utilization by reducing the expression of peroxisome proliferator-activated receptor (PPAR)α and its downstream genes required for FA oxidation (13, 14). It is unknown whether the LPS-induced reduction in cardiac energy production is mediated by inflammation.
LPS activates the JNK signaling pathway (15, 16). JNK is a stress-activated protein kinase (17) that phosphorylates c-Jun, which then forms homo- or heterodimers with c-Fos or activating transcription factor, forming the activating protein 1 complex (18). LPS activates JNK in the heart, but it is not clear whether this pathway leads to impaired cardiac FA oxidation. Inhibition of JNK improves FA oxidation in the liver (19). Thus, we hypothesized that JNK activation reduces cardiac FA oxidation and causes energy deficiency during sepsis.
In our study, we found that JNK activation altered PPARα gene expression and reduced FA oxidation in mouse hearts and in a human cardiomyocyte-derived cell line. Pharmacological inhibition of JNK increased PPARα expression in WT mice, prevented sepsis-mediated PPARα down-regulation, and improved FA oxidation. Moreover, JNK inhibition prevented cardiac dysfunction despite continued induction of inflammatory markers such as TNFα, IL-1α, and IL-6. Therefore, JNK is an important modulator of fatty acid oxidation, and its inhibition prevents LPS-mediated cardiac dysfunction.
EXPERIMENTAL PROCEDURES
Chemical Reagents
All chemical reagents were obtained from Sigma. The JNK inhibitor 1,9-pyrazoloanthrone (SP600125) was obtained from Calbiochem.
Animals
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at Columbia University. C57BL/6 and JNK2−/− mice were maintained under appropriate barrier conditions in a 12-h light-dark cycle and received food and water ad libitum. Mice were anesthetized by isofluorane inhalation. Mouse hearts were harvested, flash-frozen, and stored at −80 °C until further use. All analyses involving animals were performed with at least five mice per experimental group. Mice were treated with 5 mg/kg LPS. Control animal groups were treated with saline. Food was removed after LPS or saline administration because LPS causes anorexia. Cardiac function was assessed by echocardiography 5–6 h post-LPS administration, and mice were sacrificed 2–3 h later (7–9 h post-LPS injection).
Echocardiography
Two-dimensional echocardiography was performed on anesthetized 10- to 12-week-old female (n = 6–10 per group) mice (Sonos 5500 system, Philips Medical Systems) (20). Echocardiographic images were recorded in a digital format. Images were then analyzed off-line by a single observer blinded to the respective treatments of mice (21).
Cells
A human ventricular cardiomyocyte-derived cell line, designated AC-16, was kindly provided by M. M. Davidson (Columbia University) (22). Cells were maintained in Dulbecco's Modified Eagle Medium:Nutrient Mixture F-12 (Ham) (DMEM:F12) (Lonza) supplemented with fetal bovine serum (10%) and a mixture of penicillin and streptomycin (1%). Prior to infection with recombinant adenoviruses, the medium was changed to 2% heat-inactivated horse serum and penicillin and streptomycin (1%). The cells were infected in at least quadruplicates with control adenovirus that expresses the green fluorescent protein (Ad-GFP) or the adenovirus expressing the constitutively active form of JNK2 (Ad-JNK2α2) at a multiplicity of infection of 10. Sixteen hours post-infection, cells were washed with phosphate-buffered saline, and fresh 10% fetal bovine serum containing medium was added. To assess gene expression, cell lysates were collected 48 h later and analyzed for mRNA and protein expression.
Construction of Recombinant Adenovirus Expressing a Constitutively Active Form of JNK2
The pEGFP-C1-JNK2α2 plasmid that contained the cDNA of the constitutively active JNK2 (JNK2α2) (23) was kindly provided by Albert J. Wong, MD (Stanford University). The JNK2α2 cDNA was isolated by digestion with XhoI and BamHI and was initially cloned in the pcDNA3.1 plasmid. Double digestion with XhoI and HindIII was then carried out to the pcDNA3.1-JNK2α2 to isolate the JNK2α2 cDNA and clone it in the pAdTrack-CMV plasmid. The pAdTrack-CMV-JNK2α2 plasmid was used to generate recombinant adenovirus as described previously (24).
RNA Purification and Gene Expression Analysis
Total RNA was purified from cells or hearts using the TRIzol reagent according to the instructions of the manufacturer (Invitrogen). The cDNA was synthesized using the SuperScript III First-Strand Synthesis SuperMix (Invitrogen) and was analyzed with quantitative real-time PCR that was performed with SYBR Green PCR core reagents (Stratagene). Incorporation of the SYBR Green dye into the PCR products was monitored in real time with an Mx3000 sequence detection system (Stratagene). Samples were normalized against β-actin or 18 S. The sequences of the primers are provided in supplemental Table 1.
Protein Purification and Analysis
Isolated heart tissues or cells were homogenized in radioimmune precipitation assay buffer containing protease inhibitors (1 mm benzamidine, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 5 mm ethylene glycol tetraacetic acid, 2 mm ethylene diamine tetraacetic acid; Sigma) as well as 1 mm dithiothreitol and phosphatase inhibitors (Halt phosphatase inhibitor mixture, Thermo Scientific). 25 μg of total protein extract was applied to SDS-PAGE and transferred onto nitrocellulose membranes. Antibodies were obtained from Santa Cruz Biotechnology, Inc. (β-actin, JNK) and Cell Signaling Technology, Inc. (phospho-JNK, phospho-c-Jun-Ser-63, and phospho-c-Jun-Ser-73).
FA Oxidation
FA oxidation was measured in pieces of hearts isolated from 10- to 12-week-old mice. The heart pieces were incubated at 37 °C for 2 h in modified Krebs-Ringer buffer (MKR) (115 mm NaCl, 2.6 mm KCl, 1.2 mm KH2PO4, 10 mm NaHCO3, 10 mm HEPES (pH 7.4)) that contained 2% BSA, 0.2 mmol/ml palmitate, and 10 μCi/ml 9,10-[3H]palmitate and was gassed with 95% O2 and 5% CO2. Water was then extracted with chloroform:methanol (2:1) extraction. Palmitate oxidation was determined by measuring the amount of 3H2O in the aqueous phase.
MicroRNA (miRNA) Expression Profiling and Data Analysis
RNA samples were sent to Ocean Ridge Biosciences for analysis using custom multispecies microarrays containing 697 probes covering 707 mouse mature miRNAs present in the Sanger 14.0 miRBase database. Details about the microarrays, sample processing, data preprocessing, microarray quality control, differential expression analysis, and hierarchical clustering of miRNA array data are provided in the supplementary materials.
Statistical Analysis
Comparisons between two groups were performed using unpaired two-tailed Student's t tests. All values are presented as mean ± S.E. Differences between groups were considered statistically significant at gene.
