Abstract
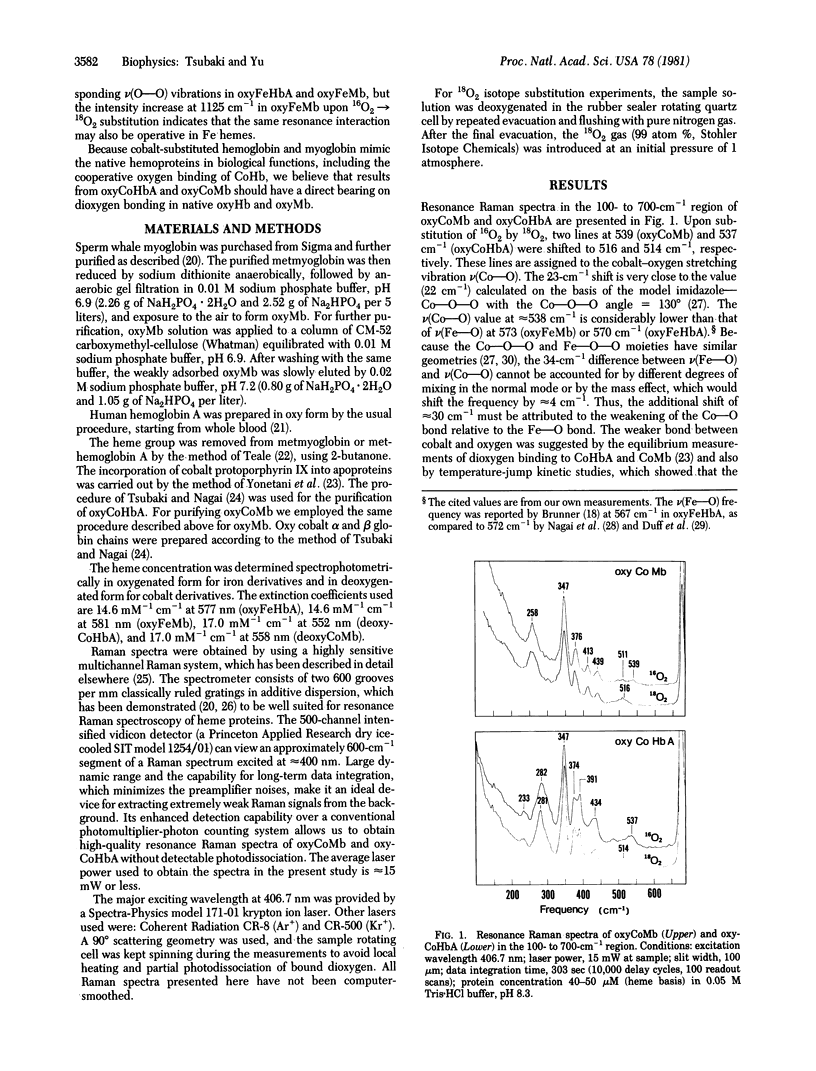
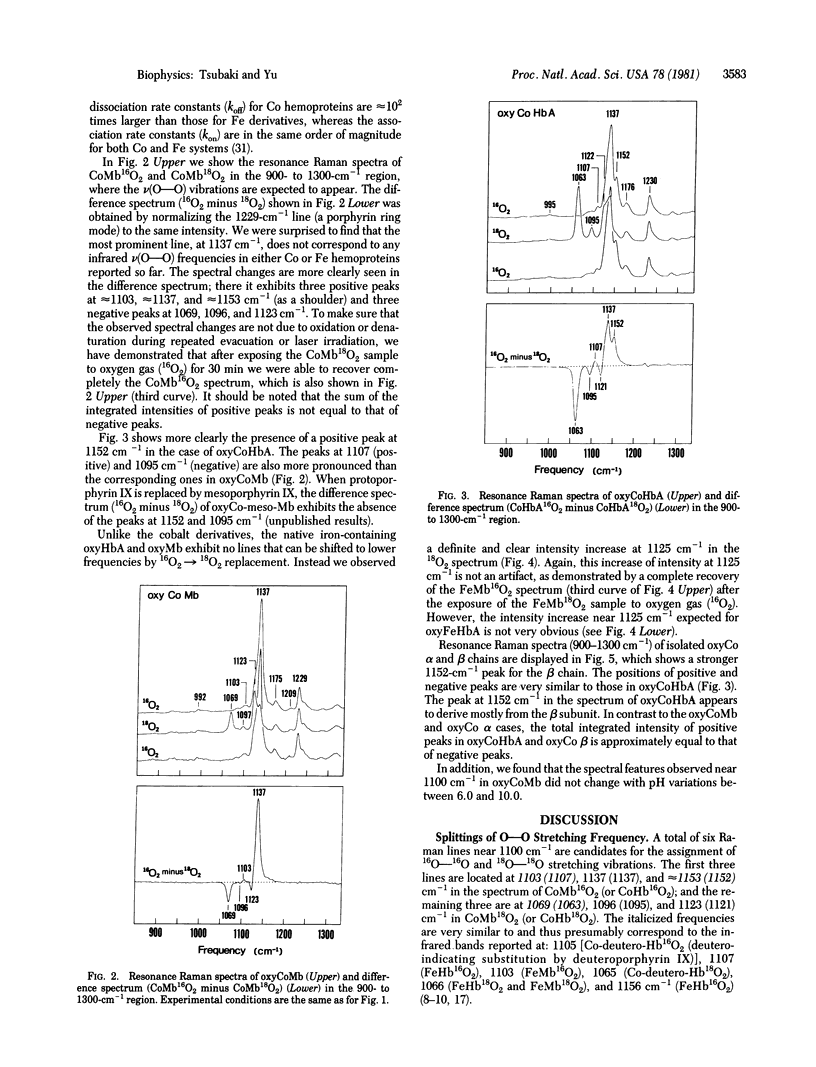
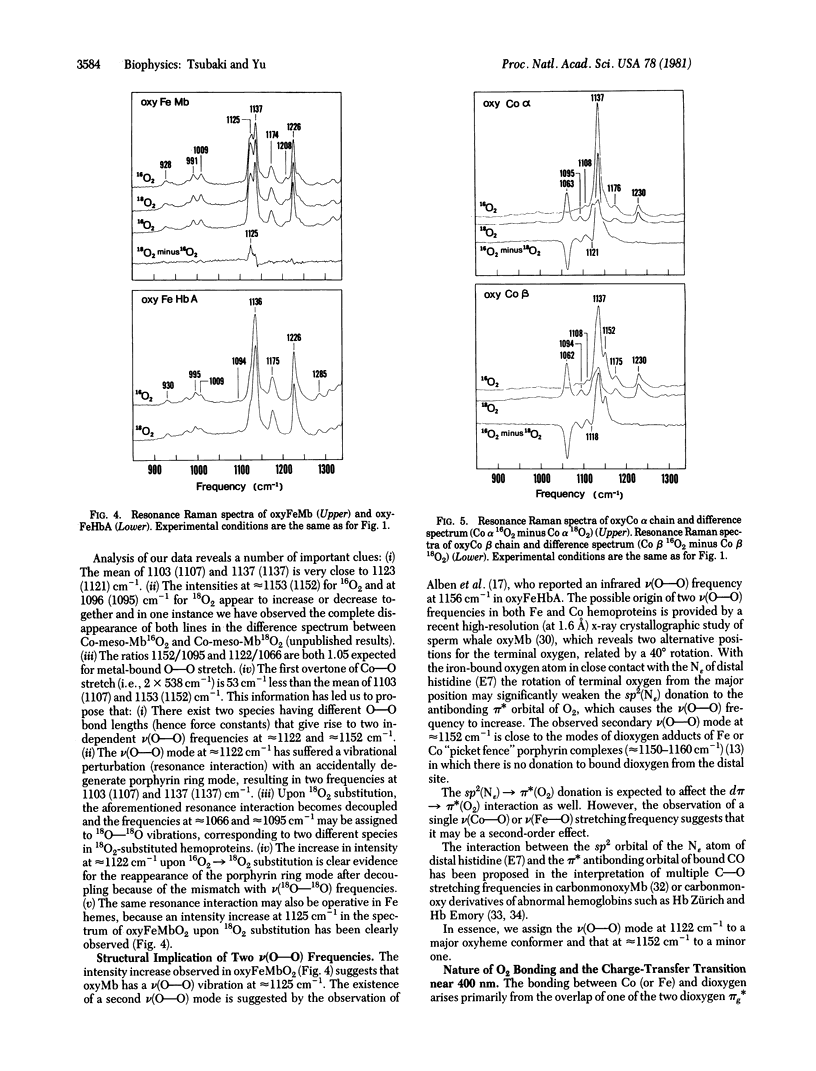
Splittings related to the stretching vibration of bound dioxygen in hemoproteins have been detected by resonance Raman spectroscopy. With excitation at 406.7 nm we observe three isotope-sensitive lines in oxycobaltmyoglobin (oxyCoMb) [or in oxycobalthemoglobin A (oxyCoHbA)] at 1103 (1107), 1137 (1137), and 1153 (1152) cm-1, of which the most intense one appears at 1137 cm-1. The first two frequencies arise from resonance interaction between a v(O--O) mode at approximately 1122 cm-1 and an accidentally degenerate porphyrin ring mode at 1123 (1121) cm-1, whereas the third one represents an "unperturbed" v(O--O) vibration from a different species. These two v(O--O) modes at approximately 1122 and approximately 1153 cm-1 shift to approximately 1066 and approximately 1096 cm-1, respectively, upon 16O2 leads to 18O2 substitution. The same resonance interaction may also occur in oxyFeMb (probably also in oxyFeHb(a), because it exhibits an intensity increase at 1125 cm-1 upon 16O2 leads to 18O2 substitution, although the v(O--O) vibrations have not been observed directly. Concomitant enhancement is observed in the v(Co--O) vibration at 539 (537( cm-1, which is considerably lower than the v(Fe--O) frequency at approximately 570 cm-1 in oxyFeMb and oxyFeHbA. The Co--O bond is longer and weaker than the Fe--O bond. Enhancement of both v(O--O) and v(Co--O) indicates the existence of a charge-transfer transition underlying the Soret band, which may be assigned as pi*(pi g*O2/xz) leads to sigma*(dz2Co/pi g*). The presence of two v(O--O) vibrations (at approximately 1122 and approximately 1152 cm-1) but only one v(Co--O) mode at approximately 538 cm-1) means that the two species in oxyCoMB or oxyCoHbA have the same Co--O bond lengths but different O--O bond lengths. The bound dioxygen in a bent end-on configuration may have two allowed orientations, which differ in the extent of sp2(N epsilon) leads to pi*(O2) donation from distal histidine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow C. H., Maxwell J. C., Wallace W. J., Caughey W. S. Elucidation of the mode of binding of oxygen to iron in oxyhemoglobin by in frared spectroscopy. Biochem Biophys Res Commun. 1973 Nov 1;55(1):91–96. doi: 10.1016/s0006-291x(73)80063-4. [DOI] [PubMed] [Google Scholar]
