Background: IL-13 promotes alternative activation (M2 polarization) in monocytes.
Results: IL-13 induces activation of Src family kinase Hck, which is required for M2 gene expression, including 15-lipoxygenase.
Conclusion: Hck acts as a key regulator controlling gene expression in alternatively activated monocytes/macrophages.
Significance: A potential new role of Src family kinase Hck in M2 polarization of monocytes/macrophages.
Keywords: Macrophages, Monocytes, p38 MAPK, Src, STAT Transcription Factor, 15-LO, Alternative Activation, Hck, Jak Kinase, MAO-A
Abstract
IL-13 is a Th2 cytokine that promotes alternative activation (M2 polarization) in primary human monocytes. Our studies have characterized the functional IL-13 receptor complex and the downstream signaling events in response to IL-13 stimulation in alternatively activated monocytes/macrophages. In this report, we present evidence that IL-13 induces the activation of a Src family tyrosine kinase, which is required for IL-13 induction of M2 gene expression, including 15-lipoxygenase (15-LO). Our data show that Src kinase activity regulates IL-13-induced p38 MAPK tyrosine phosphorylation via the upstream kinases MKK3 or MKK6. Our findings also reveal that the IL-13 receptor-associated tyrosine kinase Jak2 is required for the activation of both Src kinase as well as p38 MAPK. Further, we found that Src tyrosine kinase-mediated activation of p38 MAPK is required for Stat1 and Stat3 serine 727 phosphorylation in alternatively activated monocytes/macrophages. Additional studies identify Hck as the specific Src family member, stimulated by IL-13 and involved in regulating both p38 MAPK activation and p38 MAPK-mediated 15-LO expression. Finally we show that the Hck regulates the expression of other alternative state (M2)-specific genes (Mannose receptor, MAO-A, and CD36) and therefore conclude that Hck acts as a key regulator controlling gene expression in alternatively activated monocytes/macrophages.
Introduction
Monocytes/macrophages are crucial participants in mediating chronic inflammation. Monocytes/macrophages undergo activation to perform specific functions during inflammation and its resolution. Classic activation of macrophages (M1 polarization) is mediated by interferon-γ (IFNγ) and promotes pro-inflammatory properties that are required to kill intracellular pathogens, whereas alternative activation of macrophages (M2 polarization) is induced by the Th2 cytokines interleukin-4 (IL-4) and IL-13 and is associated with anti-inflammatory and tissue repair properties (1, 2). However, recent reports suggest that alternatively activated monocyte/macrophage can demonstrate both anti-inflammatory and pro-inflammatory properties at different stages of many diseases (2).
IL-4 and IL-13 are capable of inducing expression of the lipid-oxidizing enzyme called 15-lipoxygenase (15-LO)3 in monocytes/macrophages (3–5). 15-LO expression and activity are found in human atherosclerotic lesions (6). Through specific lipid oxidation, 15-LO generates a series of pro- and anti-inflammatory molecules, termed Hydroperoxyoctadecadienoic acids/Hydroxyoctadecadienoic acids and Hydroperoxyeicosatetraenoic acids/Hydroxyeicosatetraenoic acids. These oxidized lipid molecules have been extracted from atherosclerotic lesions and were shown to be enzyme-generated (7–9). 15-LO has been implicated in the pathogenesis of several diseases, including atherosclerosis, asthma, cancer, renal injury, and osteoporosis (10, 11). Recent studies have also suggested the involvement of 15-LO in the development of cardiac inflammation leading to heart failure (12). Our studies have defined the functional IL-13 receptor complex, association of Jaks with the receptor constituents, and the tyrosine phosphorylation of specific Stat molecules, Stat1, Stat3, Stat5, and Stat6, in response to IL-13 (13). These studies have established a novel and selective signal transduction pathway from the receptor to the nucleus in human monocytes. Understanding the signal transduction pathways that regulate the expression of 15-LO will offer potentially novel approaches for manipulating these pathways to regulate inflammatory processes.
Our studies revealed that IL-13 signaling in monocytes requires the involvement of the membrane-bound heterodimeric IL-13 receptor complex (composed of IL-13Rα1 and IL-4Rα) and the receptor-associated tyrosine kinases Jak2 and Tyk2, which are activated in response to IL-13 stimulation and influence the 15-LO expression in monocytes (5, 13). Recently we reported the existence of two distinct bifurcating parallel regulatory pathways downstream of the IL-13 receptor. We suggested that an IL-13Rα1-Tyk2-ERK1/2-dependent pathway exists in human monocytes, which acts in parallel with the IL-4Rα-Jak2-dependent signaling cascade to regulate the 15-LO gene expression in response to IL-13 stimulation (14). The IL-13Rα1-Tyk2-dependent signaling pathway is required for ERK1/2 MAPK activation and ERK1/2 MAPK-mediated transcriptional regulation of 15-LO by two different transcription factors, Cyclic AMP (cAMP)-responsive element binding protein and Egr-1 (14).
We showed that IL-13 induces p38 MAPK activation and subsequent phosphorylation of serine 727 residues on Stat1 and Stat3 in addition to tyrosine phosphorylation (15). Furthermore, our studies also demonstrated the requirement of both Stat3 tyrosine and serine phosphorylation in regulating IL-13-induced 15-LO expression in primary human monocytes (16). We also reported that PKCδ and p38 MAPK are present in a signaling complex (signalosome) with tyrosine-phosphorylated Stat3 but not with Stat1 and are required for the important serine 727 phosphorylation of Stat3 (16).4
Activation of mitogen-activated protein kinases (MAPKs) and PKCs participate as regulators of inflammatory processes (17–23). To determine the roles of p38 MAPK and PKCδ in IL-13-driven signalosome formation and in regulating Stat3 dual phosphorylation and 15-LO transcription, we investigated the mechanism of p38 MAPK and PKCδ activation. Activation of MAPKs (p38, ERK, and JNK) has been reported to be dependent on Src kinases, a family of non-receptor protein-tyrosine kinases (PTKs) that are expressed either ubiquitously or predominantly in immune cells (24–30). Although Src kinases have been predominantly linked to promoting cell transformation, Src family kinases have also been implicated as critical regulators of a large number of intracellular signaling pathways in immune cells. Src kinases may also be involved in the tyrosine phosphorylation and activation of PKCδ in various cell systems both in vivo and in vitro (31, 32) and have been reported to activate Stat3 (33–35). In general Src kinases are activated by phosphorylation of Tyr-418 (a positive-regulatory autophosphorylation site), dephosphorylation of Tyr-529 (a negative-regulatory phosphorylation site), and the association with different receptors (e.g. growth factor receptors) via its SH2 domain.
In the present study, we investigated the possibility that Src family kinases participate in the signaling pathways induced by IL-13 in alternatively activated monocytes/macrophages. Our findings demonstrate that IL-13 rapidly induces activation of a Src family tyrosine kinase, which in turn regulates the activation and tyrosine phosphorylation of p38 MAPK. In contrast, PKCδ phosphorylation/activation is independent of Src kinase activity. Our data show that Src-dependent activation of p38 MAPK is mediated by the upstream kinases MKK3/6 and is required for phosphorylation of 727 serine residues on Stat1 and Stat3 molecules as well as the expression of IL-13-stimulated 15-LO in primary human monocytes. We identify Hck as the essential Src isoform for these processes. Additionally we found that Hck is also involved in modulating a panel of other alternative state (M2)-specific markers like mannose receptor, MAO-A, and CD36 (1, 2, 36). These data add novel insights into the regulation of alternative activation of monocytes/macrophages in response to IL-13 stimulation and implicate Hck as a major player in the inflammatory processes.
EXPERIMENTAL PROCEDURES
Materials
Recombinant human IL-13 and IL-4 were purchased from BIOSOURCE International (Camarillo, CA). The rabbit reticulocyte 15-LO antibody, cross-reacting with human 15-LO, was raised in sheep and was obtained as a gift from Dr. Joseph Cornicelli (Parke-Davis). Anti-phospho-tyrosine-Stat (pY701-Stat1 and pY705-Stat3) and anti-phosphoserine-Stat (p-Ser-727-Stat1 and p-Ser-727-Stat3), anti-phospho-(Thr-202/Tyr-204)-ERK1/2, anti-phospho-(Thr-180/Tyr-182)-p38 MAPK and total p38 MAPK, and anti-phospho (Ser-189/207)-MKK3/6 and -MKK3 antibodies were purchased from Cell Signaling Technology (Beverly, MA) and diluted 1:1000 according to the manufacturer's protocol. Stat1 and Stat3 monoclonal antibodies were from BD Transduction Laboratories (Lexington, KY). The other primary antibodies used in this study were as follows. Antibodies against different isoforms of Src family protein-tyrosine kinases like Lyn, Fyn, Hck, Fgr, and Yes, mouse anti-human p-Tyr (PY99), anti-phospho-(Tyr-411)-Hck (affinity-purified goat polyclonal antibody raised against a short amino acid sequence containing phosphorylated Tyr-411 of Hck of human origin), rabbit anti-human PKCδ (C-20) and β-tubulin from Santa Cruz Biotechnology (Santa Cruz, CA), and rabbit anti-human phospho-Src [pY418] (produced against a chemically synthesized phosphopeptide derived from the region of Src that contains tyrosine 418) from BIOSOURCE. Pharmacological inhibitors such as SKF86002, SU6656, and PP2 along with PP3 (the inactive structural analog of PP2) were purchased from Calbiochem (La Jolla, CA). The inhibitors were dissolved in DMSO and stored either at 4 °C or at −20 °C as concentrated stock solutions according to the manufacturer's instructions.
Isolation of Human Monocytes
Human peripheral blood monocytes were isolated either by separation of mononuclear cells followed by adherence to BCS-coated flasks as described earlier (5) or by Ficoll-Hypaque sedimentation followed by countercurrent centrifugal elutriation (69, 70). Peripheral blood monocytes purified by these two methods were identical in response to IL-13 and consistently >95% CD14+. These studies complied with all relevant federal guidelines and institutional policies regarding the use of human subjects.
Analysis of MR Expression by FACS
FACS analyses were performed to assess the expression of mannose receptor on the surface of monocytes. Monocytes were harvested from plates by incubating with cell dissociation buffer (Invitrogen) for 10 min at 37 °C and then washed in PBS. The cell pellet was re-suspended in PBS, and then 2 × 106 cells were preincubated with 4% normal goat serum for 30 min at 4 °C. The 2 × 106 cells were spun down and then re-suspended in 4% normal goat serum in PBS and incubated with FITC-conjugated mouse anti-human CD206 (macrophage mannose receptor (MR)) mAb (BD Biosciences) for 30 min at 4 °C according to a protocol supplied by the manufacturer. Finally, the cells were washed, re-suspended in PBS, and then analyzed using a FACScanTM (Becton Dickinson, Mountain View, CA). An appropriate nonspecific isotype antibody was used as a negative control.