RESULTS
LPS-mediated Activation of JNK-induced Inflammation Inhibited FA Oxidation and Compromised Cardiac Function in C57BL/6 Mice
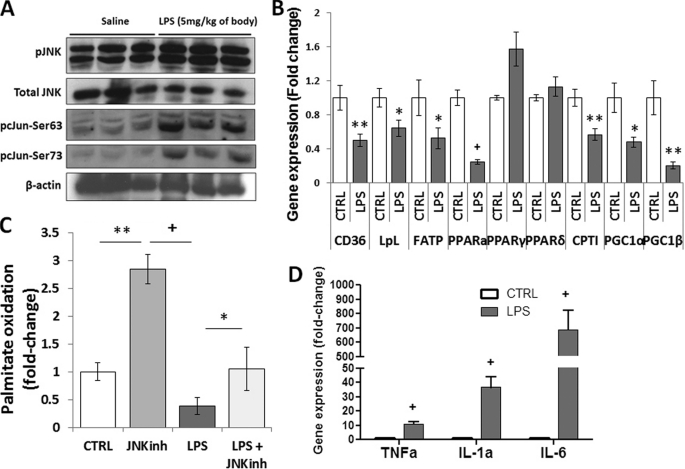
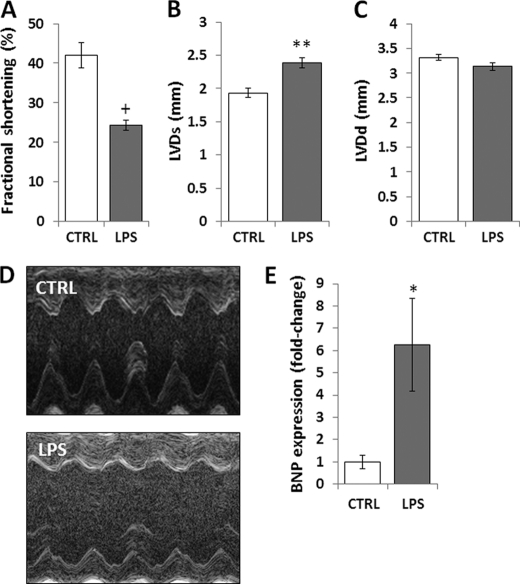
To activate JNK, female C57BL/6 mice underwent intraperitoneal injection of LPS (5 mg/kg). LPS-treated mice had increased phosphorylation/activation of JNK and its substrate, c-Jun (Fig. 1A), as shown before (25, 26). Total JNK protein levels were not affected. Consistent with previous studies (14, 27–29), mRNA levels of genes associated with FA metabolism were significantly down-regulated (Fig. 1B). Specifically, PPARα, CD36, lipoprotein lipase (LpL), fatty acid transport protein (FATP), carnitine palmitoyl-transferase (CPT)-1β, PPARγ coactivator (PGC)-1α, and PGC-1β mRNA levels were reduced by 75% (p < 0.001), 50% (p < 0.01), 36% (p < 0.05), 48% (p < 0.05), 43% (p < 0.01), 52% (p < 0.05), and 80% (p < 0.01), respectively. Cardiac PPARγ and PPARδ mRNA levels were not affected by LPS treatment. Consistent with this gene expression profile, FA oxidation (Fig. 1C) and ATP levels (supplemental Fig. 1) were reduced by 61% (p < 0.05) and 45% (p < 0.05), respectively, in heart tissue obtained from LPS-treated mice. LPS also increased cardiac expression of inflammatory markers. TNFα was increased 11-fold (p < 0.001); IL-1α, 37-fold (p < 0.001); and IL-6, 689-fold (p < 0.001) (Fig. 1D). Reduced cardiac FA oxidation and increased inflammation were associated with impaired cardiac function in LPS-treated mice. This was shown by reduced fractional shortening (p < 0.001) (Fig. 2, A and D) and increased left ventricular end-systolic diameter (p < 0.01) (B and D), whereas the left ventricular diastolic diameter was not affected (C and D). Moreover, brain natriuretic peptide (BNP) mRNA levels were increased 6.3-fold (p < 0.05) in LPS-treated animals (Fig. 2E). Thus, LPS-mediated JNK activation compromised cardiac function, and this was associated with impaired FA metabolism and elevated inflammation.
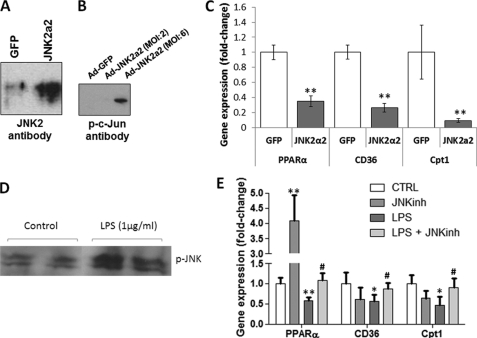
FIGURE 1.
LPS activates JNK, inhibits fatty acid oxidation, and induces inflammation. A, Western blot analysis of pJNK, total JNK, pc-Jun-Ser-63, pc-Jun-Ser-73, and β-actin protein levels obtained from hearts of 10- to 12-week-old C57BL/6 mice that were treated with 5 mg/kg LPS. (B) CD36, LpL, FATP, PPARα, PPARγ, PPARδ, CPT-1β, PGC-1α, and PGC-1β mRNA levels in hearts of 10- to 12-week-old C57BL/6 mice that were treated with 5 mg/kg LPS. n = 5; *, p < 0.05; **, p < 0.01; +, p < 0.001. C, [3H]palmitic acid oxidation in cardiac muscle of C57BL/6 mice treated with 5 mg/kg JNK inhibitor (JNKinh) (SP600125), 5 mg/kg LPS, or a combination of LPS and JNK inhibitor. n = 4; *, p < 0.05. D, TNFα, IL-1α, and IL-6 mRNA levels in hearts of 10- to 12-week-old C57BL/6 mice that were treated with 5 mg/kg LPS. n = 5; +, p < 0.001.
FIGURE 2.
Treatment of C57BL/6 mice with LPS impairs cardiac function. A–D, fractional shortening (A), left ventricular systolic diameter (LVDs) (B), and left ventricular diastolic diameter (LVDd) (C) as measured by two-dimensional echocardiography of 10- to 12-week-old C57BL/6 mice that were treated with 5 mg/kg LPS. D, photographs of echocardiograms from LPS-treated C57BL/6 mice. E, cardiac BNP mRNA levels of 10- to 12-week-old C57BL/6 mice that were treated with 5 mg/kg LPS. Control mice were treated with saline. n = 6–8; *, p < 0.05; **, p < 0.01; +, p < 0.001.
