Abstract
The future of tissue engineering and cell-based therapies for tissue regeneration will likely rely on our ability to generate functional vascular networks in vivo. In this regard, the search for experimental models to build blood vessel networks in vivo is of utmost importance 1. The feasibility of bioengineering microvascular networks in vivo was first shown using human tissue-derived mature endothelial cells (ECs) 2-4; however, such autologous endothelial cells present problems for wide clinical use, because they are difficult to obtain in sufficient quantities and require harvesting from existing vasculature. These limitations have instigated the search for other sources of ECs. The identification of endothelial colony-forming cells (ECFCs) in blood presented an opportunity to non-invasively obtain ECs 5-7. We and other authors have shown that adult and cord blood-derived ECFCs have the capacity to form functional vascular networks in vivo 7-11. Importantly, these studies have also shown that to obtain stable and durable vascular networks, ECFCs require co-implantation with perivascular cells. The assay we describe here illustrates this concept: we show how human cord blood-derived ECFCs can be combined with bone marrow-derived mesenchymal stem cells (MSCs) as a single cell suspension in a collagen/fibronectin/fibrinogen gel to form a functional human vascular network within 7 days after implantation into an immunodeficient mouse. The presence of human ECFC-lined lumens containing host erythrocytes can be seen throughout the implants indicating not only the formation (de novo) of a vascular network, but also the development of functional anastomoses with the host circulatory system. This murine model of bioengineered human vascular network is ideally suited for studies on the cellular and molecular mechanisms of human vascular network formation and for the development of strategies to vascularize engineered tissues.
Keywords: Bioengineering, Issue 53, vascular network, blood vessel, vasculogenesis, angiogenesis, endothelial progenitor cells, endothelial colony-forming cells, mesenchymal stem cells, collagen gel, fibrin gel, tissue engineering, regenerative medicine
Protocol
1. Preparation
- Make EGM-2 medium (500 mL)
- Add 100 mL of fetal bovine serum (FBS) to 395 mL of Endothelial Basal Medium (EBM-2).
- Add 5 mL of 100x Glutamine-penicillin-streptomycin solution (GPS).
- Add all the EGM-2 SingleQuots supplements except for hydrocortisone (i.e., VEGF, hFGF-B, R-IGF-1, hEGF, Heparin, ascorbic acid, and GA-1000).
- Filter sterilized with a 0.2-μm pore size vacuum filter.
- Make MSCGM medium (500 mL)
- Add 5 mL of 100x GPS to 440 mL of Mesenchymal Stem Cell Basal Medium (MSCBM).
- Add all the content of the MSCGM SingleQuots kit.
- Add hFGF-B aliquot from EGM-2 SingleQuots supplements.
- Filter sterilized with a 0.2-μm pore size vacuum filter.
- Make DMEM medium (500 mL)
- Add 50 mL of fetal bovine serum (FBS) to 440 mL of 1x high glucose Dulbecco's Modified Eagle Medium (DMEM).
- Add 5 mL of 100x GPS.
- Add 5 mL of nonessential amino acid solution.
- Filter sterilized with a 0.2-μm pore size vacuum filter.
- Make collagen/fibronectin solution (3.6 mL; made same day of injection)
- Add 0.4 mL of 10x DMEM to 150 μL of distilled H2O.
- Add 100 μL of 1M HEPES (25 mM final).
- Carefully, add 2.4 mL of bovine collagen (5 mg/mL stock; 3 mg/mL final) and mix gently on ice.
- Adjust the pH to neutral by adding 1N NaOH solution (approximately 30 μL).
- Use phenol red indicator to assess neutral pH; alternative, use pH test paper to monitor pH.
- Add 120 μL of human fibronectin (1 mg/mL stock; 30 μg/mL final)
- Add 0.4 mL of FBS (10% final)
- keep solution on ice until use.
- Make fibrinogen solution (1 mL; made same day of injection)
- Add 30 mg of powdered fibrinogen to 1 mL of 0.9N NaCl (pH 7.4) solution (30 mg/mL final).
- Prior to use, incubate the fibrinogen solution at 37° C for 30 minutes.
- Mix gently, without vortexing, prior to its addition to the collagen/fibronectin gel.
- Make thrombin solution (200 mL)
- Add 1 KU of powdered thrombin to 200 mL of 0.9% NaCl normal saline (50 U/mL).
- Filter sterilized with a 0.2-μm pore size syringe filter and store at -20° C until use.
- Prior to use, dilute with 0.9% NaCl normal saline to a final concentration of 10 U/mL.