IP and Immunoblotting
Peripheral blood monocytes were pretreated with inhibitors (30 min) and then treated with IL-13 (1–2 nm) for different time intervals as indicated. Total and postnuclear extracts were prepared by previously published protocols (13, 71). After determining the protein concentration using the Bio-Rad protein assay reagent (Hercules, CA), lysate proteins (50 μg/well) were resolved by 8% SDS-PAGE and subjected to immunoblotting as described previously (14). 15-LO protein was detected on Western blots following a previously described protocol (5). Immunoprecipitation (IP) experiments were performed according to our previously published method (16) using anti-p38 MAPK or anti-PKCδ antibodies for 2 h at 4 °C followed by addition of prewashed Protein A-Sepharose beads (Sigma) at 4 °C overnight. In several experiments, immunoblots were stripped and reprobed to assess equal loading according to our previously published protocol (13).
RNA Extraction and Quantitative Real-time PCR Analysis
Monocytes (5 × 106 in 2 ml of 10% BCS/DMEM) were plated in 6-well culture plates. 2 h after plating, cells were treated with 1–2 nm recombinant IL-13 for 24 h. In some experiments, monocytes were pre-treated with Src kinase inhibitors for 30 min followed by IL-13 treatment for 24 h. Total cellular RNA was extracted using the RNeasy mini kit from Qiagen (Valencia, CA), and real-time quantitative RT-PCR was performed according to established protocols (15). The sequences of the primers used were: 15-LO forward, 5′-GCT GGA AGG ATC TAG ATG ACT-3′ and 15-LO reverse, 5′-TGG CTA CAG AGA ATG ACG TTG-3′; CD36 forward, 5′-CAG AGG CTG ACA ACT TCA CAG-3′ and CD36 reverse, 5′-AGG GTA CGG AAC CAA ACT CAA-3′; and MAO-A forward, 5′-GCC AAG ATT CAC TTC AGA CCA GAG-3′ and MAO-A reverse, 5′-TGC TCC TCA CAC CAG TTC TTC TC-3′. GAPDH was used as an internal control with the primer sequences of forward 5′-CAC CAA CTG CTT AGC ACC CC-3′ and reverse 5′-TGG TCA TGA GTC CTT CCA CG-3′.
DNA-binding Activity of Stat1 and Stat3
A TransAMTM Stat family kit (Active Motif) was used to evaluate the DNA-binding activity of Stat1 and Stat3 according to the instruction provided by the supplier. Briefly, nuclear extracts were incubated in a 96-well assay plate precoated with immobilized oligonucleotide containing the Stat consensus binding site (5′-TTCCCGGAA-3′). The active form of Stat1 and Stat3 contained in nuclear extracts specifically binds the oligonucleotide. The wild-type (WT) consensus oligonucleotide was provided with the kit as a competitor for Stat binding to monitor the specificity of the assay by preventing Stat binding to the probe immobilized on the plate. Conversely, the mutated consensus oligonucleotide was provided as a control and expected to have a limited competition with the Stat consensus binding site. The detection of activated Stats (Stat1 and Stat3) was carried out with specific Stat1 and Stat3 primary antibodies and an HRP-conjugated secondary antibody supplied in the kit. After incubation with the developing solution for the recommended time, the reaction was stopped and the colorimetric readout was taken at 450 nm with a reference wavelength of 655 nm using a SynergyTM HT Multi-Mode Microplate Reader from Biotek (Vermont, USA).
p38MAP Kinase Assay
The activity of p38 MAPK was analyzed by using a p38 MAPK assay kit from Cell Signaling Technology. Phosphorylated p38 MAPK in the cell extract was selectively immunoprecipitated from 200 μg of lysate using immobilized (cross-linked to agarose hydrazide beads) dual phospho (Thr-180/Tyr-182)-p38 MAPK monoclonal antibody. The immune complex was washed thoroughly and resuspended in kinase buffer containing 1 μg of ATF-2 fusion protein as p38 MAPK substrate and 200 μm cold ATP. The assay mixture was incubated at 30 °C for 30 min and terminated by adding SDS sample buffer. The ATF-2 phosphorylation was analyzed by Western blotting with a phospho-ATF-2 (Thr-71) rabbit polyclonal antibody and chemiluminescent detection.
Treatment of Monocytes with Jak2 and Tyk2 Antisense ODNs
The antisense oligodeoxyribonucleotides (ODNs) sequences for human Jak2 and Tyk2 were selected based on our previously published literature (5). Control ODNs for Jak2 and Tyk2 consisted of complementary sense ODNs. All ODNs were end-modified (phosphorothioated, three bases of 5′ and 3′) oligonucleotides to limit DNA degradation and all were HPLC-purified before use (Invitrogen).
The sequences of the ODNs are as follows: Jak2 antisense, 5′-TCT TAA CTC TGT TCT CGT TC-3′; Jak2 sense, 5′-GAA CGA GAA CAG AGT TAA GA-3′; Tyk2 antisense, 5′-CCA ACT TTA TGT GCA ATG TG-3′; and Tyk2 sense, 5′-CAC ATT GCA CAT AAA GTT GG-3′.
Primary human monocytes (5 × 106 cells/well) were plated in 6-well culture plates and transfected with Jak2 and Tyk2 sense and antisense ODNs at 2 μm concentration using Mirus TransIt-Oligo Transfection Reagent (Mirus Bio Corp., Madison, WI) according to the manufacturer's protocols, and the incubation was continued for 48 h. For the transfection control, cells were incubated with the transfection reagent alone for 48 h. After this treatment, monocytes were stimulated with IL-13 for another 15 min to study the activation/phosphorylation of Src kinase and p38 MAPK.
Treatment of Monocytes with Hck Antisense ODNs
Human monocytes were plated (5 × 106 in 2 ml of 10% BCS/DMEM) as described above in 6-well plates. The antisense ODN sequence for human Hck was selected based on prior literature and successfully used before to selectively inhibit the expression of endogenous Hck (43). The Hck antisense ODN sequence was 5′-GAA CTT GGA CTT CAT GCA CCC-3′. The complementary Hck sense ODN (5′-GGG TGC ATG AAG TCC AAG TTC-3′) was used as control. Antisense and sense ODNs for human Hck were purchased from Invitrogen. All ODNs were totally phosphorothioated as above to limit DNA degradation and were HPLC-purified before use. Hck antisense or sense ODNs were boiled for 2–3 min and then cooled at room temperature before being added to the cells. For Hck ODN treatment, human monocytes were treated with Hck antisense or sense ODN sequences (either 5 or 10 μm concentration) for 48 h with one re-feed at 24 h prior to the addition of IL-13 either for 15 min or 24 h. Cells were then lysed or utilized for further experimentation.
Data Analysis
The number of experiments analyzed is indicated in each figure. Band intensities were quantified by densitometric analyses using a laser densitometer (MICROTEK ScanMaker 8700, Cerritos, CA) and NIH Image software Program. Differences among experimental groups (for dose responses) were analyzed using one-way analysis of variance. The significance of observations was calculated using unpaired Student's t test analysis, and p < 0.05 was considered statistically significant.
RESULTS
IL-13 Induces Phosphorylation of Src Tyrosine Kinase in Primary Human Monocytes
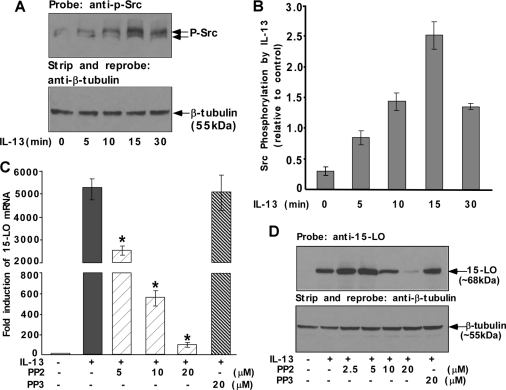
To determine the role of Src tyrosine kinase in alternative monocyte activation, we first examined activation of Src kinase following IL-13 treatment utilizing an antibody that specifically recognizes the autophosphorylation site of Src kinase (phospho-tyrosine at position 418). After treatment with IL-13 for different time intervals, the cells were lysed and the whole cell lysates were subjected to immunoblot analysis using the anti-phospho-Src antibody. IL-13 induced the phosphorylation of Src kinase at its autophosphorylation site in a time-dependent manner thus indicating Src activation (Fig. 1, A and B). The phosphorylated form of Src kinase was detected as early as 5 min, and the peak of phosphorylation was ∼15 min after IL-13 treatment with the signal diminishing afterward (Fig. 1, A and B). This result suggests that the activation of Src tyrosine kinase in IL-13-stimulated human monocytes is one of the early signaling events leading to various downstream cellular processes.
FIGURE 1.
Src kinase is phosphorylated by IL-13 and required for IL-13-induced 15-LO expression in monocytes. Monocytes (5 × 106 per group) (A, C, and D) were either directly treated with IL-13 (1 nm) for different time intervals as indicated (A) and for 24 h (C and D) or pretreated with Src kinase inhibitor PP2 or with PP3, the inactive structural analog of PP2 (C and D) for 30 min at various doses followed by stimulation with IL-13 for 24 h. In A, cells were lysed and the whole cell extracts (50 μg/lane) were loaded on an 8% SDS-PAGE gel and immunoblotted with a rabbit polyclonal anti-phospho-Src (Tyr-418) antibody (upper panel). The same blot was stripped and reprobed with a rabbit polyclonal anti-β-tubulin antibody (lower panel). The results are representative of three independent experiments. In B, densitometric quantification of Western blot results (shown in arbitrary units) were conducted with NIH Image and are presented in the adjacent panels as means ± S.D. (n = 3). In C, total cellular RNA extracts were prepared and subjected to quantitative real-time RT-PCR analysis. After normalization with GAPDH amplification, the -fold induction of 15-LO mRNA expression for different groups was plotted. Data are representative of three similar experiments and shown as the means ± S.D. (n = 3). Significant differences were determined by comparing PP2- and PP3-treated groups to the IL-13-treated control (*, p < 0.002). In D, the cells were harvested and lysed. 50 μg of the postnuclear lysates was resolved by SDS-PAGE, and 15-LO protein expression was detected on Western blots with a 15-LO-specific antibody. The same blot was stripped and reprobed with an antibody against β-tubulin (lower panel of D) to assess equal loading. The results in D are representative of three independent experiments.
Src Tyrosine Kinase Activity Is Required for 15-LO Expression
To investigate the possible role of Src kinase in regulating IL-13-induced 15-LO mRNA expression, monocytes were pretreated with PP2 (Fig. 1C) or SU6656 (supplemental Fig. S1A) at several indicated doses for 30 min and then incubated with IL-13 for an additional 24 h. The results of our quantitative real-time RT-PCR experiment showed that the induction of 15-LO mRNA by IL-13 was >4000-fold (212) by 24 h, and treatment with PP2 and SU6656 both caused profound inhibition of 15-LO mRNA levels (>99% inhibition at the highest indicated doses; Fig. 1C and supplemental Fig. S1A). In contrast PP3 showed no inhibition of IL-13-driven induction of 15-LO mRNA (Fig. 1C). The mRNA levels of GAPDH were nearly identical in all the samples, indicating specificity of the response.