Glucose Metabolism Markers Indicate Reduced Glucose Utilization in Sepsis
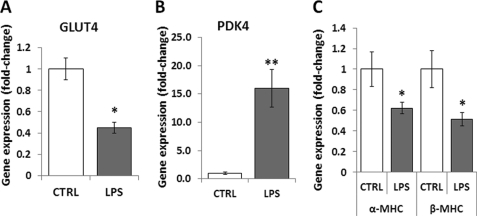
Both cardiac dysfunction in sepsis and chronic heart failure are characterized by reduced cardiac contractility. To investigate whether the mechanisms that underlie these two disease states are similar, we tested if several outcomes that have been associated with heart failure occur in cardiac dysfunction during sepsis as well. We first assessed whether glucose catabolism markers are up-regulated in our experimental model of sepsis to compensate for the suppression of FA metabolism that occurs in sepsis. Consistent with previous studies (5–7), LPS-treated hearts had a 55% decrease in glucose transporter (GLUT)4 mRNA levels (p < 0.05) (Fig. 3A) and a 16-fold (p < 0.01) increase in pyruvate dehydrogenase kinase (PDK)4 mRNA as compared with hearts of saline-treated mice (Fig. 3B). These changes indicate suppression of glucose metabolism during sepsis.
FIGURE 3.
Mechanisms of cardiac dysfunction in sepsis differ from chronic heart failure. A–C, GLUT4 (A), PDK4 (B), and αMHC and βMHC (C) mRNA levels in hearts of 10- to 12-week-old C57BL/6 mice that were treated with 5 mg/kg LPS. Control mice were treated with saline. n = 6–8; *, p < 0.05; **, p < 0.01.
Both αMHC and βMHC Gene Expression Levels Are Reduced in Sepsis
We assessed whether sepsis induces switching from the fast-contracting α-MHC isoform to the slow contracting β-MHC, which also occurs in heart failure. Both α-MHC and β-MHC mRNA levels were down-regulated in LPS-treated hearts by 38% (p < 0.05) and 49% (p < 0.05), respectively (Fig. 3C). Therefore, instead of the adaptive switching from α-MHC to β-MHC, which occurs in pressure-overload heart failure, sepsis-associated cardiac dysfunction is accompanied by reduction of mRNA levels of both myosin isoforms.
Heart Failure-associated MicroRNAs Are Not Modulated in Sepsis
A systematic analysis of cardiac miRNA profiles using an established miRNA array was performed to assess changes in miRNAs that have been associated with heart failure. Several miRNAs are up-regulated during heart failure in humans and experimental animals (30–33). The vast majority of these miRNAs were not modulated during sepsis (supplemental Table 2 and Fig. 2). Therefore, the heart failure-associated changes in miRNAs do not occur in sepsis-associated cardiac dysfunction.
JNK Inhibition Prevents Sepsis-related Cardiac Dysfunction
We attempted to use mice with a deletion of JNK2, a major cardiac JNK isoform, to test whether the JNK signaling pathway mediates the effects of LPS. First, we assessed heart function in Jnk2−/− mice. These mice have increased basal cardiac JNK1 protein levels (supplemental Fig. 3A) and impaired basal cardiac function (supplemental Fig. 3B), which was found to be comparable with cardiac dysfunction in LPS-treated C57BL/6 mice. Although treatment of these mice with LPS did not further impair the already compromised cardiac function, the hearts still had increased phosphorylation of JNK and its target c-Jun (supplemental Fig. 3C). Activation of the JNK pathway in LPS-treated JNK2−/− mice was, as expected, associated with reduced PPARα (p < 0.01), PGC-1α (p < 0.01), PGC-1β (p < 0.01), FATP (p < 0.05), and CD36 (p < 0.05) mRNA levels (supplemental Fig. 3D), as observed in LPS-treated C57BL/6 mice. Treatment of Jnk2−/− mice with LPS also dramatically increased the expression of cardiac inflammatory markers. TNFα increased 34-fold (p < 0.01); IL-1α, 21-fold (p < 0.01); and IL-6, 640-fold (p < 0.001) (supplemental Fig. 3E). Therefore, JNK1 appears to compensate when JNK2 is deleted specifically.
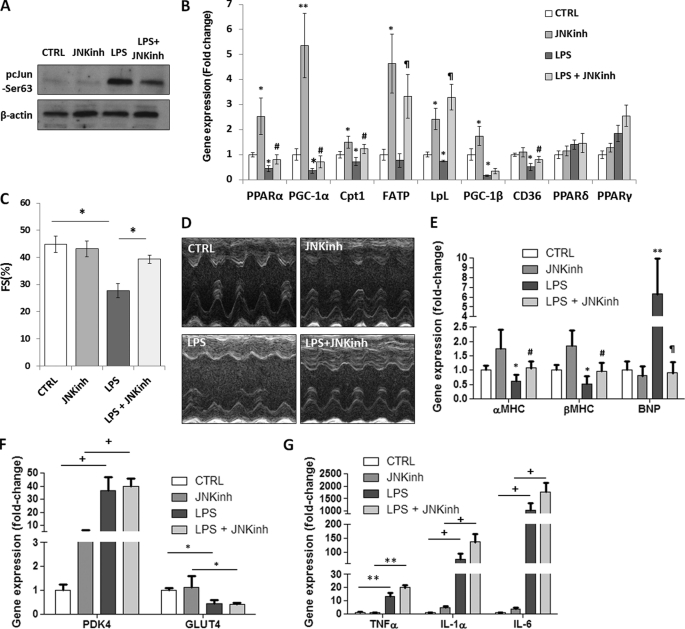
Because multiple JNK isoforms are activated by LPS, we then tested whether a general JNK inhibitor would prevent LPS-mediated impairment in FA oxidation and cardiac function. C57BL/6 mice received daily injections of 5 mg/kg JNK inhibitor (SP600125) for 5 days. On the 5th day, the mice were treated with LPS for 8 h. Reduced JNK activation was confirmed by Western blotting that showed decreased levels of phosphorylated c-Jun (pc-Jun) in mice treated with LPS and JNK inhibitor (Fig. 4A). Gene expression of PPARα, PGC-1α, CPT-1β, FATP, LpL, and CD36 was not reduced in mice that were treated with the LPS and JNK inhibitor, as compared with control wild-type mice (Fig. 4B). Mice treated with LPS and JNK inhibitor had mRNA levels of PPARα, PGC-1α, CPT-1β, FATP, LpL and CD36 increased by 80% (p < 0.05), 100% (p < 0.01), 71% (p < 0.05), 4.3-fold (p < 0.01), 4.5-fold (p < 0.01), and 59% (p < 0.05), respectively (Fig. 4B); this was associated with increased cardiac muscle FA oxidation (p < 0.05) (Fig. 1C). Of note, treatment of mice with the JNK inhibitor alone increased PPARα, PGC-1α, CPT-1β, FATP, and LpL gene expression levels by 2.5-fold (p < 0.05), 5.4-fold (p < 0.01), 50% (p < 0.05), 4.6-fold (p < 0.05), 2.4-fold (p < 0.05), respectively, as compared with saline-treated control mice (Fig. 4B). Consistent with the gene expression profile, treatment with JNK inhibitor increased cardiac FA oxidation by 2.8-fold (p < 0.01) as compared with saline-treated mice (Fig. 1C).