- Make 1% gelatin solution (500 mL)
- Add 5 g of powdered gelatin to 500 mL of Dulbecco's phosphate buffered saline (PBS).
- Autoclaved at 121 °C for 30 min.
- Filter sterilized with a 0.2-μm pore size vacuum filter.
- Coat 100-mm tissue culture plates with 1% gelatin coating solution
- Add 10 mL of 1% gelatin solution to each 100-mm tissue culture plate.
- Incubate plates at 37°C for 60 min.
- Prior to use, remove the gelatin solution and wash the plates once with PBS.
2. Culture of Human Cord Blood-Derived Endothelial Colony-Forming Cells (ECFCs)
This protocol assumes frozen vials of ECFC are available in the laboratory before this experiment. ECFC can be isolated from the mononuclear cell fraction of either umbilical cord blood or adult peripheral blood as previously described 7.
Thaw one vial of ECFCs (typically 0.5-1x106 cells in 1 mL of freezing media) taken from the liquid nitrogen storage tank and immediately dilute its content in a 15-mL conical tube containing 10 mL of DMEM medium. Spin at 1200 rpm for 5 min. Remove supernatant and resuspend the cell pellet in 10 mL of warm EGM-2 medium.
Add the 10 mL of ECFC suspension into one 1% gelatin-coated 100-mm tissue culture plate. Place the plate in a humidified incubator at 37°C and 5%CO2. Next day, gently aspirate out unbound cells and medium, and feed bound cells with 10 mL of fresh EGM-2 medium.
Feed the plate every 2-3 days with EGM-2 medium. Allow cells to expand such that the plate is covered by a confluent cellular monolayer. At confluence, subculture the cells as follows:
Aspirate out the culture medium and wash the cells with 10 mL of PBS.
Remove PBS and add 2 mL of trypsin-EDTA solution to each 100-mm plate. Gently rock the plates to evenly distribute the trypsin-EDTA solution. Incubate at 37°C and 5%CO2 for 3-5 minutes. Gently tap the plate to see the detached cells in suspension under an inverted microscope.
When cells completely detach, add 8 mL of EGM-2 medium and collect the cell solution into a 15-mL conical tube. Take 10 μL to count the cells in a haemocytometer and work out the total number of cells harvested.
Plate the cells in 1% gelatin-coated tissue culture plates at a seeding density of 5,000 cell/cm2 using EGM-2 medium. Place the plates in the incubator and feed them every 2-3 days with EGM-2 medium.
Repeat this procedure for subsequent passages. Keep track of the passage number as the cell population is expanded. ECFCs will be used between passages 4-8.
3. Culture of Human Bone Marrow-Derived Mesenchymal Stem Cells (MSCs)
This protocol assumes frozen vials of human MSC are available in the laboratory before this experiment. MSC can be isolated from bone marrow aspirates as previously described 11.
Thaw one vial of MSCs (typically 0.50-1x106 cells in 1 mL freezing media) taken from the liquid nitrogen storage tank and immediately dilute its content in a 15-mL conical tube containing 10 mL of DMEM medium. Spin at 1200 rpm for 5 min. Remove supernatant and resuspend the cell pellet in 10 mL of warm MSCGM medium.
Add the 10 mL of MSC suspension into one uncoated 100-mm tissue culture plate. Place the plate in a humidified incubator at 37°C and 5%CO2. Next day, gently aspirate out unbound cells and medium, and feed bound cells with 10 mL of fresh MSCGM medium.
Feed the plate every 2-3 days with MSCGM medium. Allow cells to expand such that the plate reaches 80% of confluent cellular monolayer. At 80% confluence, subculture the cells as follows:
Aspirate out the culture medium and wash the cells with 10 mL of PBS.
Remove PBS and add 2 mL of trypsin-EDTA solution to each 100-mm plate. Gently rock the plates to evenly distribute the trypsin-EDTA solution. Incubate at 37°C and 5%CO2 for 3-5 minutes. Gently tap the plate to see the detached cells in suspension under an inverted microscope.
When cells completely detach, add 8 mL of MSCGM medium and collect the cell solution into a 15-mL conical tube. Take 10 μL to count the cells in a haemocytometer and work out the total number of cells harvested.
Plate the cells in uncoated tissue culture plates at a seeding density of 10,000 cell/cm2 using MSCGM medium. Place the plates in the incubator and feed them every 2-3 days with MSCGM medium.
Repeat this procedure for subsequent passages. Keep track of the passage number as the cell population is expanded. MSCs will be used between passages 4-8.