Because Src kinase activity regulated IL-13-dependent 15-LO mRNA expression, we next examined whether Src kinase activity was required for the IL-13-induced expression of 15-LO protein. After pretreatment with Src kinase activity inhibitors PP2 or SU6656 for 30 min followed by incubation with IL-13 for 24 h, the monocytes were harvested and lysed, and 15-LO protein was detected on Western blots. The results shown in Fig. 1D and supplemental Fig. S1B indicate that both of the Src kinase inhibitors substantially suppressed expression of 15-LO protein in a dose-dependent manner. PP2 at 20 μm caused almost total inhibition of 15-LO induction, whereas SU6656 treatment inhibited 15-LO induction to the same extent at 5–10 μm. In contrast, PP3 had no inhibitory effect on the induction of 15-LO protein expression by IL-13 (Fig. 1D). It should be noted that the cell viability was not affected by these pharmacological inhibitors at the specified doses and times of treatment. These results show that Src kinase is a critical regulator of 15-LO gene expression in alternatively activated monocytes/macrophages in response to IL-13 treatment.
Jak2 Regulates IL-13-induced Activation/Phosphorylation of Src Kinase and p38 MAPK
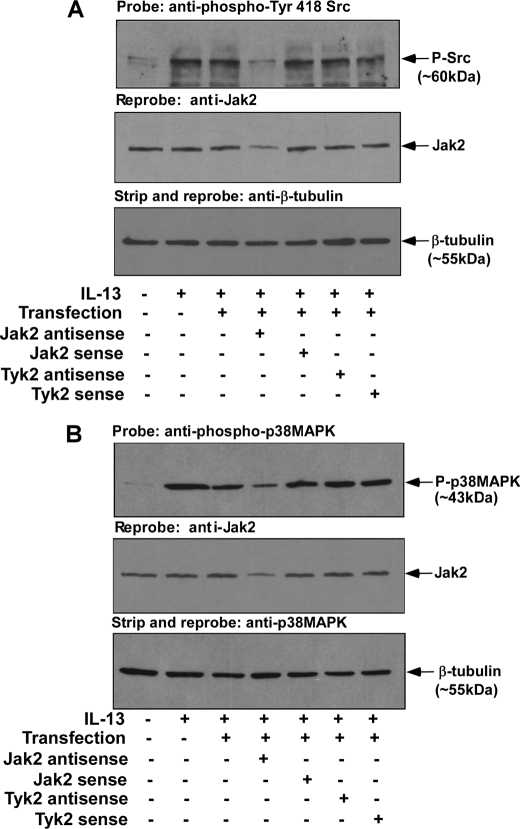
Previous studies from our group demonstrated the requirement of both Jak2 and Tyk2 kinases for the IL-13-induced expression of 15-LO in primary human monocytes (5). Because our results indicated that IL-13 stimulated activation/phosphorylation of Src PTK in human monocytes, and Src kinase activity was required for 15-LO gene expression, we next examined the requirement for Jak2 and Tyk2 kinases for the IL-13-induced signaling pathways leading to the activation/phosphorylation of Src kinase. For these studies, monocytes were transfected with antisense or sense ODNs against Jak2 or Tyk2 kinases for 48 h in the presence of Mirus TransIt-Oligo transfection reagent. After treatment, the cells were stimulated with IL-13 for another 15 min. Total cell lysates were prepared, and activation/phosphorylation of Src PTK was evaluated. Our results suggest that the antisense ODN (2 μm) against Jak2 kinase inhibited IL-13-stimulated activation/phosphorylation of Src PTK, whereas the sense ODN and the vehicle controls had no effect (Fig. 2A, upper panel). In contrast, treatment with antisense to Tyk2 (2 μm) (Fig. 2A, upper panel) had no inhibitory effect on the IL-13-induced activation/phosphorylation of Src kinase. Antisense ODN inhibition of Jak2 protein expression level was also verified by reprobing the same blot with an antibody against Jak2 kinase (Fig. 2A, middle panel). These data identify Jak2 as the upstream receptor-associated tyrosine kinase regulating Src PTK phosphorylation/activation in alternatively activated monocytes/macrophages in response to IL-13 stimulation.
FIGURE 2.
Jak2 antisense treatment inhibits activation/phosphorylation of both Src kinase and p38 MAPK in IL-13-stimulated monocytes. Monocytes were pre-treated with antisense or sense ODNs to Jak2 and Tyk2 (A and B) prior to the addition of IL-13 (2 nm) for 15 min. The cells were lysed, and 50 μg of the total cell extracts (from each sample group) were separated by 8% SDS-PAGE and immunoblotted either with a rabbit polyclonal anti-phospho-Src (Tyr-418) antibody (upper panel of A) or with anti-phospho-p38 MAPK (Thr-180/Tyr-182) antibody (upper panel of B). Blots were then reprobed with Jak2 antibody to examine the effect of antisense ODN on Jak2 expression (middle panels of A and B). The same blots were stripped and reprobed with β-tubulin antibody to assess equal loading (lower panels of A and B). The results are representative of three independent experiments.
We next investigated the requirement of Jak2 and Tyk2 kinases for the IL-13-stimulated activation/phosphorylation of p38 MAPK. By performing similar experiments as above, we showed that substantial inhibition of Jak2 expression in monocytes treated with antisense to Jak2 (2 μm) (Fig. 2B, middle panel) was also associated with down-regulation of IL-13-stimulated activation/phosphorylation of p38 MAPK (Fig. 2B, upper panel). In contrast, treatment with antisense ODNs against Tyk2 (2 μm) (Fig. 2B, upper panel) caused no inhibition of IL-13-induced activation/phosphorylation of p38 MAPK. These results confirmed the regulatory role of Jak2 kinase in controlling the IL-13-induced activation/phosphorylation of Src PTK as well as p38 MAPK.
Src Tyrosine Kinase Regulates IL-13-stimulated Activation/Phosphorylation of p38 MAPK but Not Phosphorylation of PKCδ
To explore whether Src tyrosine kinase activity is required for p38 MAPK phosphorylation/activation in IL-13-treated monocytes, we treated cells with PP2 or SU6656 for 30 min before IL-13 stimulation. Postnuclear extracts were immunoprecipitated using either p38 MAPK or PKCδ antibody, and the immunoprecipitates were subsequently immunoblotted with a general phospho-tyrosine antibody (supplemental Fig. S2, A and B). Pretreatment with PP2 or SU6656 substantially inhibited IL-13-induced p38 MAPK phosphorylation, whereas the inactive structural analog PP3 showed negligible inhibition (supplemental Fig. S2A).
Because PKCδ is also phosphorylated in response to IL-13 stimulation, we explored whether Src kinases were involved in this process. Neither PP2 nor SU6656 inhibited IL-13-induced tyrosine phosphorylation of PKCδ (supplemental Fig. S2B), suggesting that Src kinase does not regulate IL-13-dependent PKCδ activation in primary human monocytes.
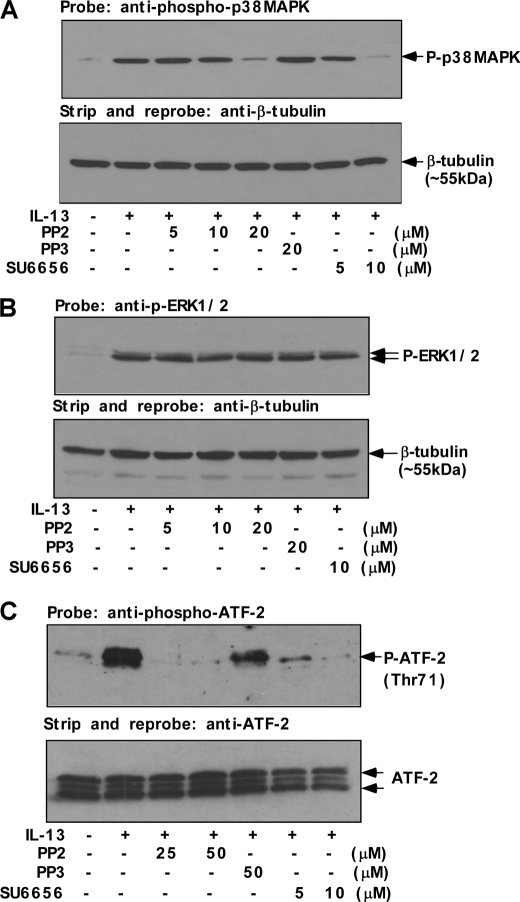
In addition to detecting phospho-tyrosine on p38 MAPK, we confirmed this finding by examining p38 MAPK phosphorylation using a phospho-p38 MAPK (Thr-180/Tyr-182) antibody (Fig. 3A). The results indicate that both PP2 (20 μm) and SU6656 (5–10 μm) markedly inhibited IL-13-induced p38 MAPK phosphorylation, and PP3, the structural analog of PP2, showed no inhibitory effect on p38 MAPK phosphorylation (Fig. 3A). To investigate whether Src kinase activity has any role in controlling the activation of ERK in IL-13-stimulated monocytes, we also performed experiments using PP2 and SU6656. Monocytes were preincubated with various doses of PP2 and SU6656 prior to IL-13 stimulation. As presented in Fig. 3B, pretreatment with PP2 or SU6656 at a dose (20 μm and 10 μm, respectively) that substantially inhibited 15-LO protein expression (Fig. 1D) and p38 MAPK activation/phosphorylation (Fig. 3A) had no inhibitory effect on IL-13-induced ERK1/2 phosphorylation. These data thus suggest that Src kinase selectively activates p38 MAPK in IL-13-stimulated monocytes.
FIGURE 3.
Src kinase is the upstream regulator of p38 MAPK activity. Human monocytes (5 × 106/group) (A–C) were either directly treated with IL-13 for 15 min (A and C) and 1 h (B) or pretreated with Src kinase inhibitors SU6656 and PP2 or its inactive structural analog PP3 for 30 min at various indicated doses followed by IL-13 treatment for 15 min (A and C) and 1 h (B). A, postnuclear extracts (50 μg/lane) were resolved by 8% SDS-PAGE and immunoblotted with anti-phospho-p38 MAPK (Thr-180/Tyr-182) antibody (upper panel). The blot was subsequently stripped and reprobed with β-tubulin antibody to assess equal loading (lower panel). B, the cells were lysed, and 50 μg of the cell extracts (from each sample group) were separated by SDS-PAGE and immunoblotted with anti-phospho-ERK1/2 antibody (upper panel of B). The same blot was stripped and reprobed with β-tubulin antibody (lower panel) as a loading control. C, p38 MAPK enzymatic activity was measured in postnuclear extracts by selective IP using immobilized phospho-p38 MAPK (Thr-180/Tyr-182) antibody followed by incubation of IP pellets in kinase buffer containing ATF-2 fusion protein and cold ATP. The immunoprecipitates were resolved by SDS-PAGE and ATF-2 phosphorylation was detected using anti-phospho-ATF-2 (Thr-71) antibody by Western blotting. The same blot was stripped and reprobed with ATF-2 antibody as a loading control (lower panel).
Because Thr-180/Tyr-182 dual phosphorylation is reported to be associated with the kinase activity of p38 MAPK (15, 37–39), we performed assays of p38 MAPK enzymatic activity. ATF-2 has been reported to be a downstream substrate of p38 MAPK, in an in vitro kinase assay (40). As shown in Fig. 3C, immunoprecipitated phospho-p38 MAPK obtained in the absence of IL-13 treatment showed very low levels of ATF-2 phosphorylation, whereas after IL-13 treatment the activity was significantly enhanced, suggesting that phosphorylated p38 MAPK in IL-13-stimulated cells was enzymatically active. To confirm that p38 MAPK-mediated phosphorylation of ATF-2 is controlled by Src family tyrosine kinase, we pretreated cells with Src kinase activity inhibitors PP2 or SU6656 for 30 min before IL-13 stimulation. It should be noted here that the higher doses of PP2 do not induce any adverse effect in monocytes within the stipulated time of these experiments. The addition of both Src kinase inhibitors dramatically reduced the activity of p38 MAPK (Fig. 3C). In contrast, PP3 showed far less effect on p38 MAPK-mediated ATF-2 phosphorylation. These results clearly demonstrate the role of Src kinase in selectively regulating the IL-13-induced phosphorylation and enzymatic activity of p38 MAPK without affecting PKCδ and ERK1/2 phosphorylation.