FIGURE 4.
JNK inhibition prevents LPS-mediated reduction in cardiac fatty acid oxidation and improves heart function despite elevated inflammation. A, Western blot analysis of pc-Jun-Ser-63 and β-actin protein levels obtained from hearts of 10- to 12-week-old C57BL/6 mice that were treated with 5 mg/kg LPS or a combination of LPS and 5 mg/kg JNK inhibitor (JNKinh) (SP600125). Control cells were treated with saline. B, PPARα, PGC-1α,CPT-1β, FATP, LpL, PGC-1β, CD36, PPARδ, and PPARγ mRNA levels in hearts of 10- to 12-week-old C57BL/6 mice that were treated with 5 mg/kg LPS or a combination of LPS and 5 mg/kg JNK inhibitor (SP600125). n = 5; *, p < 0. 05 versus control; **, p < 0.01 versus control; #, p < 0.05 versus LPS; ¶, p < 0.01 versus LPS. C, fractional shortening as measured by two-dimensional echocardiography of 10- to 12-week-old C57BL/6 mice that were treated with 5 mg/kg LPS or a combination of 5 mg/kg LPS and 5 mg/kg JNK inhibitor (SP600125). n = 5; *, p < 0.05. D, photographs of echocardiograms of 10- to 12-week-old C57BL/6 mice that were treated with 5 mg/kg LPS or a combination of 5 mg/kg LPS and 5 mg/kg JNK inhibitor (SP600125). E, cardiac αMHC, βMHC, and BNP mRNA levels of 10- to 12-week-old C57BL/6 mice that were treated with 5 mg/kg LPS or a combination of LPS and 5 mg/kg JNK inhibitor (SP600125). n = 4; *, p < 0.05 versus control; **, p < 0.01 versus control; #, p < 0.05 versus LPS; ¶, p < 0.01 versus LPS. F, cardiac PDK4 and GLUT4 mRNA levels of 10- to 12-week-old C57BL/6 mice that were treated with 5 mg/kg LPS or a combination of LPS and 5 mg/kg JNK inhibitor (SP600125). n = 4; *, p < 0.05; +, p < 0.001. G, TNFα, IL-1α, and IL-6 mRNA levels in hearts of 10- to 12-week-old C57BL/6 mice that were treated with 5 mg/kg LPS or a combination of 5 mg/kg LPS and 5 mg/kg JNK inhibitor (SP600125). n = 5; **, p < 0.01; +, p < 0.001.
JNK inhibition also prevented LPS-induced cardiac dysfunction as seen by a lack of a fractional shortening defect (Fig. 4C & 4D). Moreover, as compared with saline-treated control mice, αMHC and βMHC mRNA levels were not reduced and BNP mRNA levels were not increased in mice treated with combination of LPS and JNK inhibitor (Fig. 4E). The observed improvement in cardiac function was not associated with indications of improved glucose catabolism. The LPS-mediated increase of PDK4 and reduction of GLUT4 mRNA levels was not reversed in LPS-treated mice that were injected with JNK inhibitor (Fig. 4F). In addition, cardiac mRNA levels of TNFα, IL-1α, and IL-6 remained elevated in mice that were treated with a combination of JNK inhibitor and LPS (Fig. 4G). Thus, JNK inhibitor-mediated improvement in cardiac function of LPS-treated mice was not accompanied either by a change in the glucose catabolism gene expression profile or by the alleviation of inflammation.
A Constitutively Active Form of JNK Reduced Gene Expression of PPARα and Its Target Genes
To test whether activation of JNK alone would alter the expression of FA oxidation genes, we treated AC16 cells with a recombinant adenovirus expressing a constitutively active form of JNK2 (Ad-JNK2α2), which has the ability to be autophosphorylated (23). Adenovirus-mediated JNK2α2 expression (Fig. 5A) and phosphorylation of the JNK target, c-Jun (Fig. 5B), were confirmed by Western blotting of protein lysates. Ad-JNK2α2 infection reduced PPARα, CD36, and CPT-1β mRNA levels by 65% (p < 0.01), 68% (p < 0.01), and 84% (p < 0.01), respectively (Fig. 5C).
FIGURE 5.
Adenovirus- or LPS-mediated activation of JNK in cardiomyocytes alters the expression of genes that are associated with fatty acid metabolism. A, Western blot analysis of JNK2 in AC-16 cells that were treated with adenovirus expressing constitutively active JNK2α2. Control cells were treated with adenovirus expressing GFP. B, Western blot analysis of pc-Jun in AC-16 cells that were treated with adenovirus expressing constitutively active JNK2α2 at multiplicity of infection 2 or 6. Control cells were treated with adenovirus expressing GFP. C, PPARα, CD36 and CPT-1β mRNA levels determined by quantitative RT-PCR analysis of AC16 cells treated with adenovirus expressing JNK2α2. Control cells were treated with adenovirus (multiplicity of infection 6) expressing GFP. n = 4. *, p < 0.05; **, p < 0.01 as compared with cells that were treated with Ad-GFP. D, Western blot analysis of pJNK in AC16 cells that were treated with LPS. E, PPARα, CD36, and CPT-1β mRNA levels in AC16 cells that were treated with 100 nm JNK inhibitor (SP600125), LPS, or a combination of LPS and the JNK inhibitor (SP600125). The mRNA levels were determined by quantitative RT-PCR analysis. n = 6. *, p < 0.05; **, p < 0.01 as compared with cells that were not treated with LPS; #, p < 0.05 as compared with cells that were treated with LPS only.
LPS-mediated Activation of JNK Down-regulated the Expression of PPARα and Its Target Genes in a Cardiomyocyte-derived Cell Line
AC16 cell treatment with 1 μg/ml LPS induced phosphorylation of JNK (Fig. 5D). The treatment also reduced the mRNA levels of PPARα by 42% (p < 0.01) (Fig. 5E) and its targets CD36 by 46% (p < 0.05) and CPT-1β by 26% (p < 0.05) (E). Down-regulation of PPARα, CD36, and CPT-1β mRNA levels was prevented by the combined treatment of AC16 cells with LPS and 100 nm JNK inhibitor (Fig. 5E). Treatment of the cells with the JNK inhibitor only stimulated the expression of PPARα 4.1-fold (Fig. 5E).
DISCUSSION
Severe sepsis is a major cause of death in intensive care units (34) and leads to cardiac dysfunction (1, 2). Sepsis leads to reduced substrate oxidation and increased cardiac inflammation. Most (35–43) but not all (44–46) studies also report reduced cardiac ATP production in sepsis. The differences between the two groups of studies may be attributed to the different sepsis induction methods and time courses that were followed in each case. Thus, although cardiac ATP levels seem to be unchanged in models of sepsis that are assessed later than 18–24 h post-induction of sepsis by cecal ligation puncture (35, 36), they are reduced when assessed earlier (37–39) or when an endotoxin injection is used instead of cecal ligation puncture as the sepsis induction method (40–44). Cardiac ATP and fatty acid oxidation levels were reduced in our model, in which sepsis was induced by endotoxin injection, and effects were assessed 5–8 h post-injection. Thus, cardiac energy deficiency occurs in our model.