4. Resuspension of Cells in Collagen/Fibronectin/Fibrinogen Solution (Day 0)
Prior to the experiment, make sure there are enough ECFCs and MSCs in culture; 0.8x106 ECFCs and 1.2x106 MSCs will be required for each implant and mouse.
Aspirate out the medium of each culture plate and wash the cells with 10 mL of PBS. Remove PBS and add 2 mL of trypsin-EDTA solution to each 100-mm plate. Gently rock the plates to evenly distribute the trypsin-EDTA solution. Incubate for 3-5 minutes. Gently tap the plate to see the detached cells in suspension under an inverted microscope.
When cells completely detach, add 8 mL of DMEM medium and collect the cell solution into a 15-mL conical tube. Take 10 μL to count the cells in a haemocytometer and work out the total number of ECFCs and MSCs harvested.
Transfer 4x106 ECFCs (5x 0.8x106 cells) and 6x106 MPCs (5x 1.2x106 cells) together into a single 50-mL conical tube. This is the total amount of cells required for five individual implants and mice. Centrifuge at 1200 rpm and remove the supernatant.
Gently, add 100 uL of Fibrinogen solution to 0.9 mL of collagen/fibronectin solution (3 mg/mL final fibrinogen concentration); keep the mixture on ice.
Resuspend the cell pellet on 1 mL of ice cold collagen/fibronectin/fibrinogen solution; mix the cells very gently to avoid bubbles. Load the mixture into a 1-mL sterile syringe, and place a 26-gauge needle with its cap on the tip of the syringe. Keep the loaded syringe on ice until injection.
5. Injection into Immunodeficient Nude Mouse (Day 0)
All animal experiments will be carried out with 6-week old athymic nude (nu/nu) mice.
Prior to the injection, anesthetize the immunodeficient mice by placing them into a gas chamber delivering isoflurane. Allow the mice to inhale the isoflurane for approximately 2 minutes until they are anesthetized and unresponsive to toe pinch (monitor their heart beats by inspection).
For each mouse, inject 50 μL of thrombin solution (10 U/mL) subcutaneously into the upper dorsal region using a 26-gauge needle.
In the same place where thrombin was injected, inject 200 μL of the cell mixture using a 26-gauge needle. Collagen will gel at 37°C and fibrinogen will form fibrin gel in the presence of thrombin. As a result, the implant should form a small, but appreciable, bump under the skin.
After the injection, place the mice on a layer of gauze for comfort and warmth and observe them until they become ambulatory. Then after, observe the mice daily for the first three days.
6. Harvesting (Day 7)
One week after the injections, euthanize the mice by placing them into a gas chamber delivering compressed CO2 gas.
Once euthanized, cut open the skin near the area of the injection and surgically remove the gel plug. Digital photographs of the retrieved gel plugs with a scale are advised.
Place the harvested gel plugs into histological cassette and deep them into 10% neutral buffered formalin overnight at room temperature.
After fixation, wash the 10% neutral buffered formalin away with distilled H2O and place the histological cassettes at 4°C in PBS until histological evaluation.
7. Evaluation: Histology (H&E) and Immunohistochemistry (hCD31)
For histological evaluation, the implants are embedded in paraffin and sectioned (7 μm-thick sections) using standard histological procedures.
Quantify microvessel density by evaluation of Hematoxilin and Eosin (H&E) stained sections taken from the middle part of the implants. Standard protocols for H&E staining can be found elsewhere. Microvessels can be identified as lumenal structures containing red blood cells. Report microvessels density as the average number of red blood cell-filled microvessels from the fields analyzed and expressed as vessels/mm2.
To demonstrate the human nature of the microvascular vessels, sections of the retrieved implant should be immunohistochemically stained with a human-specific CD31 (hCD31) antibody using standard staining protocols. We recommend using the monoclonal mouse anti-human CD31 antibody from DakoCytomation (Clone JC70A; cat. # M0823) at a 1:100 dilution. The human specificity of this antibody has been confirmed by the negative reaction obtained with a diversity of mouse tissue sections that were stained in parallel 7,11. Of note, mouse blood vessels are often seen inside the implants (specially around the border), but mouse vessels will not stain positive with this antibody. In addition, the presence of human MSC can be detected by immunohistochemical staining with human-specific antibodies against CD90 or α-Smooth muscle actin (α-SMA). Human MSC are found both in the perivascular region of newly-formed blood vessels and interstitially located throughout the implant 11.