MKK3/6 Acts Upstream of p38 MAPK Pathway in Mediating the Regulation of IL-13- induced 15-LO Expression
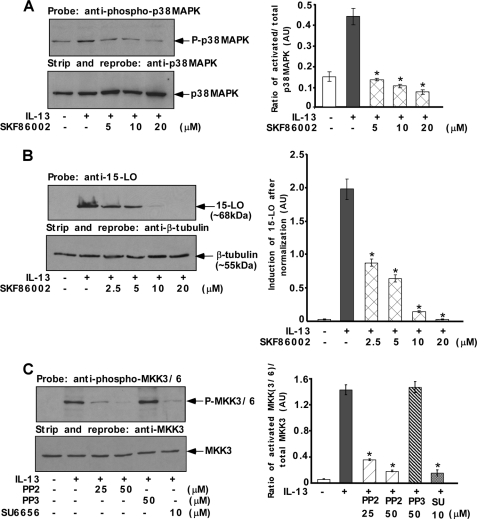
Our previously published studies demonstrated that the dual phosphorylation and activation of p38 MAPK is a critical event in IL-13 signaling leading to the induction of 15-LO in alternatively activated monocytes/macrophages (15). To examine whether the dual phosphorylation and activation of p38 MAPK by IL-13 is mediated by the immediate upstream kinases MKK3/6, we pretreated monocytes with the MKK3/6 inhibitor, SKF86002, before IL-13 stimulation. Our results showed that p38 MAPK dual phosphorylation/activation was down-regulated by SKF86002 in a dose-dependent manner suggesting the involvement of MKK3/6 as an upstream kinase in regulating the activation of p38 MAPK (Fig. 4A). To investigate whether MKK3/6 enzymatic activities are required for IL-13 induction of 15-LO expression, we pretreated monocytes with the same MKK3/6 inhibitor SKF86002 before IL-13 stimulation. We observed that pretreatment of monocytes with SKF86002 resulted in a profound and dose-dependent inhibition of IL-13-induced 15-LO expression (Fig. 4B).
FIGURE 4.
p38 MAPK-mediated regulation of 15-LO expression requires Src kinase-dependent activation of MKK3/6. Monocytes (5 × 106 per group) (A–C) were either pretreated with the MKK3/6 inhibitor, SKF86002 (A and B) for 30 min or pretreated with Src kinase inhibitors SU6656 or PP2 or its inactive structural analog PP3 for 30 min (C) at various indicated doses followed by IL-13 treatment for either 15 min (A and C) or 24 h (B). In the upper panels of A and B, postnuclear extracts (50 μg/lane) were resolved by 8% SDS-PAGE and immunoblotted with anti-phospho-p38 MAPK (Thr-180/Tyr-182) and anti-15-LO antibodies, respectively. The blots were subsequently stripped and reprobed with anti-p38 MAPK and anti-β-tubulin antibodies to assess equal loading (lower panels of A and B, respectively). The results are representative of three similar experiments. In C, postnuclear extracts (50 μg/lane) were resolved by SDS-PAGE and immunoblotted with anti-phospho-MKK3/6 antibody. The blot was subsequently stripped and reprobed with anti-MKK3 antibody to assess equal loading (lower panel). The results are representative of three identical experiments performed. In A–C, densitometric quantification of Western blot results (shown in arbitrary units, AU) were conducted with NIH Image and are presented in the adjacent panels as means ± S.D. (n = 3). Significant differences were determined by comparing each group to the IL-13-treated monocytes as the control (*, p < 0.0005).
Src Tyrosine Kinase Regulates IL-13-stimulated Activation/Phosphorylation of MKK3/6
To determine further whether Src kinase acts upstream of the MKK3/6-p38 MAPK signaling pathway to regulate IL-13-stimulated 15-LO expression, we examined the effects of inhibiting Src kinase activity on the IL-13-induced activation of MKK3/6. We pretreated cells with the Src kinase activity inhibitors PP2 or SU6656 for 30 min before IL-13 stimulation. As shown in Fig. 4C, activation of MKK3/6 by IL-13 was significantly inhibited by treatment of primary monocytes with the Src kinase inhibitors PP2 and SU6656. In contrast, PP3, a negative structural analog of PP2, had no inhibitory effect on MKK3/6 phosphorylation/activation by IL-13 (Fig. 4C).
Src Tyrosine Kinase Is Involved in Stat1 and Stat3 Serine Phosphorylation
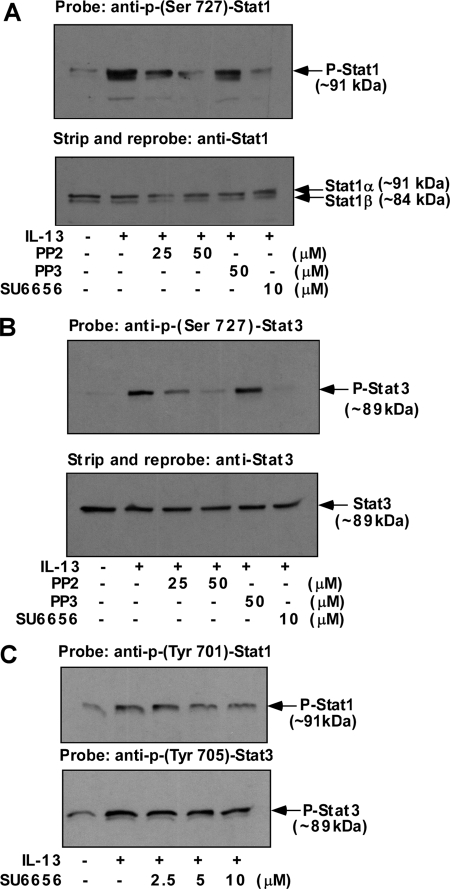
Previously we demonstrated that p38 MAPK activity was required for the serine phosphorylation of both Stat1 and Stat3 (15), and here we report the involvement of Src kinase in regulating p38 MAPK phosphorylation/activity. We therefore investigated whether Src kinase activity regulates Stat1 and Stat3 serine phosphorylation. The results of a representative experiment are shown in Fig. 5. Monocytes were pretreated with either PP2 or SU6656 for 30 min prior to the addition of IL-13. The serine phosphorylation status of both Stat1 and Stat3 was determined by Western blots using phosphoserine-Stat specific antibodies. The results indicate that both PP2 and SU6656 inhibited Stat1 serine phosphorylation (Fig. 5A). The degree of inhibition was nearly complete at 50 μm PP2 and 10 μm SU6656. In contrast PP3 showed a negligible effect on Stat1 serine phosphorylation (Fig. 5A).
FIGURE 5.
Src kinase activity is required for IL-13-induced Stat1 and Stat3 Ser-727 phosphorylation. Monocytes (5 × 106/group) (A–C) were either directly treated with IL-13 or pre-treated with Src kinase inhibitors SU6656 (A–C) or PP2 and its inactive structural analog PP3 (A and B) at the indicated doses for 30 min followed by stimulation with IL-13 for 15 min (C) or 1 h (A and B). Whole cell extracts (50 μg/lane) were resolved by SDS-PAGE, and the Ser-727 phosphorylations of both Stat1 and Stat3 were detected by immunoblotting using p-Ser-727 Stat1 (A) or p-Ser-727 Stat3 antibodies (B). Blots were then stripped and reprobed with Stat1 (lower panel of A) or Stat3 (lower panel of B) to assess equal loading. Whole cell lysates were also analyzed for p-Tyr-701 Stat1 and p-Tyr-705 Stat3 (C).
We conducted similar experiments where we studied the effect of Src inhibitors on Stat3 serine phosphorylation. The cells were similarly pretreated with PP2 or SU6656 before IL-13 stimulation. Subsequently, phosphoserine Stat3 was detected on Western blots, as shown in Fig. 5B. The results demonstrated that both PP2 (50 μm) and SU6656 (10 μm) had a similar effect on Stat3 serine phosphorylation (almost complete inhibition) as that exhibited on Stat1 serine phosphorylation in Fig. 5A.
To evaluate whether Src kinase activity had any effects on Stat1 and Stat3 tyrosine phosphorylation, the phosphorylation status of Stat proteins was assessed using anti-phospho-tyrosine antibodies specific for either Stat1 or Stat3. Pretreatment of SU6656 up to 10 μm (the dose that blocked both Stat1 and Sta3 serine phosphorylation almost completely) had no inhibitory effects on IL-13-induced Stat1 or Stat3 tyrosine phosphorylation (Fig. 5C).
IL-13 Induction of Stat1 and Stat3 DNA-binding Activity Is Regulated by Src Tyrosine Kinase Activity in Human Monocytes
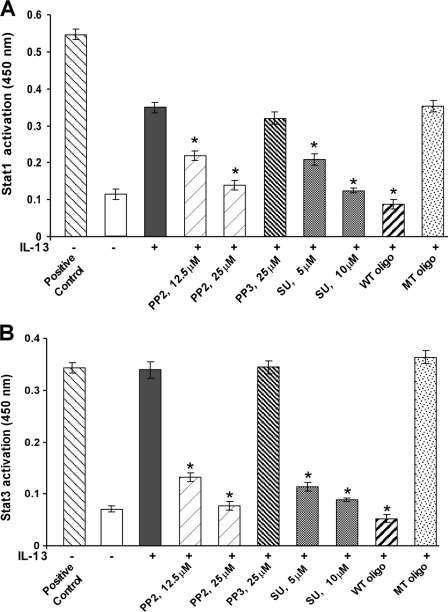
Earlier we demonstrated that activation of p38 MAPK by IL-13 is required for maximal Stat1 and Stat3 DNA-binding activities (15) and that p38 MAPK-mediated Stat1 and Stat3 serine phosphorylation is important for maximal DNA-binding activities (15). Using a Stat3 serine 727 mutant we further confirmed the contribution of Stat serine phosphorylation in enhanced DNA-binding activities in primary human monocytes (16). Because we already demonstrated in Fig. 3 that Src kinase acts upstream of the p38 MAPK signaling pathway, we therefore studied the role of Src kinase activity on IL-13-stimulated Stat1 and Stat3 DNA-binding activity in primary monocytes. Using an ELISA-based TransAMTM method and employing specific Stat1 and Stat3 antibodies, we demonstrated that IL-13 treatment enhanced both Stat1 and Stat3 DNA-binding activity in nuclear protein extracts of monocytes (Fig. 6, A and B). Furthermore, pretreatment of monocytes with Src kinase inhibitors significantly reduced the extent of both Stat1 (Fig. 6A) and Stat3 (Fig. 6B) DNA-binding activities in a dose-dependent manner. The wild-type (WT) consensus oligonucleotides also significantly reduced the Stat1 and Stat3 activation. Conversely, PP3 and the mutated (MT) consensus oligonucleotides showed negligible effects on both Stat1 and Stat3 DNA-binding activities. These results establish the involvement of Src tyrosine kinase activity in regulating both Stat1 and Stat3 DNA binding in response to IL-13.