It has been suggested that either energy deficiency (37–52) or inflammation (9–12) might compromise heart function and lead to cardiac failure. However, anti-inflammatory therapies have failed to protect from sepsis-mediated organ failure (53–58). Therefore, we hypothesized that reduced energy production was a major cause of decreased heart function during sepsis. A major signal transduction mechanism that has been associated with tissue energy homeostasis (19) and is also activated during sepsis (15, 16) is the JNK pathway. Thus, we investigated if JNK interferes with the cardiac energy production process. Activation of JNK in both cell culture and septic animals inhibited the expression of genes associated with FA metabolism. In addition, inhibition of the JNK pathway increased PPARα expression and induced FA oxidation gene expression. Moreover, it prevented sepsis-mediated impairment of FA oxidation and improved cardiac function, despite increased inflammation.
Both sepsis-related cardiac dysfunction and chronic heart failure have reduced cardiac output. To investigate whether the two diseases share similar pathophysiological mechanisms, we assessed if pathways that are altered in chronic heart failure are similarly affected in the hearts of LPS-treated mice. Besides reduced FA oxidation, chronic heart failure is characterized by elevated glucose utilization (59–65), a reduced αMHC:βMHC ratio (66–68), and up-regulation of several miRNAs (30–33). Our study shows that none of these changes occur in the hearts of LPS-treated mice. Thus, the mechanisms that underlie sepsis-associated cardiac dysfunction and chronic heart failure are different.
The mechanisms that mediate the down-regulation of FA oxidation in heart failure and sepsis are not well understood. In heart failure there is reduced expression of FA oxidation-associated genes, which are regulated by PPARα and PGC-1α (69). Both PPARα (70) and PGC-1α (71) protein levels are significantly decreased in the myocardium from humans with end-stage heart failure, but the mechanisms for these changes are unclear. LPS-induced reduction in FA utilization is associated with a reduced expression of genes required for FA oxidation in heart (14, 27, 72), muscle (72, 73), diaphragm (74), kidney (75), and adipose tissue (27, 72, 73). Cardiac PPARα mRNA levels are reduced by LPS, and this may account for the reduction of cardiac FA metabolism (76–78). Whether these energetic changes are secondary to increased production of inflammatory cytokines or because of another effect of LPS within the heart was not known.
One pathway that mediates the effects of LPS is the JNK signaling pathway (15, 16). Our study shows that activation of the JNK pathway is associated with inhibition of the expression of FA metabolism-associated genes both in vitro and in vivo. Inhibition of JNK increased FA oxidation in several tissues (19, 79–81), but this has not been studied in the heart with or without LPS. There are three JNK isoforms: JNK1 and JNK2, which are ubiquitously expressed, and JNK3, which is expressed predominantly in neurons (82). In hearts of LPS-treated Jnk2−/− mice, we found that JNK1 compensates for JNK2 loss. This is consistent with previous findings in the liver and adipose tissue of LPS-treated mice (83, 84). Therefore, we treated C57BL/6 mice with SP600125 to achieve general inhibition of the JNK signaling pathway. JNK inhibitor treatment increased expression of PPARα in C57BL/6 mice. It also prevented cardiac dysfunction of LPS-treated mice. The improvement in cardiac function by JNK inhibition was associated with increased cardiac FA oxidation, as shown by [3H]palmitate oxidation and mRNA levels of FA oxidation markers such as PPARα, PGC-1α, CD36, FATP, LpL, and CPT-1β. Therefore, JNK activation may be the pathway through which LPS blocks FA oxidation. Alternatively, the JNK inhibitor may release the suppressive effect of LPS on PPARα expression by compensating for a parallel JNK-independent mechanism that is also triggered by LPS and compromises fatty acid oxidation. JNK inhibition suffices to restore FA oxidation to normal levels and results in the prevention of cardiac dysfunction in sepsis. On the other hand, the expression of glucose oxidation markers such as PDK4 and GLUT4 in mice treated with LPS and JNK inhibitor did not change as compared with mice treated with LPS only. This observation implies that glucose utilization, which is inhibited by LPS, does not improve by treatment with the JNK inhibitor. Thus, JNK inhibition prevented LPS-mediated reduction of FA oxidation and rescued heart function.
JNK inhibition may also be beneficial for cardiac function in sepsis because it prevents other pathological processes, such as abnormalities in calcium handling or production of reactive oxygen species. Sepsis-mediated JNK activation has been associated with the induction of both mechanisms (85), and this may be associated with arrhythmias that occur in sepsis (86–89). Septic arrhythmias can be attributed to both abnormal calcium handling and reactive oxygen species production (90). However, the investigation of these mechanisms is beyond the scope of this study, which focused only on cardiac muscle function.
Previous studies showed that knockout animals for inflammation-related genes are resistant to LPS-mediated cardiac dysfunction and mortality (9–12). In our studies, however, the improvement in cardiac function of LPS-treated mice that underwent JNK inhibition was not associated with alleviation of inflammation. TNFα, IL-1α, and IL-6 cardiac mRNA levels remained high in LPS-treated mice despite JNK inhibition. This finding indicates that although local inflammation is a component of sepsis, it may not be the major reason for sepsis-mediated heart dysfunction. If this hypothesis is correct, it may explain why anti-inflammatory therapies have not improved mortality in septic patients (53–58).
We confirmed our in vivo observations with studies in cultured cells. To do this, we activated the JNK signaling pathway in cells either using a recombinant adenovirus that expresses a constitutively active JNK2 or treating the cells with LPS. We showed that activation of JNK alone reduced FA oxidation gene expression. JNK signaling was inhibited in LPS-treated cells using a pharmacologic JNK inhibitor. Loss of JNK activation prevented the inhibition of expression of FA oxidation-associated genes. Thus, we conclude that LPS-driven reduction in FA oxidation is mediated by JNK signaling.