8. Representative Results
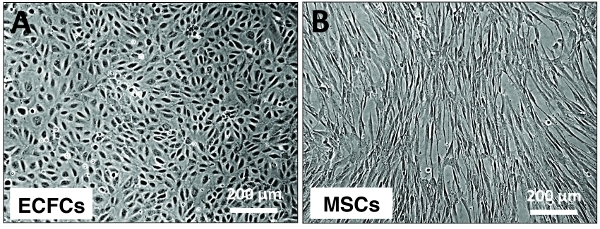 Figure 1. Typical appearance of ECFC and MSC cultures. Phase contrast micrographs displaying the typical appearance of ECFCs and MSCs in culture. (A) Confluent monolayer of cord blood-derived ECFCs displaying the characteristic cobble-stone morphology of endothelial cells. (B) Human bone marrow-derived MSCs displaying a spindle shape morphology. Scaler bars, 200 um.
Figure 1. Typical appearance of ECFC and MSC cultures. Phase contrast micrographs displaying the typical appearance of ECFCs and MSCs in culture. (A) Confluent monolayer of cord blood-derived ECFCs displaying the characteristic cobble-stone morphology of endothelial cells. (B) Human bone marrow-derived MSCs displaying a spindle shape morphology. Scaler bars, 200 um.
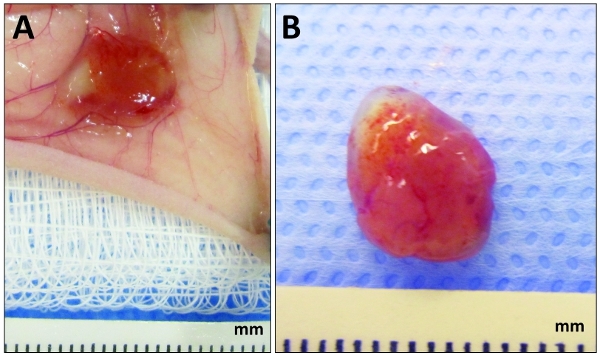 Figure 2. Appearance of explanted plugs at day 7. Human cord blood-derived ECFCs and bone marrow-derived MSCs were embedded in collagen/fibronectin/fibrin gel and implanted subcutaneously into nude mice as described in the text. (A) After 7 days, once the mouse has been euthanized, cut open the skin near the area of the injection and expose the cell/gel plug by flipping the skin. (B) Appearance of the plug surgically removed from the mouse and prior to formalin fixation. The red color of the implant is an indication of vascularization.
Figure 2. Appearance of explanted plugs at day 7. Human cord blood-derived ECFCs and bone marrow-derived MSCs were embedded in collagen/fibronectin/fibrin gel and implanted subcutaneously into nude mice as described in the text. (A) After 7 days, once the mouse has been euthanized, cut open the skin near the area of the injection and expose the cell/gel plug by flipping the skin. (B) Appearance of the plug surgically removed from the mouse and prior to formalin fixation. The red color of the implant is an indication of vascularization.
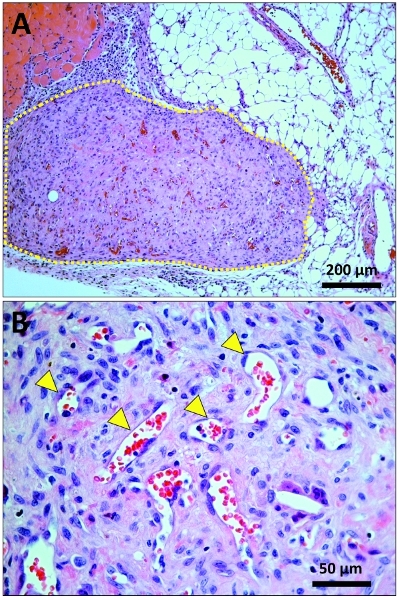 Figure 3. Histological identification of vascular network in explanted plugs. Hematoxilin and Eosin (H&E) stained sections taken from the middle part of the explanted plugs. (A) Low magnification (10x) micrograph displaying the implant (marked by a yellow dashed line) in the context of surrounding host tissues (i.e., adipose tissue and skeletal muscle). (B) High magnification (40x) micrograph displaying multiple microvessels (yellow arrowhead pointing at some of them) inside the plug; microvessels can be identified as lumenal structures containing red blood cells.
Figure 3. Histological identification of vascular network in explanted plugs. Hematoxilin and Eosin (H&E) stained sections taken from the middle part of the explanted plugs. (A) Low magnification (10x) micrograph displaying the implant (marked by a yellow dashed line) in the context of surrounding host tissues (i.e., adipose tissue and skeletal muscle). (B) High magnification (40x) micrograph displaying multiple microvessels (yellow arrowhead pointing at some of them) inside the plug; microvessels can be identified as lumenal structures containing red blood cells.