FIGURE 6.
IL-13-induced Stat1 and Stat3 DNA-binding activity requires Src tyrosine kinase activity in monocytes. Human monocytes (5 × 106/group) were either pretreated with Src kinase inhibitors SU6656 or PP2 or its inactive structural analog PP3 for 30 min at various indicated doses followed by IL-13 stimulation for 1 h or directly exposed to IL-13 for the same period of time. 5 μg of nuclear extracts was run in duplicate to perform an immunodetection of activated Stat1 (A) and activated Stat3 (B) using a TransAMTM Stat family kit. Nuclear extracts from IFNγ-stimulated COS-7 cells and IL-6-stimulated HepG2 cells were used as positive controls for activated Stat1 and Stat3, respectively. The wild-type (WT) and mutated (MT) consensus oligonucleotides were used to monitor the specificity of the assay. Data are means ± S.E. of three separate experiments. Significant differences were determined by comparing each group to the IL-13-treated monocytes as the control (*, p < 0.005).
IL-13 Induces Tyrosine Phosphorylation of Hck
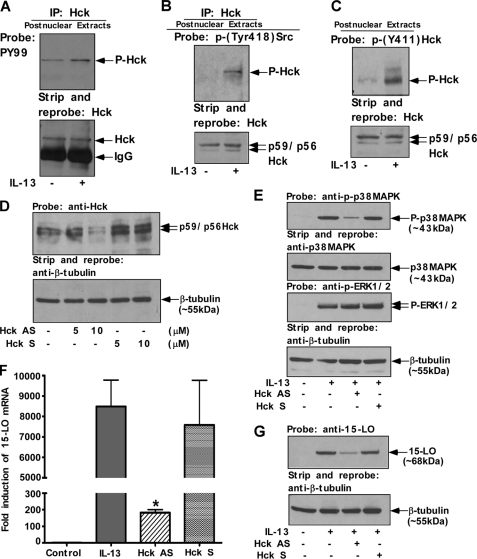
Several members of the Src family PTKs are constitutively expressed in mononuclear phagocytes and have been shown to play important roles in the signal transduction pathways of several monocyte/macrophage-activating factors (41). In agreement with previously published articles (42), we detected constitutive expression of Src family PTKs like Lyn, Fgr, Hck, Fyn, and Yes in postnuclear lysates of primary human monocytes (supplemental Fig. S3A). To explore the biochemical mechanisms leading to monocyte activation by IL-13, we investigated whether IL-13 activated a specific member of the Src family PTKs. Monocytes were activated with 2 nm IL-13 for 15 min, and postnuclear extracts were immunoprecipitated using Hck, Fgr, and Lyn antibodies, respectively, and the immunoprecipitates were subsequently immunoblotted with a general phospho-tyrosine antibody (Fig. 7A and supplemental Fig. S3, B and C). As shown in Fig. 7A and supplemental Fig. S3, B and C (upper panels), a basal level of tyrosine phosphorylation was present in unstimulated monocytes (for all of these members). Activation of monocytes by IL-13 resulted in a rapid induction of tyrosine phosphorylation for Hck, but the level of tyrosine phosphorylation for both Fgr and Lyn was unchanged. Stripping of these blots followed by direct immunoblotting with Hck, Fgr, and Lyn antibodies demonstrated almost equal amounts of immunoprecipitated protein in all the cases (Fig. 7A and supplemental Fig. S3, B and C, lower panels). To confirm these results we immunoprecipitated Hck from the postnuclear extracts, and the immunoprecipitates were then immunoblotted with a general Src phospho-tyrosine antibody that specifically recognizes the autophosphorylation site of all the members of Src family PTKs (phospho-tyrosine at position 418) (Fig. 7B). Our results demonstrated that IL-13 induced the phosphorylation of Hck at its autophosphorylation site (Fig. 7B). In another experiment, Hck activation/phosphorylation by IL-13 stimulation was re-confirmed by direct immunoblotting of postnuclear extracts using phospho-Hck (Tyr-411) antibody for detection of Tyr-411 phosphorylated Hck of human origin (Fig. 7C). These results demonstrated that IL-13 induces Hck tyrosine phosphorylation in alternatively activated monocytes/macrophages and suggest that Hck may be involved in IL-13 signal transduction in human monocytes.
FIGURE 7.
Activation of Hck, the specific Src PTK isoform, regulates both p38 MAPK activation/phosphorylation and 15-LO gene expression in IL-13-stimulated monocytes. Monocytes (10 × 106/group) (A and B) and (5 × 106/group) (C) were either incubated in medium alone or directly treated with IL-13 (2 nm) for 15 min. Postnuclear extracts were prepared and subjected to IP with Hck (goat polyclonal) antibody (A and B). The immunoprecipitates were resolved by SDS-PAGE for immunoblotting with the anti-phospho-tyrosine antibody, PY99 (mouse monoclonal) (A, upper panel). HRP-conjugated donkey anti-mouse, pre-absorbed secondary antibody from Affinity Bioreagents Inc. (Golden, CO), was used to develop the blot. In the upper panel of B, immunoprecipitates were resolved by 8% SDS-PAGE and immunoblotted with the rabbit polyclonal anti-phospho-Src (Tyr-418) antibody. HRP-conjugated donkey anti-rabbit, pre-absorbed antibody from Affinity Bioreagents Inc., was used as a secondary antibody. The blots were subsequently stripped and reprobed with Hck antibody to assess equal immunoprecipitation (lower panels of A and B). In the upper panel of C, postnuclear extracts (50 μg/lane) were resolved by 8% SDS-PAGE and immunoblotted with anti-phospho-Hck (Tyr-411) antibody (goat polyclonal). The blot was then stripped and reprobed with anti-Hck antibody as a loading control (lower panel). In D–G, monocytes (5 × 106/group) were pre-treated with antisense (AS) or sense (S) ODNs to Hck either at indicated concentrations or at 10 μm for 48 h prior to the addition of IL-13 (1 nm) for either 15 min (E) or 24 h (F and G). D, cells were lysed and 50 μg of the postnuclear extracts (from each sample group) was separated by 8% SDS-PAGE and immunoblotted with anti-Hck antibody to examine the effect of antisense ODN on Hck expression (upper panel of D). The same blot was stripped and reprobed with an antibody against β-tubulin to assess equal loading (lower panel of D). In E, postnuclear extracts (50 μg/lane) were resolved by SDS-PAGE and immunoblotted with anti-phospho-p38 MAPK (Thr-180/Tyr-182) antibody. The blot was then stripped and reprobed with anti-p38 MAPK antibody. In another experiment, cells were lysed and 50 μg of the postnuclear extracts (from each sample group) was separated by 8% SDS-PAGE and immunoblotted with anti-phospho-ERK1/2 antibody. The same blot was stripped and reprobed with β-tubulin antibody to assess equal loading. The results are representative of three identical experiments performed. F, total cellular RNA extracts were prepared and subjected to real-time quantitative RT-PCR analysis. After normalization with GAPDH amplification, the -fold induction of 15-LO mRNA expression for different groups was plotted. Data are the means ± S.D. (n = 3). Significant differences were determined by comparing the antisense (AS) or sense (S) ODNs (to Hck)-treated groups to the IL-13-treated control (*, p < 0.004). In G, the cells were harvested and lysed. 50 μg of the postnuclear lysates was resolved by SDS-PAGE, and 15-LO protein expression was detected on Western blots with a 15-LO-specific antibody (upper panel of G). The 15-LO blot was stripped and reprobed with an antibody against β-tubulin (lower panel of G) to assess equal loading. The results are representative of three independent experiments.
Hck Regulates IL-13 Induction of p38 MAPK Activation and 15-LO Expression
To further test the functional role of Hck, we next investigated the necessity of this specific member of the Src family PTKs for the IL-13-induced signaling pathways leading to expression of 15-LO. We examined the effect of blocking Hck expression by using antisense ODNs on both p38 MAPK activation and 15-LO gene expression. This previously published antisense ODN sequence was reported to be effective against the Src kinase isoform Hck (43). Representative results of the effect of antisense ODN treatment on the expression of Hck are shown in Fig. 7D (upper panel). As previously reported (43) antisense ODN was shown to selectively inhibit Hck protein expression, without affecting the expression of β-tubulin. When normalized for sample loading, as determined by the β-tubulin blot (Fig. 7D, lower panel) the antisense ODN caused ∼10% inhibition of Hck protein expression at 5 μm compared with the sense control, whereas at 10 μm concentration it caused ∼70% inhibition of Hck protein expression (Fig. 7D, upper panel). Treatment with Hck sense ODN did not inhibit Hck protein expression (Fig. 7D, upper panel).
To investigate whether Hck is directly controlling p38 MAPK phosphorylation/activation, we treated cells with Hck antisense ODNs (10 μm) for 48 h and then stimulated with IL-13 for 15 min. Postnuclear extracts were prepared and directly immunoblotted with phospho-p38 MAPK (Thr-180/Tyr-182) antibody (Fig. 7E, first panel). Our results (Fig. 7E, first panel) indicate that down-regulation of Hck expression by treatment with Hck-specific antisense ODN also abrogated IL-13-induced p38 MAPK phosphorylation in a significant manner (p < 0.002). These results are consistent with our previous finding indicating a regulatory role of Src kinases on p38 MAPK phosphorylation in alternatively activated monocytes/macrophages.
To demonstrate the specific role of Hck in regulating p38 MAPK phosphorylation/activation, we further checked the effect of Hck antisense ODNs on ERK1/2 phosphorylation/activation by performing a similar experiment in IL-13-induced monocytes. Our results (Fig. 7E, third panel) indicate that down-regulation of Hck expression by treatment with Hck-specific antisense ODNs has no inhibitory effect on IL-13-stimulated ERK1/2 MAPK phosphorylation/activation. These results thus confirm that Hck is not required for IL-13-mediated activation of ERK1/2 and selectively required for p38 MAPK-mediated regulation of 15-LO expression in IL-13-treated primary human monocytes.
To test the role of Hck in regulating 15-LO gene expression, we treated cells with the Hck-specific antisense or control sense ODN for 48 h. After treatment, the cells were exposed to IL-13 for another 24 h to determine the effect of Hck antisense treatment on 15-LO gene expression (Fig. 7, F and G). Total cellular RNA or proteins were extracted for real-time RT-PCR or Western blot analysis, respectively. Our results indicate that the Hck-specific antisense ODN significantly inhibited the IL-13-mediated induction of 15-LO mRNA expression (Fig. 7F) (*, p < 0.004) and protein expression (Fig. 7G) (∼73%), whereas the sense ODN had no inhibitory effect on 15-LO gene expression (Fig. 7, F and G). Our results thus clearly establish that depriving monocytes of the Src PTK family member Hck blocks IL-13-mediated signaling to 15-LO gene expression.