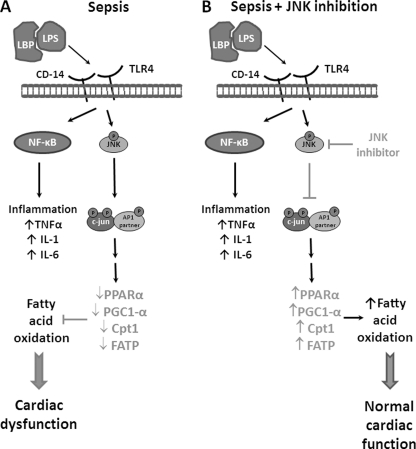
In conclusion, we have found that activation of JNK suppresses FA oxidation in the heart. Inhibition of JNK signaling in mice increases PPARα expression and prevents LPS-mediated reduction in cardiac energy production and mechanical dysfunction. Moreover, our studies suggest that localized inflammation and impaired energy production are separate events in this process. In addition, they suggest that inflammation is not the direct cause of reduced heart function and decreased FA oxidation. Inhibition of the JNK pathway may be a therapeutic approach to prevent reduced FA oxidation and rescue heart function during sepsis. A schematic representation of a proposed pathway that mediates the effects of sepsis-induced JNK activation on cardiac FA oxidation and heart function is depicted in Fig. 6. LPS induces the production of inflammatory cytokines and also activates the JNK signaling pathway. Activation of JNK and its target protein c-Jun down-regulates the expression of genes associated with FA oxidation. This decreases cardiac energy production and eventually compromises heart function. These defects are prevented by JNK inhibition, which protects cardiac function in sepsis despite elevated inflammation. Thus, our data implicate JNK as a suitable target for the treatment of cardiac dysfunction in sepsis and perhaps in other settings associated with reduced FA oxidation.
FIGURE 6.
Proposed model. A, schematic model that explains the role of the JNK signaling pathway in the inhibition of fatty acid oxidation and relevant gene expression that leads to cardiac dysfunction during sepsis. Binding of the complex that consists of LPS and lipopolysaccharide binding protein (LBP) on the TLR4 and CD14 receptors activates the JNK signaling pathway. In addition, inflammatory pathways are activated via stimulation of the NF-κB signaling pathway. The JNK signaling pathway leads to down-regulation of the expression of fatty acid oxidation-associated genes and eventually causes cardiac dysfunction. B, inhibition of JNK prevents sepsis-mediated down-regulation of cardiac fatty acid oxidation, thus it protects cardiac function in sepsis despite increased levels of inflammatory cytokines.
Supplementary Material
This study was supported by National Heart, Lung, and Blood Institute Grants HL45095 and 73029 (to I. J. G.), HL48739 (V. I. Z.), and T32 HL007343 (to R. K.). This work was also supported by American Heart Association Postdoctoral Grant 10POST4440032 (to K. D.), by an American Diabetes Association mentored postdoctoral grant ADA 07-07-MN-22 (to I. J. G. and K. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental experimental procedures, references, Figs. 1–3, and Tables 1 and 2.
The microRNA profiling data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database under accession number GSE29914.
- FA
- fatty acid
- CD
- cluster of differentiation
- PPAR
- peroxisome proliferator-activated receptor
- LpL
- lipoprotein lipase
- FATP
- fatty acid transport protein
- CPT
- carnitine palmitoyl-transferase
- PGC
- peroxisome proliferator-activated receptor γ coactivator
- BNP
- brain natriuretic peptide
- GLUT
- glucose transporter
- PDK
- pyruvate dehydrogenase kinase
- miRNA
- microRNA
- Ad
- adenovirus.
REFERENCES
- 1. Annane D., Bellissant E., Cavaillon J. M. (2005) Lancet 365, 63–78 [DOI] [PubMed] [Google Scholar]
- 2. Levy M. M., Fink M. P., Marshall J. C., Abraham E., Angus D., Cook D., Cohen J., Opal S. M., Vincent J. L., Ramsay G. (2003) Crit. Care Med. 31, 1250–1256 [DOI] [PubMed] [Google Scholar]
- 3. Ballard F. B., Danforth W. H., Naegle S., Bing R. J. (1960) J. Clin. Invest. 39, 717–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neubauer S. (2007) N. Engl. J. Med. 356, 1140–1151 [DOI] [PubMed] [Google Scholar]
- 5. Tessier J. P., Thurner B., Jüngling E., Lückhoff A., Fischer Y. (2003) Cardiovasc. Res. 60, 119–130 [DOI] [PubMed] [Google Scholar]
- 6. Ling P. R., Bistrian B. R., Mendez B., Istfan N. W. (1994) Metabolism 43, 279–284 [DOI] [PubMed] [Google Scholar]
- 7. Schilling J., Lai L., Sambandam N., Dey C. E., Leone T. C., Kelly D. P. (2011) Circ. Heart Fail. 4, 474–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akira S., Takeda K., Kaisho T. (2001) Nat. Immunol. 2, 675–680 [DOI] [PubMed] [Google Scholar]
- 9. Carlson D. L., Willis M. S., White D. J., Horton J. W., Giroir B. P. (2005) Crit. Care Med. 33, 1021–1028 [DOI] [PubMed] [Google Scholar]
- 10. Fallach R., Shainberg A., Avlas O., Fainblut M., Chepurko Y., Porat E., Hochhauser E. (2010) J. Mol. Cell. Cardiol. 48, 1236–1244 [DOI] [PubMed] [Google Scholar]
- 11. Knuefermann P., Nemoto S., Misra A., Nozaki N., Defreitas G., Goyert S. M., Carabello B. A., Mann D. L., Vallejo J. G. (2002) Circulation 106, 2608–2615 [DOI] [PubMed] [Google Scholar]
- 12. Ohlsson K., Björk P., Bergenfeldt M., Hageman R., Thompson R. C. (1990) Nature 348, 550–552 [DOI] [PubMed] [Google Scholar]
- 13. Maitra U., Chang S., Singh N., Li L. (2009) Mol. Immunol. 47, 420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feingold K., Kim M. S., Shigenaga J., Moser A., Grunfeld C. (2004) Am. J. Physiol. Endocrinol. Metab. 286, E201–207 [DOI] [PubMed] [Google Scholar]
- 15. Hambleton J., Weinstein S. L., Lem L., DeFranco A. L. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 2774–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanghera J. S., Weinstein S. L., Aluwalia M., Girn J., Pelech S. L. (1996) J. Immunol. 156, 4457–4465 [PubMed] [Google Scholar]
- 17. Weston C. R., Davis R. J. (2007) Curr. Opin. Cell Biol. 19, 142–149 [DOI] [PubMed] [Google Scholar]