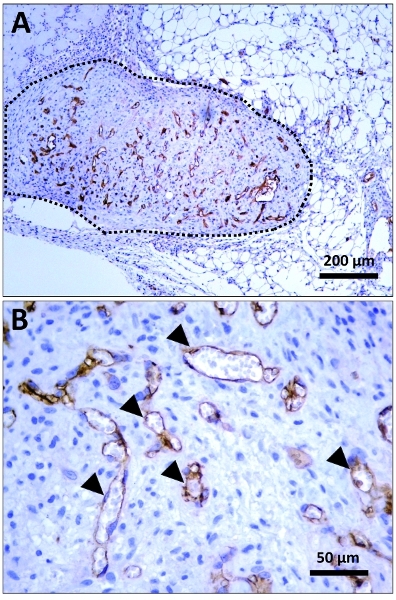 Figure 4. Immunohistochemical identification of human lumens. Immunohistochemically stained sections taken from the middle part of the explanted plugs. Staining was carried out using a monoclonal mouse anti-human CD31 (hCD31) antibody from DakoCytomation (Clone JC70A; cat. # M0823) at a 1:100 dilution; cell nuclei were counterstained with hematoxilin. (A) Low magnification (10x) micrograph displaying the implant (delineated by a black dashed line) in the context of surrounding host tissues. Human specific, CD31-positive microvessels are stained in brown (peroxidase staining). (B) High magnification (40x) micrograph displaying multiple human microvessels (black arrowhead pointing at some hCD31-positive lumens) inside the plug.
Figure 4. Immunohistochemical identification of human lumens. Immunohistochemically stained sections taken from the middle part of the explanted plugs. Staining was carried out using a monoclonal mouse anti-human CD31 (hCD31) antibody from DakoCytomation (Clone JC70A; cat. # M0823) at a 1:100 dilution; cell nuclei were counterstained with hematoxilin. (A) Low magnification (10x) micrograph displaying the implant (delineated by a black dashed line) in the context of surrounding host tissues. Human specific, CD31-positive microvessels are stained in brown (peroxidase staining). (B) High magnification (40x) micrograph displaying multiple human microvessels (black arrowhead pointing at some hCD31-positive lumens) inside the plug.
Discussion
This is an experimental model of bioengineering human vascular networks. The main characteristics of this model are: 1) microvessels are formed from human cells isolated from post-natal tissues (i.e., blood and bone marrow); 2) microvessels are formed in an adult animal; and 3) microvessels do not arise from pre-existing (host) vessels but instead they are formed, de novo, from single cells suspended in a suitable gel.
Angiogenesis plays an important role in this assay because connections to the murine vasculature are needed to achieve red blood cell-filled vessels, one of the functional read-out in this assay. In addition, host myeloid cells are recruited to the implant in the early days post transplantation, and they are necessary for the formation of the new vascular bed 12.
This experimental model offers a versatile, quantifiable, and relatively simple model system to study post-natal formation of human vascular networks in vivo. Additionally, this assay is simple to perform and it does not require an incision or surgical procedure. The model could be used to study the vasculogenic potential of different sources of human endothelial and perivascular cells. The model could be used to screen for anti- and/or pro-vasculogenic compounds. Finally, the model can be used to study the role(s) of specific genes in the formation and function of a vascular network composed of human endothelium.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was partially supported by NIH grant K99EB009096-01A1 to J.M.-M.
References
- Melero-Martin JM, Bischoff J. Chapter 13. An in vivo experimental model for postnatal vasculogenesis. Methods Enzymol. 2008;445:303–329. doi: 10.1016/S0076-6879(08)03013-9. [DOI] [PubMed] [Google Scholar]
- Koike N. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138–139. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- Schechner JS. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci USA. 2000;97:9191–9196. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nö r, E J. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81:453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DA. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- Melero-Martin JM. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- Au P. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111:1302–1305. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder MC. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traktuev D. Robust Functional Vascular Network Formation In Vivo by Cooperation of Adipose Progenitor and Endothelial Cells. Circ Res. 2009;104:1410–1420. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- Melero-Martin JM. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero-Martin JM. Host Myeloid Cells Are Necessary for Creating Bioengineered Human Vascular Networks In Vivo. Tissue Eng Part A. 2010;16:2457–2466. doi: 10.1089/ten.tea.2010.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]