Expression of M2 Genes Like MAO-A and CD36 Is Controlled by Src Kinase Activation in IL-13-stimulated Monocytes
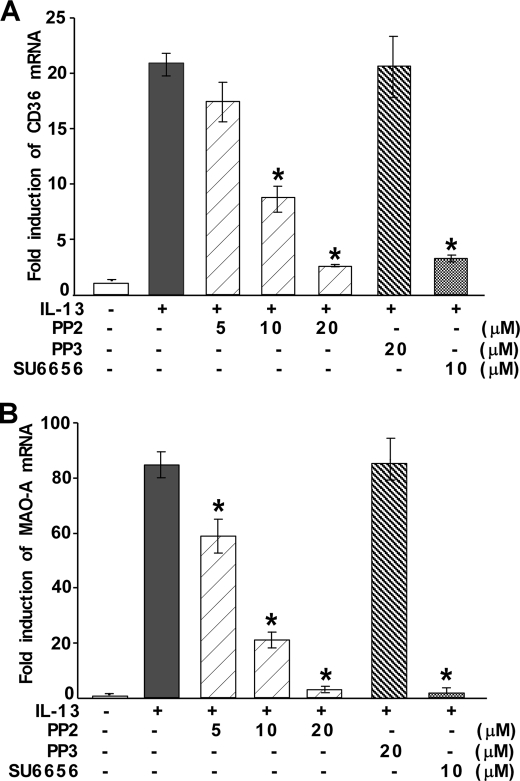
To explore the possible role of Src kinase in regulating the expression of alternative state (M2)-specific genes (like MAO-A and CD36) other than 15-LO, monocytes were pretreated with PP2 or SU6656 (Fig. 8, A and B) at several indicated doses for 30 min and then incubated with IL-13 for an additional 24 h. The results of our quantitative real-time PCR experiment showed that both CD36 and MAO-A mRNA are induced after IL-13 stimulation (Fig. 8, A and B). Pretreatment with PP2 (10–20 μm) and SU6656 (10 μm) caused significant down-regulation of both MAO-A and CD36 mRNA levels (*, p < 0.001) in IL-13-induced monocytes (Fig. 8, A and B). In contrast, the inactive structural analog PP3 showed no inhibition of IL-13-mediated induction of either MAO-A or CD36 mRNA (Fig. 8, A and B). These results thus indicate that IL-13-induced activation of Src kinase is essential for the expression of M2 genes like MAO-A and CD36.
FIGURE 8.
Src kinase activity is required for IL-13-induced expression of CD36 and MAO-A. Human monocytes (5 × 106/group) (A and B) were either directly treated with IL-13 for 24 h or pretreated with Src kinase inhibitors SU6656 and PP2 or its inactive structural analog PP3 for 30 min at various indicated doses followed by IL-13 treatment for 24 h. Total cellular RNA extracts were prepared and subjected to quantitative real-time PCR analysis. After normalization with GAPDH, the -fold induction of CD36 (A) and MAO-A (B) mRNA for different groups was plotted. Data are representative of three independent experiments and shown as the means ± S.D. (n = 3). Significant differences for both the experiments were determined by comparing SU6656-, PP2-, and PP3-treated groups to the IL-13-treated control (*, p < 0.001).
The Src Kinase Hck Is Required for the Expression of Other Alternative State (M2)-specific Genes
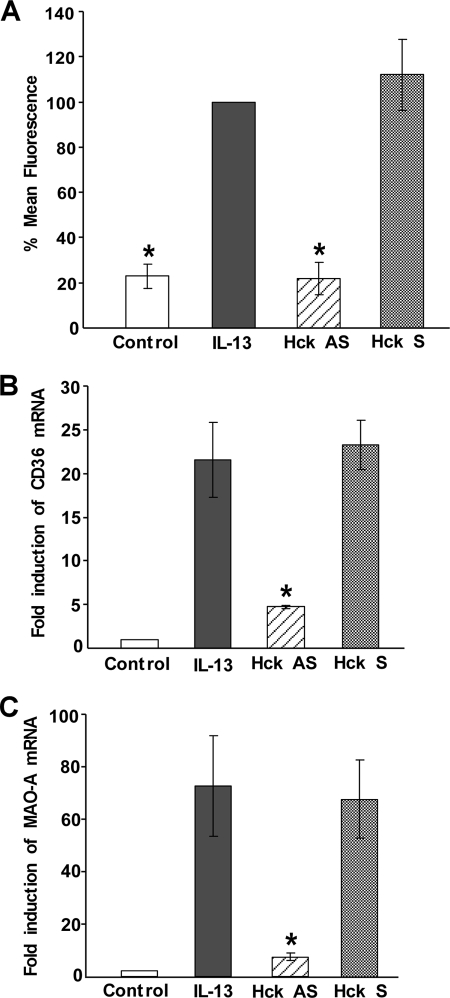
To further investigate the importance of Hck as a key regulator of alternative monocyte activation, we first analyzed the effect of Hck-specific antisense ODN treatment on the expression of MR, a universal marker of alternative monocyte/macrophage activation (2, 36, 44). For this purpose monocytes were pretreated with Hck-specific antisense and sense ODNs before stimulation with IL-13. After 5 days of incubation, the expression of MR was analyzed by FACS. MR expression was remarkably enhanced (∼75%) after IL-13 stimulation (Fig. 9A). Hck-specific antisense ODN significantly reduced the IL-13-mediated up-regulation of the MR expression (*, p < 0.005) (Fig. 9A), whereas the sense ODN had no inhibitory effect on the MR expression level (Fig. 9A).
FIGURE 9.
Hck regulates the expression of mannose receptor, CD36, and MAO-A in alternatively activated monocytes by IL-13. In A, human monocytes (5 × 106/group) were either incubated for 5 days in the presence or absence of IL-13 (2 nm) or pre-treated with Hck-specific antisense (AS) or sense (S) ODNs at 10 μm for 48 h prior to the addition of IL-13 (2 nm) for 5 days and then re-fed with the same antisense and sense after 24 h of IL-13 treatment. After 5 days of IL-13 stimulation, the expression of mannose receptor was evaluated by FACS. Data are mean ± S.E. of three independent experiments. Significant differences were determined by comparing each group to the IL-13-treated group (mean fluorescence was considered as 100%) as the control (*, p < 0.005). In B and C, monocytes (5 × 106/group) were pre-treated with Hck-specific antisense or sense ODNs at 10 μm for 48 h prior to the addition of IL-13 (2 nm) for 24 h. Total cellular RNA extracts were prepared and subjected to quantitative real-time PCR analysis. After normalization with GAPDH, the -fold induction of CD36 (B) and MAO-A (C) mRNA for different groups was plotted. Data are the means ± S.D. (n = 3). Significant differences for both the experiments were determined by comparing the antisense or sense ODNs (to Hck)-treated groups to the IL-13-stimulated control (*, p < 0.015).
Using the same Hck-specific antisense ODN, we further checked whether Hck had any effect in regulation of other markers of alternative activation like CD36 (36, 44, 45) or MAO-A (2, 46, 47). Our quantitative real-time PCR experiment data showed that the levels of both CD36 and MAO-A mRNA are up-regulated after IL-13 stimulation (Fig. 9, B and C). Hck-specific antisense ODN treatment profoundly down-regulated the IL-13-stimulated mRNA expression of CD36 and MAO-A (*, p < 0.015), the two alternative state (M2)-specific genes, whereas the sense ODN had no inhibitory effect (Fig. 9, B and C). These results thus demonstrate that activation of Hck by IL-13 is not limited to the regulation of 15-LO pathway, but rather has a common regulatory role on the alternative activation of monocytes/macrophages.
To examine whether Hck is also involved in the regulation of alternative activation of monocytes by IL-4, we checked the role of Hck in regulating IL-4-induced 15-LO expression. We followed the same antisense approach as described above and treated the cells with the Hck-specific antisense or control sense ODN as mentioned previously. After treatment, the cells were exposed to IL-4 for another 24 h to determine the effect of Hck antisense treatment on 15-LO mRNA expression (supplemental Fig. S4). Results from our real-time quantitative PCR experiment indicated that the Hck-specific antisense ODN significantly inhibited the IL-4-mediated induction of 15-LO mRNA expression (supplemental Fig. S4) (*, p < 0.008), whereas the sense ODN control had no effect on IL-4-stimulated 15-LO mRNA expression (supplemental Fig. S4). These data thus suggest that the proposed mechanism is also true for IL-4-stimulated monocytes and, therefore, is a general effect on the alternative activation pathway. In the model presented in Fig. 10, we show the requisite role for Hck-MKK3/6-p38 MAPK-dependent signaling cascade for regulating both Stat1 and Stat3 serine phosphorylation and Stat1/3-mediated M2 (15-LO, MR, MAO-A, and CD36) gene expression in alternatively activated monocytes/macrophages.
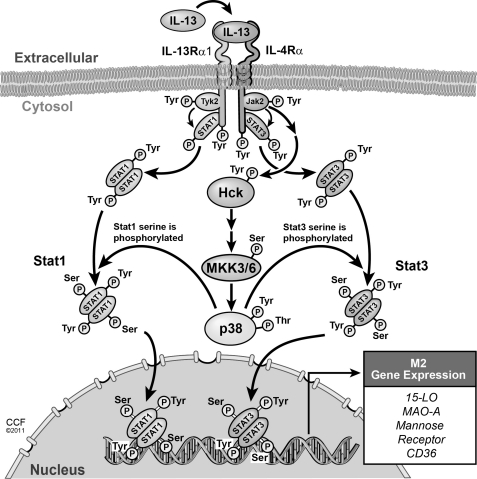
FIGURE 10.
Proposed model shows the requirement of the IL-4Rα-Jak2-mediated signaling pathway for IL-13-induced M2 gene expression in human monocytes/macrophages. IL-13 stimulates the Src kinase Hck via the IL-4Rα-Jak2-dependent signaling pathway leading to the activation of the Hck-MKK3/6-p38 MAPK signaling cascade. Consequently Stat1 and Stat3 are maximally activated, and gene expression of 15-LO, mannose receptor, MAO-A, and CD36 is induced.
DISCUSSION
Src cytoplasmic tyrosine kinases have been intensively studied for many years, either in the form of viral Src (v-Src), encoded by the v-src transforming gene of Rous Sarcoma Virus, or in the form of cellular Src (c-Src), encoded by the c-src gene, the cellular homolog or proto-oncogene form of oncogenic v-src. Initially studied because of the ability of v-src to cause cell transformation, Src kinases have more recently been implicated in many signaling pathways in immune cells such as growth, differentiation, and gene transcription (48). In non-transformed, normal cells Src is not activated without stimulation but mutations such as in transforming v-src contribute to constitutive enzyme activation.
Src family PTKs have been shown to be critical factors for regulating the signal transduction pathways in response to several monocyte/macrophage-activating factors (49–51). Earlier reports indicated the catalytic activation/tyrosine phosphorylation of p59 Hck in human monocytes in response to IL-2 (42). In this study we show that IL-13-stimulated activation of Src family kinases at the autophosphorylation site (Tyr-418) is a unique early signaling event in peripheral blood monocytes. In this report we focus on the contributions of Src family tyrosine kinases to the IL-13-driven alternative M2 macrophage activation and report that Src is a critical regulator of IL-13-stimulated p38 MAPK phosphorylation/activation, Stat1 and Stat3 serine phosphorylation, and downstream 15-LO expression. We further identify Hck as the Src isoform involved in these processes. Our results thus provide evidence that Hck is a major signaling kinase in alternatively activated human monocytes/macrophages by IL-13 and implicate a central role for Src family kinases in atherogenesis and chronic inflammation.