- 18. Vogt P. K. (2001) Oncogene 20, 2365–2377 [DOI] [PubMed] [Google Scholar]
- 19. Yu X. X., Murray S. F., Watts L., Booten S. L., Tokorcheck J., Monia B. P., Bhanot S. (2008) Am. J. Physiol. Endocrinol. Metab. 295, E436–445 [DOI] [PubMed] [Google Scholar]
- 20. Takuma S., Suehiro K., Cardinale C., Hozumi T., Yano H., Shimizu J., Mullis-Jansson S., Sciacca R., Wang J., Burkhoff D., Di Tullio M. R., Homma S. (2001) Am. J. Physiol. Heart Circ. Physiol. 280, H2364–2370 [DOI] [PubMed] [Google Scholar]
- 21. Wang C. Y., Mazer S. P., Minamoto K., Takuma S., Homma S., Yellin M., Chess L., Fard A., Kalled S. L., Oz M. C., Pinsky D. J. (2002) Circulation 105, 1609–1614 [DOI] [PubMed] [Google Scholar]
- 22. Davidson M. M., Nesti C., Palenzuela L., Walker W. F., Hernandez E., Protas L., Hirano M., Isaac N. D. (2005) J. Mol. Cell. Cardiol. 39, 133–147 [DOI] [PubMed] [Google Scholar]
- 23. Tsuiki H., Tnani M., Okamoto I., Kenyon L. C., Emlet D. R., Holgado-Madruga M., Lanham I. S., Joynes C. J., Vo K. T., Wong A. J. (2003) Cancer Res. 63, 250–255 [PubMed] [Google Scholar]
- 24. Drosatos K., Sanoudou D., Kypreos K. E., Kardassis D., Zannis V. I. (2007) J. Biol. Chem. 282, 19556–19564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peng T., Zhang T., Lu X., Feng Q. (2009) Cardiovasc. Res. 81, 733–741 [DOI] [PubMed] [Google Scholar]
- 26. Ramana K. V., Willis M. S., White M. D., Horton J. W., DiMaio J. M., Srivastava D., Bhatnagar A., Srivastava S. K. (2006) Circulation 114, 1838–1846 [DOI] [PubMed] [Google Scholar]
- 27. Bagby G. J., Spitzer J. A. (1980) Am. J. Physiol. 238, H325–330 [DOI] [PubMed] [Google Scholar]
- 28. Memon R. A., Bass N. M., Moser A. H., Fuller J., Appel R., Grunfeld C., Feingold K. R. (1999) Biochim. Biophys. Acta 1440, 118–126 [DOI] [PubMed] [Google Scholar]
- 29. Memon R. A., Fuller J., Moser A. H., Smith P. J., Feingold K. R., Grunfeld C. (1998) Am. J. Physiol. 275, E64–72 [DOI] [PubMed] [Google Scholar]
- 30. Matkovich S. J., Van Booven D. J., Youker K. A., Torre-Amione G., Diwan A., Eschenbacher W. H., Dorn L. E., Watson M. A., Margulies K. B., Dorn G. W., 2nd. (2009) Circulation 119, 1263–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Rooij E., Marshall W. S., Olson E. N. (2008) Circ. Res. 103, 919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Rooij E., Sutherland L. B., Liu N., Williams A. H., McAnally J., Gerard R. D., Richardson J. A., Olson E. N. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 18255–18260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Rooij E., Sutherland L. B., Qi X., Richardson J. A., Hill J., Olson E. N. (2007) Science 316, 575–579 [DOI] [PubMed] [Google Scholar]
- 34. Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001) Crit. Care Med. 29, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 35. Hotchkiss R. S., Song S. K., Neil J. J., Chen R. D., Manchester J. K., Karl I. E., Lowry O. H., Ackerman J. J. (1991) Am. J. Physiol. 260, C50–57 [DOI] [PubMed] [Google Scholar]
- 36. Pasque M. K., Murphy C. E., Van Trigt P., Pellom G. L., Currie W. D., Wechsler A. S. (1983) Arch. Surg. 118, 1437–1440 [DOI] [PubMed] [Google Scholar]
- 37. Astiz M., Rackow E. C., Weil M. H., Schumer W. (1988) Circ. Shock 26, 311–320 [PubMed] [Google Scholar]
- 38. Chen H. W., Hsu C., Lu T. S., Wang S. J., Yang R. C. (2003) Shock 20, 274–279 [DOI] [PubMed] [Google Scholar]
- 39. Tang C., Liu M. S. (1996) Am. J. Physiol. 270, R254–263 [DOI] [PubMed] [Google Scholar]
- 40. Bronsveld W., van Lambalgen A. A., van Velzen D., van den Bos G. C., Koopman P. A., Thijs L. G. (1985) Cardiovasc. Res. 19, 455–464 [DOI] [PubMed] [Google Scholar]
- 41. Chang L., Zhao J., Yang J., Zhang Z., Du J., Tang C. (2003) Eur. J. Pharmacol. 473, 171–176 [DOI] [PubMed] [Google Scholar]
- 42. Levy B., Mansart A., Bollaert P. E., Franck P., Mallie J. P. (2003) Intensive Care Med. 29, 292–300 [DOI] [PubMed] [Google Scholar]
- 43. Supinski G. S., Callahan L. A. (2006) Am. J. Respir. Crit. Care Med. 173, 1240–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Supinski G. S., Murphy M. P., Callahan L. A. (2009) Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1095–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Escames G., López L. C., Ortiz F., López A., García J. A., Ros E., Acuña-Castroviejo D. (2007) FEBS J. 274, 2135–2147 [DOI] [PubMed] [Google Scholar]
- 46. Ashrafian H., Redwood C., Blair E., Watkins H. (2003) Trends Genet. 19, 263–268 [DOI] [PubMed] [Google Scholar]
- 47. Hearse D. J. (1979) Am. J. Cardiol. 44, 1115–1121 [DOI] [PubMed] [Google Scholar]
- 48. Kapelko V. I., Lakomkin V. L., Korchazhkina O. V., Pisarenko O. I. (1996) Mol. Cell. Biochem. 163–164, 131–136 [DOI] [PubMed] [Google Scholar]
- 49. Ruiz-Lozano P., Smith S. M., Perkins G., Kubalak S. W., Boss G. R., Sucov H. M., Evans R. M., Chien K. R. (1998) Development 125, 533–544 [DOI] [PubMed] [Google Scholar]
- 50. Tuunanen H., Engblom E., Naum A., Någren K., Hesse B., Airaksinen K. E., Nuutila P., Iozzo P., Ukkonen H., Opie L. H., Knuuti J. (2006) Circulation 114, 2130–2137 [DOI] [PubMed] [Google Scholar]
- 51. Sack M. N., Rader T. A., Park S., Bastin J., McCune S. A., Kelly D. P. (1996) Circulation 94, 2837–2842 [DOI] [PubMed] [Google Scholar]
- 52. Razeghi P., Young M. E., Alcorn J. L., Moravec C. S., Frazier O. H., Taegtmeyer H. (2001) Circulation 104, 2923–2931 [DOI] [PubMed] [Google Scholar]
- 53. Bone R. C., Fisher C. J., Jr., Clemmer T. P., Slotman G. J., Metz C. A., Balk R. A. (1987) N. Engl. J. Med. 317, 653–658 [DOI] [PubMed] [Google Scholar]
- 54. Luce J. M., Montgomery A. B., Marks J. D., Turner J., Metz C. A., Murray J. F. (1988) Am. Rev. Respir. Dis. 138, 62–68 [DOI] [PubMed] [Google Scholar]