- Cerdonio M., Congiu-Castellano A., Mogno F., Pispisa B., Romani G. L., Vitale S. Magnetic properties of oxyhemoglobin. Proc Natl Acad Sci U S A. 1977 Feb;74(2):398–400. doi: 10.1073/pnas.74.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Halbert T. R., Suslick K. S. Nature of O2 and CO binding to metalloporphyrins and heme proteins. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3333–3337. doi: 10.1073/pnas.73.10.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff L. L., Appelman E. H., Shriver D. F., Klotz I. M. Steric disposition of O2 in oxyhemoglobin as revealed by its resonance Raman spectrum. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1098–1103. doi: 10.1016/0006-291x(79)91148-3. [DOI] [PubMed] [Google Scholar]
- Eicher H., Trautwein A. Electronic structure and quadrupole splittings of ferrous iron in hemoglobin. J Chem Phys. 1969 Mar 15;50(6):2540–2551. doi: 10.1063/1.1671413. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Mildvan A. S., Yonetani T., Srivastava T. S. EPR study of 17O nuclear hyperfine interaction in cobalt-oxyhemoglobin: conformation of bound oxygen. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1005–1012. doi: 10.1016/0006-291x(75)90774-3. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Hewitt J. A., Wootton J. F. Alteration of functional properties associated with the change in quaternary structure in unliganded haemoglobin. J Mol Biol. 1975 Apr 5;93(2):203–218. doi: 10.1016/0022-2836(75)90128-x. [DOI] [PubMed] [Google Scholar]
- Kirchner R. F., Loew G. H. Semiempirical calculations of model oxyheme: Variation of calculated electromagnetic properties with electronic configuration and oxygen geometry. J Am Chem Soc. 1977 Jul 6;99(14):4639–4647. doi: 10.1021/ja00456a020. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Kyogoku Y., Iizuka T., Saito M. I. Nature of the iron-ligand bond in ferrous low spin hemoproteins studied by resonance Raman scattering. J Am Chem Soc. 1976 Aug 18;98(17):5169–5173. doi: 10.1021/ja00433a019. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Houtchens R. A., Caughey W. S. Structure of carboxymyoglobin in crystals and in solution. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6042–6046. doi: 10.1073/pnas.76.12.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell J. C., Caughey W. S. Infrared evidence for similar metal-dioxygen bonding in iron and cobalt oxyhemoglobins. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1309–1314. doi: 10.1016/0006-291x(74)90340-4. [DOI] [PubMed] [Google Scholar]
- Maxwell J. C., Volpe J. A., Barlow C. H., Caughey W. S. Infrared evidence for the mode of binding of oxygen to iron of myoglobin from heart muscle. Biochem Biophys Res Commun. 1974 May 7;58(1):166–171. doi: 10.1016/0006-291x(74)90906-1. [DOI] [PubMed] [Google Scholar]
- Nagai K., Kitagawa T., Morimoto H. Quaternary structures and low frequency molecular vibrations of haems of deoxy and oxyhaemoglobin studied by resonance raman scattering. J Mol Biol. 1980 Jan 25;136(3):271–289. doi: 10.1016/0022-2836(80)90374-5. [DOI] [PubMed] [Google Scholar]
- Pauling L., Coryell C. D. The Magnetic Properties and Structure of Hemoglobin, Oxyhemoglobin and Carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A. 1936 Apr;22(4):210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. E. Structure and refinement of oxymyoglobin at 1.6 A resolution. J Mol Biol. 1980 Oct 5;142(4):531–554. doi: 10.1016/0022-2836(80)90262-4. [DOI] [PubMed] [Google Scholar]
- Reed C. A., Cheung S. K. On the bonding of FeO2 in hemoglobin and related dioxygen complexes. Proc Natl Acad Sci U S A. 1977 May;74(5):1780–1784. doi: 10.1073/pnas.74.5.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Tsubaki M., Nagai K. Effect of removal of a salt-bridge on the oxygen binding properties and the electronic structure of heme in cobalt-iron hybrid hemoglobin. J Biochem. 1979 Oct;86(4):1029–1035. doi: 10.1093/oxfordjournals.jbchem.a132596. [DOI] [PubMed] [Google Scholar]