The Src family consists of nine members. Among them Hck, Fgr, and Lyn are the predominant Src PTKs in circulating monocytes and macrophages (42, 52, 53). Increased Hck gene expression has been reported in LPS-stimulated human peripheral blood monocyte-derived macrophages (50). Furthermore, a combination of LPS and IFNγ induced enhanced expression of Hck and Lyn in murine bone marrow-derived macrophages (51). The present study is the first report of Hck phosphorylation/activation downstream of IL-13, an initiator of alternative activation. Using the Hck-specific antisense ODN, we further confirmed the role of Hck as the specific isoform of the Src PTK family regulating both p38 MAPK phosphorylation/activation and p38 MAPK-mediated 15-LO expression in alternative activation of monocytes/macrophages by IL-13. This is a unique feature of alternative M2 macrophage activation and explores novel pathways by which the Th2 cell cytokine IL-13 mediates its effect on monocyte/macrophage cell biology.
Although it is difficult to establish a consensus set of markers for alternative activation of monocytes/macrophages in humans, genes that are regulated by IL-4 and IL-13 include mannose receptor (MR) (36, 44), Alox 15 (encodes 12/15-lipoxygenase) (3, 4, 47), MAO-A (46, 47), CD36 (36, 44, 45), and FN1 (44, 47) among others. Our results demonstrate the important role of Hck in modulating the expression of the MR, a well established, universal marker of alternative activation. We further show that Hck controls the expression of CD36 and MAO-A, two other markers in alternatively activated monocytes/macrophages in human. These observations thus indicate Hck as critical for regulating alternative activation of monocytes/macrophages.
Previous studies have identified p38 MAPK as downstream target of Src signaling in various cell types (24, 28, 29, 37, 54); however, this is the first report, to our knowledge, that a member of the Src family kinases is required for p38 MAPK phosphorylation/activation in alternatively activated monocytes. PKCδ activation and tyrosine phosphorylation have also been reported to be dependent on the activity of Src PTKs in various systems (31, 32, 55–57). Although IL-13-dependent expression of 15-LO requires the activation of both of these kinases in human monocytes, our results show that Src kinases regulate the activation of p38 MAPK and not PKCδ. Moreover, we determined that Src kinase-dependent activation of p38 MAPK is mediated by the MAPK kinase family members, MKK3/6, the two main upstream activators of p38 MAPK (58–61).
Previously we demonstrated that IL-13 induction of 15-LO expression in human monocytes requires tyrosine phosphorylation/activation of Jak2 and Tyk2 (5). Our results further showed that Jak2 and Tyk2 are associated with IL-4Rα and IL-13Rα1, respectively, in the heterodimeric IL-13 receptor complex (13). The results of our Jak2/Tyk2 antisense experiment clearly indicate the existence of an IL-4Rα-Jak2-Src-p38 MAPK-dependent signaling pathway in primary monocytes, which acts in parallel with the IL-13Rα1-Tyk2-ERK1/2-dependent cascade to regulate the 15-LO gene expression in monocytes in response to IL-13 stimulation (14). Because the IL-4Rα-Jak2-Src-dependent signaling pathway does not regulate the activation of PKCδ in IL-13-treated monocytes, it is highly probable that the parallel and requisite IL-13Rα1-Tyk2-mediated signaling cascade is involved in the activation of PKCδ by IL-13.
Src kinases can regulate the activation of a variety of transcription factors, including Stats (33–35, 62, 63). Activation of Src kinases can lead to the activation of Stat1 (35), Stat3 (33–35), and Stat5 (64, 65), which is reflected by the phosphorylation of those Stats on specific tyrosine and serine residues. When Src kinases become activated, they interact with receptor-associated tyrosine kinases (Jaks) (63, 66, 67) and other intracellular signaling pathways like MAPKs (24–30, 38), which phosphorylate Stat1 and/or Stat3 on the Ser-727 residue to provide them with optimal transcriptional activity (15, 16). Our previous results indicated the requirement for both Stat3 Tyr-705 and Ser-727 phosphorylation for maximal Stat3 DNA binding as well as in IL-13-induced 15-LO expression in primary human monocytes (16). Here we demonstrate that the activation of a Src kinase family member induces both Stat1 and Stat3 DNA-binding activity by stimulating the serine phosphorylation of both Stat1 and Stat3 in primary monocytes. The Src kinase Hck has been implicated previously in regulating Stat3 tyrosine phosphorylation in Sf9 insect cells (68). In contrast we observe no Hck regulation of Stat3 tyrosine phosphorylation, but instead it regulates the secondary serine 727 phosphorylation of both Stat1 and Stat3 in a p38 MAPK-dependent pathway. This is a novel regulatory role for Hck in the IL-13-mediated activation pathway.
In the present study we show the requirement of Src-MKK3/6-dependent activation of p38 MAPK in regulating the process of both Stat1 and Stat3 serine 727 phosphorylation after IL-13 stimulation (Fig. 10 and supplemental Fig. S5). Previously, we efficiently transfected primary monocytes with the phosphomutants of Stat3 and demonstrated the requirement of both tyrosine and serine phosphorylation of Stat3 in regulating IL-13-induced expression of 15-LO (16). Ongoing research from our laboratory is therefore focused on investigating the mechanism of p38 MAPK-mediated serine phosphorylation of Stat1 in greater detail and to explore the role of Stat1 (using phosphomutant constructs) as a transcription factor in IL-13-induced 15-LO expression; by performing similar studies to those conducted for Stat3 (16).
In summary, we have elucidated a novel pathway for regulating 15-LO expression in IL-13-driven alternatively activated monocytes/macrophages. Our results show the involvement of a specific Src PTK family member Hck as a downstream target from the IL-13 receptor and an upstream regulator of p38 MAPK activation. We further show the existence of a Src-MKK3/6-p38 MAPK-mediated signaling pathway in controlling the process of 15-LO induction in monocytes/macrophages. In addition, we identify Hck as the master regulatory component in modulating a panel of other alternative state (M2)-specific markers. Thus our results demonstrate for the first time, a central role for the Src kinase Hck in regulating alternative activation of monocytes/macrophages. In Fig. 10 and in supplemental Fig. S5, we present schematic models of the IL-13 signaling machinery in alternatively activated monocytes/macrophages leading to 15-LO expression. In these models we suggest bifurcating pathways downstream of the heterodimeric IL-13 receptor complex. In one pathway IL-13 stimulates Stat3 tyrosine phosphorylation by the IL-4Rα-Jak2-mediated signaling and in the other Stat1 is tyrosine phosphorylated by the IL-13Rα1-Tyk2-dependent signaling cascade.4 Src-MKK3/6-mediated regulation of p38 MAPK activation is required for both Stat3 and Stat1 serine phosphorylation. Stat3 serine phosphorylation is a critical regulatory step in 15-LO gene transcription and requires the formation of a signaling complex (signalosome) containing PKCδ, tyrosine-phosphorylated Stat3, and p38 MAPK (16).4 These studies reveal unexplored mechanisms of IL-13 signaling and provide deeper insights into the generation of alternatively activated monocytes/macrophages.
Supplementary Material
Acknowledgments
We thank Meenakshi Shukla for providing freshly isolated monocytes for this study. We also acknowledge Dr. Valentin Yakubenko for his help in performing the FACS analysis. We are grateful to Suman Kundu, Dr. Anny Mulya, and Dr. Linda Hsi for their help during the preparation of this report. Authors are also thankful to David Schumick (Center for medical art and photography, Cleveland Clinic) for preparing the models.
This work was supported, in whole or in part, by National Institutes of Health Grants HL051068 and HL087018 (to M. K. C.). This work was also supported by the National Center for Research Resources (Grant Center for Translational Science Activities 1UL1RR024989).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
A. Bhattacharjee and M. K. Cathcart, unpublished observation.
- 15-LO
- 15-lipoxygenase
- ODN
- oligodeoxyribonucleotide
- PTK
- protein-tyrosine kinase
- MKK
- MAPK kinase
- Hck
- hematopoietic cell kinase
- MR
- mannose receptor
- IP
- immunoprecipitation
- v-Src
- viral Src
- BCS
- bovine calf serum
- PP2
- 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- PP3
- 4-amino-7-phenylpyrazol[3,4-d]pyrimidine.