- 55. Annane D., Bellissant E., Bollaert P. E., Briegel J., Confalonieri M., De Gaudio R., Keh D., Kupfer Y., Oppert M., Meduri G. U. (2009) JAMA 301, 2362–2375 [DOI] [PubMed] [Google Scholar]
- 56. Fisher C. J., Jr., Dhainaut J. F., Opal S. M., Pribble J. P., Balk R. A., Slotman G. J., Iberti T. J., Rackow E. C., Shapiro M. J., Greenman R. L., et al. (1994) JAMA 271, 1836–1843 [PubMed] [Google Scholar]
- 57. Opal S. M., Fisher C. J., Jr., Dhainaut J. F., Vincent J. L., Brase R., Lowry S. F., Sadoff J. C., Slotman G. J., Levy H., Balk R. A., Shelly M. P., Pribble J. P., LaBrecque J. F., Lookabaugh J., Donovan H., Dubin H., Baughman R., Norman J., DeMaria E., Matzel K., Abraham E., Seneff M. (1997) Crit. Care Med. 25, 1115–1124 [DOI] [PubMed] [Google Scholar]
- 58. Reinhart K., Karzai W. (2001) Crit. Care Med. 29, S121–125 [DOI] [PubMed] [Google Scholar]
- 59. Stanley W. C., Recchia F. A., Lopaschuk G. D. (2005) Physiol. Rev. 85, 1093–1129 [DOI] [PubMed] [Google Scholar]
- 60. Neglia D., De Caterina A., Marraccini P., Natali A., Ciardetti M., Vecoli C., Gastaldelli A., Ciociaro D., Pellegrini P., Testa R., Menichetti L., L'Abbate A., Stanley W. C., Recchia F. A. (2007) Am. J. Physiol. Heart Circ. Physiol. 293, H3270–3278 [DOI] [PubMed] [Google Scholar]
- 61. Lionetti V., Stanley W. C., Recchia F. A. (2011) Cardiovasc. Res. 90, 202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bishop S. P., Altschuld R. A. (1970) Am. J. Physiol. 218, 153–159 [DOI] [PubMed] [Google Scholar]
- 63. Taegtmeyer H., Overturf M. L. (1988) Hypertension 11, 416–426 [DOI] [PubMed] [Google Scholar]
- 64. Takeyama D., Kagaya Y., Yamane Y., Shiba N., Chida M., Takahashi T., Ido T., Ishide N., Takishima T. (1995) Cardiovasc. Res. 29, 763–767 [PubMed] [Google Scholar]
- 65. Christe M. E., Rodgers R. L. (1994) J. Mol. Cell. Cardiol. 26, 1371–1375 [DOI] [PubMed] [Google Scholar]
- 66. Mercadier J. J., Lompré A. M., Wisnewsky C., Samuel J. L., Bercovici J., Swynghedauw B., Schwartz K. (1981) Circ. Res. 49, 525–532 [DOI] [PubMed] [Google Scholar]
- 67. Boluyt M. O., O'Neill L., Meredith A. L., Bing O. H., Brooks W. W., Conrad C. H., Crow M. T., Lakatta E. G. (1994) Circ. Res. 75, 23–32 [DOI] [PubMed] [Google Scholar]
- 68. Ng W. A., Grupp I. L., Subramaniam A., Robbins J. (1991) Circ. Res. 68, 1742–1750 [DOI] [PubMed] [Google Scholar]
- 69. Huss J. M., Kelly D. P. (2004) Circ. Res. 95, 568–578 [DOI] [PubMed] [Google Scholar]
- 70. Karbowska J., Kochan Z., Smoleński R. T. (2003) Cell. Mol. Biol. Lett. 8, 49–53 [PubMed] [Google Scholar]
- 71. Sihag S., Cresci S., Li A. Y., Sucharov C. C., Lehman J. J. (2009) J. Mol. Cell. Cardiol. 46, 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lu B., Moser A., Shigenaga J. K., Grunfeld C., Feingold K. R. (2010) Biochem. Biophys. Res. Commun. 391, 1737–1741 [DOI] [PubMed] [Google Scholar]
- 73. Feingold K. R., Marshall M., Gulli R., Moser A. H., Grunfeld C. (1994) Arterioscler. Thromb. 14, 1866–1872 [DOI] [PubMed] [Google Scholar]
- 74. Feingold K. R., Moser A., Patzek S. M., Shigenaga J. K., Grunfeld C. (2009) J. Lipid Res. 50, 2055–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Feingold K. R., Wang Y., Moser A., Shigenaga J. K., Grunfeld C. (2008) J. Lipid Res. 49, 2179–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dreyer C., Keller H., Mahfoudi A., Laudet V., Krey G., Wahli W. (1993) Biol. Cell 77, 67–76 [DOI] [PubMed] [Google Scholar]
- 77. Keller H., Dreyer C., Medin J., Mahfoudi A., Ozato K., Wahli W. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 2160–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fruchart J. C. (2009) Atherosclerosis 205, 1–8 [DOI] [PubMed] [Google Scholar]
- 79. Hirosumi J., Tuncman G., Chang L., Görgün C. Z., Uysal K. T., Maeda K., Karin M., Hotamisligil G. S. (2002) Nature 420, 333–336 [DOI] [PubMed] [Google Scholar]
- 80. Singh R., Wang Y., Xiang Y., Tanaka K. E., Gaarde W. A., Czaja M. J. (2009) Hepatology 49, 87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yang R., Wilcox D. M., Haasch D. L., Jung P. M., Nguyen P. T., Voorbach M. J., Doktor S., Brodjian S., Bush E. N., Lin E., Jacobson P. B., Collins C. A., Landschulz K. T., Trevillyan J. M., Rondinone C. M., Surowy T. K. (2007) J. Biol. Chem. 282, 22765–22774 [DOI] [PubMed] [Google Scholar]
- 82. Davis R. J. (2000) Cell 103, 239–252 [DOI] [PubMed] [Google Scholar]
- 83. Tuncman G., Hirosumi J., Solinas G., Chang L., Karin M., Hotamisligil G. S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 10741–10746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rozo A. V., Vijayvargia R., Weiss H. R., Ruan H. (2008) Diabetologia 51, 1493–1504 [DOI] [PubMed] [Google Scholar]
- 85. Ceylan-Isik A. F., Zhao P., Zhang B., Xiao X., Su G., Ren J. (2010) J. Mol. Cell. Cardiol. 48, 367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Patel D., Duke K., Light R. B., Jacobs H., Mink S. N., Bose D. (2000) J. Crit. Care 15, 64–72 [DOI] [PubMed] [Google Scholar]
- 87. Fairchild K. D., Srinivasan V., Moorman J. R., Gaykema R. P., Goehler L. E. (2011) Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R330–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Duncan D. J., Yang Z., Hopkins P. M., Steele D. S., Harrison S. M. (2010) Cell Calcium 47, 378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schwartz A., Gurman G., Cohen G., Gilutz H., Brill S., Schily M., Gurevitch B., Shoenfeld Y. (2002) Eur. J. Intern. Med. 13, 434. [DOI] [PubMed] [Google Scholar]
- 90. Kim Y. M., Guzik T. J., Zhang Y. H., Zhang M. H., Kattach H., Ratnatunga C., Pillai R., Channon K. M., Casadei B. (2005) Circ. Res. 97, 629–636 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.