- Tsubaki M., Srivastava R. B., Yu N. T. Temperature dependence of resonance Raman spectra of metmyoglobin and methemoglobin azide. Detection of resonance-enhanced bound azide vibrations and iron-azide stretch. Biochemistry. 1981 Feb 17;20(4):946–952. doi: 10.1021/bi00507a047. [DOI] [PubMed] [Google Scholar]
- Tucker P. W., Phillips S. E., Perutz M. F., Houtchens R., Caughey W. S. Structure of hemoglobins Zürich [His E7(63)beta replaced by Arg] and Sydney [Val E11(67)beta replaced by Ala] and role of the distal residues in ligand binding. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1076–1080. doi: 10.1073/pnas.75.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS J. J. NATURE OF THE IRON-OXYGEN BOND IN OXYHAEMOGLOBIN. Nature. 1964 Apr 4;202:83–84. doi: 10.1038/202083b0. [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B., Wittenberg B. A., Peisach J., Blumberg W. E. On the state of the iron and the nature of the ligand in oxyhemoglobin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1846–1853. doi: 10.1073/pnas.67.4.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Kayne F. J., Yonetani T. Studies on cobalt myoglobins and hemoglobins. II. Kinetic studies of reversible oxygenation of cobalt myoglbins and hemoglobins by the temperature jump relaxation method. J Biol Chem. 1974 Feb 10;249(3):691–698. [PubMed] [Google Scholar]
- Yammoto T., Palmer G. The valence and spin state of iron in oxyhemoglobin as inferred from resonance Raman spectroscopy. J Biol Chem. 1973 Jul 25;248(14):5211–5213. [PubMed] [Google Scholar]
- Yonetani T., Yamamoto H., Woodrow G. V., 3rd Studies on cobalt myoglobins and hemoglobins. I. Preparation and optical properties of myoglobins and hemoglobins containing cobalt proto-, meso-, and deuteroporphyrins and thermodynamic characterization of their reversible oxygenation. J Biol Chem. 1974 Feb 10;249(3):682–690. [PubMed] [Google Scholar]
- Yu N. T., Tsubaki M. Resonance Raman spectra of manganese myoglobin and its azide complex. Assignment of a new charge-transfer band to azide (pi) to porphyrin (pi) transition. Biochemistry. 1980 Sep 30;19(20):4647–4653. doi: 10.1021/bi00561a017. [DOI] [PubMed] [Google Scholar]