REFERENCES
- 1. Gordon S. (2003) Nat. Rev. Immunol. 3, 23–35 [DOI] [PubMed] [Google Scholar]
- 2. Gordon S., Martinez F. O. (2010) Immunity 32, 593–604 [DOI] [PubMed] [Google Scholar]
- 3. Conrad D. J., Kuhn H., Mulkins M., Highland E., Sigal E. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nassar G. M., Morrow J. D., Roberts L. J., 2nd, Lakkis F. G., Badr K. F. (1994) J. Biol. Chem. 269, 27631–27634 [PubMed] [Google Scholar]
- 5. Roy B., Cathcart M. K. (1998) J. Biol. Chem. 273, 32023–32029 [DOI] [PubMed] [Google Scholar]
- 6. Cathcart M. K., Folcik V. A. (2000) Free Radic. Biol. Med. 28, 1726–1734 [DOI] [PubMed] [Google Scholar]
- 7. Brooks C. J., Steel G., Gilbert J. D., Harland W. A. (1971) Atherosclerosis 13, 223–237 [DOI] [PubMed] [Google Scholar]
- 8. Folcik V. A., Nivar-Aristy R. A., Krajewski L. P., Cathcart M. K. (1995) J. Clin. Invest. 96, 504–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harland W. A., Gilbert J. D., Steel G., Brooks C. J. (1971) Atherosclerosis 13, 239–246 [DOI] [PubMed] [Google Scholar]
- 10. Kuhn H., Walther M., Kuban R. J. (2002) Prostaglandins Other Lipid Mediat. 68–69, 263–290 [DOI] [PubMed] [Google Scholar]
- 11. Klein R. F., Allard J., Avnur Z., Nikolcheva T., Rotstein D., Carlos A. S., Shea M., Waters R. V., Belknap J. K., Peltz G., Orwoll E. S. (2004) Science 303, 229–232 [DOI] [PubMed] [Google Scholar]
- 12. Kayama Y., Minamino T., Toko H., Sakamoto M., Shimizu I., Takahashi H., Okada S., Tateno K., Moriya J., Yokoyama M., Nojima A., Yoshimura M., Egashira K., Aburatani H., Komuro I. (2009) J. Exp. Med. 206, 1565–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roy B., Bhattacharjee A., Xu B., Ford D., Maizel A. L., Cathcart M. K. (2002) J. Leukoc Biol. 72, 580–589 [PubMed] [Google Scholar]
- 14. Bhattacharjee A., Mulya A., Pal S., Roy B., Feldman G. M., Cathcart M. K. (2010) J. Immunol. 185, 5211–5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu B., Bhattacharjee A., Roy B., Xu H. M., Anthony D., Frank D. A., Feldman G. M., Cathcart M. K. (2003) Mol. Cell Biol. 23, 3918–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhattacharjee A., Xu B., Frank D. A., Feldman G. M., Cathcart M. K. (2006) J. Immunol. 177, 3771–3781 [DOI] [PubMed] [Google Scholar]
- 17. Alexander M., Daniel T., Chaudry I. H., Schwacha M. G. (2004) J. Cell Physiol. 201, 35–44 [DOI] [PubMed] [Google Scholar]
- 18. Chen X. L., Xia Z. F., Wei D., Han S., Ben D. F., Wang G. Q. (2003) Burns 29, 533–539 [DOI] [PubMed] [Google Scholar]
- 19. Maung A. A., Fujimi S., Miller M. L., MacConmara M. P., Mannick J. A., Lederer J. A. (2005) J. Leukoc. Biol. 78, 565–573 [DOI] [PubMed] [Google Scholar]
- 20. McCloskey C. A., Kameneva M. V., Uryash A., Gallo D. J., Billiar T. R. (2004) Shock 22, 380–386 [DOI] [PubMed] [Google Scholar]
- 21. Murr M. M., Yang J., Fier A., Gallagher S. F., Carter G., Gower W. R., Jr., Norman J. G. (2003) J. Gastrointest. Surg 7, 20–25 [DOI] [PubMed] [Google Scholar]
- 22. Abe J. (2007) J. Mol. Cell. Cardiol. 43, 404–408 [DOI] [PubMed] [Google Scholar]
- 23. Poole D. P., Matsuyama H., Nguyen T. V., Eriksson E. M., Fowler C. J., Furness J. B. (2007) Gastroenterology 133, 1229–1239 [DOI] [PubMed] [Google Scholar]
- 24. Thobe B. M., Frink M., Choudhry M. A., Schwacha M. G., Bland K. I., Chaudry I. H. (2006) Am. J. Physiol. Cell Physiol. 291, C476-C482 [DOI] [PubMed] [Google Scholar]
- 25. Lim M. J., Seo Y. H., Choi K. J., Cho C. H., Kim B. S., Kim Y. H., Lee J., Lee H., Jung C. Y., Ha J., Kang I., Kim S. S. (2007) Arch. Biochem. Biophys. 465, 197–208 [DOI] [PubMed] [Google Scholar]
- 26. Touyz R. M., He G., Wu X. H., Park J. B., Mabrouk M. E., Schiffrin E. L. (2001) Hypertension 38, 56–64 [DOI] [PubMed] [Google Scholar]
- 27. Ishida M., Ishida T., Thomas S. M., Berk B. C. (1998) Circ. Res. 82, 7–12 [DOI] [PubMed] [Google Scholar]
- 28. Callera G. E., Touyz R. M., Tostes R. C., Yogi A., He Y., Malkinson S., Schiffrin E. L. (2005) Hypertension 45, 773–779 [DOI] [PubMed] [Google Scholar]
- 29. Frey M. R., Golovin A., Polk D. B. (2004) J. Biol. Chem. 279, 44513–44521 [DOI] [PubMed] [Google Scholar]
- 30. Kyaw M., Yoshizumi M., Tsuchiya K., Kagami S., Izawa Y., Fujita Y., Ali N., Kanematsu Y., Toida K., Ishimura K., Tamaki T. (2004) Mol. Pharmacol. 65, 832–841 [DOI] [PubMed] [Google Scholar]
- 31. Rosenzweig T., Aga-Mizrachi S., Bak A., Sampson S. R. (2004) Cell Signal. 16, 1299–1308 [DOI] [PubMed] [Google Scholar]
- 32. Gschwendt M., Kielbassa K., Kittstein W., Marks F. (1994) FEBS Lett. 347, 85–89 [DOI] [PubMed] [Google Scholar]
- 33. Yeh M., Gharavi N. M., Choi J., Hsieh X., Reed E., Mouillesseaux K. P., Cole A. L., Reddy S. T., Berliner J. A. (2004) J. Biol. Chem. 279, 30175–30181 [DOI] [PubMed] [Google Scholar]
- 34. Norkina O., Dolganiuc A., Shapiro T., Kodys K., Mandrekar P., Szabo G. (2007) J. Leukoc. Biol. 82, 752–762 [DOI] [PubMed] [Google Scholar]
- 35. Cirri P., Chiarugi P., Marra F., Raugei G., Camici G., Manao G., Ramponi G. (1997) Biochem. Biophys. Res. Commun. 239, 493–497 [DOI] [PubMed] [Google Scholar]
- 36. Yakubenko V. P., Bhattacharjee A., Pluskota E., Cathcart M. K. (2011) Circ. Res. 108, 544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yogi A., Callera G. E., Montezano A. C., Aranha A. B., Tostes R. C., Schiffrin E. L., Touyz R. M. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 1960–1967 [DOI] [PubMed] [Google Scholar]
- 38. McMullen M., Keller R., Sussman M., Pumiglia K. (2004) Oncogene 23, 1275–1282 [DOI] [PubMed] [Google Scholar]
- 39. Cuenda A., Rousseau S. (2007) Biochim. Biophys. Acta 1773, 1358–1375 [DOI] [PubMed] [Google Scholar]
- 40. Raingeaud J., Gupta S., Rogers J. S., Dickens M., Han J., Ulevitch R. J., Davis R. J. (1995) J. Biol. Chem. 270, 7420–7426 [DOI] [PubMed] [Google Scholar]
- 41. Musso T., Johnston J. A., Linnekin D., Varesio L., Rowe T. K., O'Shea J. J., McVicar D. W. (1995) J. Exp. Med. 181, 1425–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bosco M. C., Curiel R. E., Zea A. H., Malabarba M. G., Ortaldo J. R., Espinoza-Delgado I. (2000) J. Immunol. 164, 4575–4585 [DOI] [PubMed] [Google Scholar]
- 43. English B. K., Ihle J. N., Myracle A., Yi T. (1993) J. Exp. Med. 178, 1017–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martinez F. O., Gordon S., Locati M., Mantovani A. (2006) J. Immunol. 177, 7303–7311 [DOI] [PubMed] [Google Scholar]
- 45. Berry A., Balard P., Coste A., Olagnier D., Lagane C., Authier H., Benoit-Vical F., Lepert J. C., Séguéla J. P., Magnaval J. F., Chambon P., Metzger D., Desvergne B., Wahli W., Auwerx J., Pipy B. (2007) Eur. J. Immunol. 37, 1642–1652 [DOI] [PubMed] [Google Scholar]
- 46. Chaitidis P., Billett E. E., O'Donnell V. B., Fajardo A. B., Fitzgerald J., Kuban R. J., Ungethuem U., Kühn H. (2004) J. Immunol. 173, 4821–4827 [DOI] [PubMed] [Google Scholar]
- 47. Chaitidis P., O'Donnell V., Kuban R. J., Bermudez-Fajardo A., Ungethuem U., Kühn H. (2005) Cytokine 30, 366–377 [DOI] [PubMed] [Google Scholar]
- 48. Roskoski R., Jr. (2005) Biochem. Biophys. Res. Commun. 331, 1–14 [DOI] [PubMed] [Google Scholar]
- 49. Ghazizadeh S., Fleit H. B. (1994) J. Immunol. 152, 30–41 [PubMed] [Google Scholar]
- 50. Ziegler S. F., Wilson C. B., Perlmutter R. M. (1988) J. Exp. Med. 168, 1801–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boulet I., Ralph S., Stanley E., Lock P., Dunn A. R., Green S. P., Phillips W. A. (1992) Oncogene 7, 703–710 [PubMed] [Google Scholar]
- 52. Lowell C. A. (2004) Mol. Immunol. 41, 631–643 [DOI] [PubMed] [Google Scholar]
- 53. Okutani D., Lodyga M., Han B., Liu M. (2006) Am. J. Physiol. Lung Cell Mol. Physiol. 291, L129–141 [DOI] [PubMed] [Google Scholar]
- 54. Khadaroo R. G., Parodo J., Powers K. A., Papia G., Marshall J. C., Kapus A., Rotstein O. D. (2003) Surgery 134, 242–246 [DOI] [PubMed] [Google Scholar]
- 55. Denning M. F., Dlugosz A. A., Threadgill D. W., Magnuson T., Yuspa S. H. (1996) J. Biol. Chem. 271, 5325–5331 [DOI] [PubMed] [Google Scholar]
- 56. Benes C., Soltoff S. P. (2001) Am. J. Physiol. Cell Physiol. 280, C1498-C1510 [DOI] [PubMed] [Google Scholar]
- 57. Kronfeld I., Kazimirsky G., Lorenzo P. S., Garfield S. H., Blumberg P. M., Brodie C. (2000) J. Biol. Chem. 275, 35491–35498 [DOI] [PubMed] [Google Scholar]
- 58. Moriguchi T., Kuroyanagi N., Yamaguchi K., Gotoh Y., Irie K., Kano T., Shirakabe K., Muro Y., Shibuya H., Matsumoto K., Nishida E., Hagiwara M. (1996) J. Biol. Chem. 271, 13675–13679 [DOI] [PubMed] [Google Scholar]
- 59. Dérijard B., Raingeaud J., Barrett T., Wu I. H., Han J., Ulevitch R. J., Davis R. J. (1995) Science 267, 682–685 [DOI] [PubMed] [Google Scholar]
- 60. Raingeaud J., Whitmarsh A. J., Barrett T., Dérijard B., Davis R. J. (1996) Mol. Cell Biol. 16, 1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chabaud-Riou M., Firestein G. S. (2004) Am. J. Pathol. 164, 177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rane S. G., Reddy E. P. (2002) Oncogene 21, 3334–3358 [DOI] [PubMed] [Google Scholar]
- 63. Gharavi N. M., Alva J. A., Mouillesseaux K. P., Lai C., Yeh M., Yeung W., Johnson J., Szeto W. L., Hong L., Fishbein M., Wei L., Pfeffer L. M., Berliner J. A. (2007) J. Biol. Chem. 282, 31460–31468 [DOI] [PubMed] [Google Scholar]
- 64. Okutani Y., Kitanaka A., Tanaka T., Kamano H., Ohnishi H., Kubota Y., Ishida T., Takahara J. (2001) Oncogene 20, 6643–6650 [DOI] [PubMed] [Google Scholar]
- 65. Klejman A., Schreiner S. J., Nieborowska-Skorska M., Slupianek A., Wilson M., Smithgall T. E., Skorski T. (2002) EMBO J. 21, 5766–5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bhattacharya S., Ray R. M., Johnson L. R. (2006) Biochem. J. 397, 437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Proietti C., Salatino M., Rosemblit C., Carnevale R., Pecci A., Kornblihtt A. R., Molinolo A. A., Frahm I., Charreau E. H., Schillaci R., Elizalde P. V. (2005) Mol. Cell Biol. 25, 4826–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schreiner S. J., Schiavone A. P., Smithgall T. E. (2002) J. Biol. Chem. 277, 45680–45687 [DOI] [PubMed] [Google Scholar]
- 69. Wahl L. M., Katona I. M., Wilder R. L., Winter C. C., Haraoui B., Scher I., Wahl S. M. (1984) Cell Immunol. 85, 373–383 [DOI] [PubMed] [Google Scholar]
- 70. Wahl S. M., Katona I. M., Stadler B. M., Wilder R. L., Helsel W. E., Wahl L. M. (1984) Cell Immunol. 85, 384–395 [DOI] [PubMed] [Google Scholar]
- 71. Rosen R. L., Winestock K. D., Chen G., Liu X., Hennighausen L., Finbloom D. S. (1996) Blood 88, 1206–1214 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.