Abstract
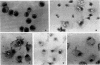
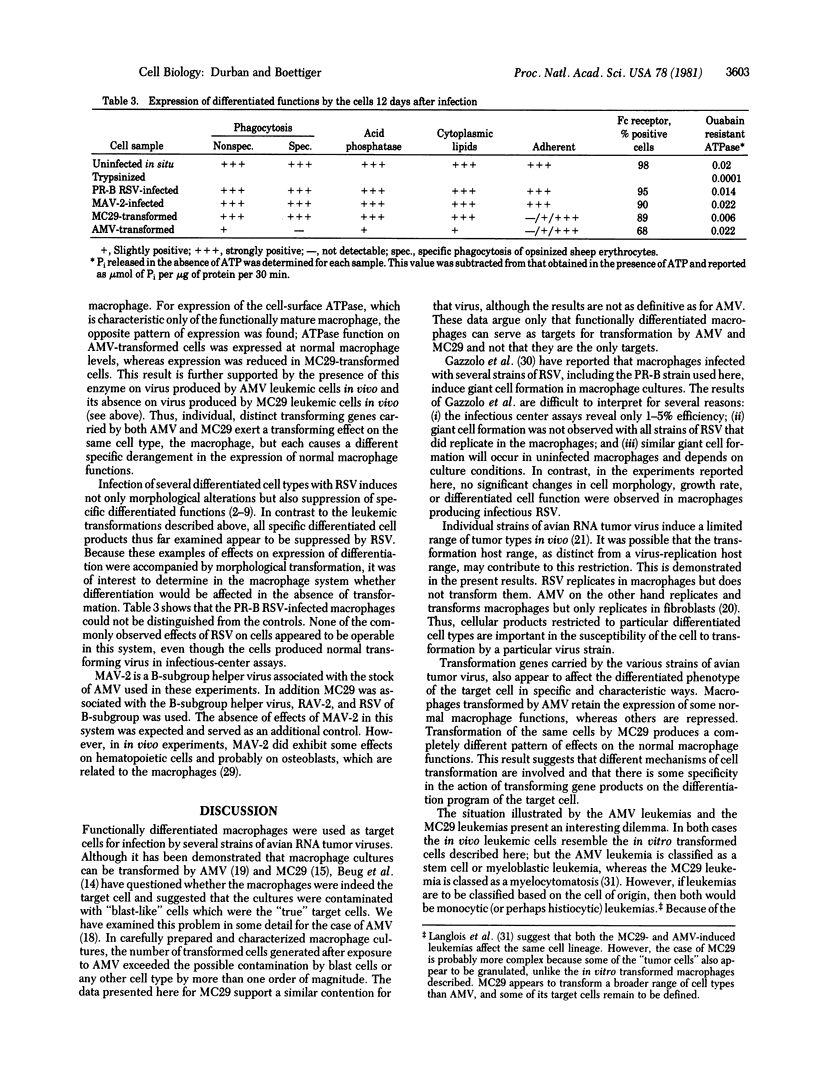
Functionally differentiated chicken macrophages were derived by in vitro differentiation of embryonic yolk sac cells and were characterized by several macrophage-specific cell markers. Uniform, infected, virus-producing cultures were obtained after exposure of these macrophages to avian myoblastosis virus (AMV), avian myelocytomatosis virus (MC29), myeloblastosis-associated virus (MAV-2), and Prague strain of Rous sarcoma virus (PR-B RSV). Both AMV and MC29 induced morphological transformation typical of the in vivo leukemias induced by these virus strains. Analysis of the expression of macrophage-specific markers in these two transformed cell types demonstrated that different markers of the mature macrophage were suppressed by each virus, even though the parental cell immediately preceding the transformation event was a mature macrophage in both cases. Cells infected with PR-B RSV and MAV-2 showed no observable difference from uninfected macrophages in terms of morphological characteristics, growth rate, or expression of the differentiated functions of macrophages. Ths system provides demonstrations of a cell type that produces infectious, transforming RSV but fails to respond by functional alterations induced by the transforming gene, src.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALUDA M. A., GOETZ I. E. Morphological conversion of cell cultures by avian myeloblastosis virus. Virology. 1961 Oct;15:185–199. doi: 10.1016/0042-6822(61)90234-3. [DOI] [PubMed] [Google Scholar]
- BEARD J. W. AVIAN VIRUS GROWTHS AND THEIR ETIOLOGIC AGENTS. Adv Cancer Res. 1963;7:1–127. doi: 10.1016/s0065-230x(08)60982-3. [DOI] [PubMed] [Google Scholar]
- Banerjee R. K., Racker E. Solubilization, purification, and characterization of a nucleoside triphosphatase from avian myeloblastosis virus. J Biol Chem. 1977 Oct 10;252(19):6700–6700. [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Durban E. M. Progenitor-cell populations can be infected by RNA tumor viruses, but transformation is dependent on the expression of specific differentiated functions. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1249–1254. doi: 10.1101/sqb.1980.044.01.135. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Roby K., Brumbaugh J., Biehl J., Holtzer H. Transformation of chicken embryo retinal melanoblasts by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1977 Aug;11(4):881–890. doi: 10.1016/0092-8674(77)90299-9. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Temin H. M. Light inactivation of focus formation by chicken embryo fibroblasts infected with avian sarcoma virus in the presence of 5-bromodeoxyuridine. Nature. 1970 Nov 14;228(5272):622–624. doi: 10.1038/228622a0. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Moscovici M. G., Moscovici C. Isolation of complementary DNA unique to the genome of avian myeloblastosis virus (AMV). Virology. 1980 May;103(1):112–122. doi: 10.1016/0042-6822(80)90130-0. [DOI] [PubMed] [Google Scholar]
- Durban E. M., Boettiger D. Replicating, differentiated macrophages can serve as in vitro targets for transformation by avian myeloblastosis virus. J Virol. 1981 Jan;37(1):488–492. doi: 10.1128/jvi.37.1.488-492.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOULDS L. The histologic analysis of mammary tumors of mice. J Natl Cancer Inst. 1956 Dec;17(6):701–801. [PubMed] [Google Scholar]
- Fiszman M. Y., Fuchs P. Temperature-sensitive expression of differentiation in transformed myoblasts. Nature. 1975 Apr 3;254(5499):429–431. doi: 10.1038/254429a0. [DOI] [PubMed] [Google Scholar]
- Gazzolo L., Moscovici M. G., Moscovici C. Replication of avian sarcoma viruses in chicken macrophages. Virology. 1974 Apr;58(2):514–525. doi: 10.1016/0042-6822(74)90085-3. [DOI] [PubMed] [Google Scholar]
- Graf T. Two types of target cells for transformation with avian myelocytomatosis virus. Virology. 1973 Aug;54(2):398–413. doi: 10.1016/0042-6822(73)90152-9. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Biehl J., Yeoh G., Meganathan R., Kaji A. Effect of oncogenic virus on muscle differentiation. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4051–4055. doi: 10.1073/pnas.72.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois A. J., Fritz R. B., Heine U., Beard D., Bolognesi D. P., Beard J. W. Response of bone marrow to MC29 avian leukosis virus in vitro. Cancer Res. 1969 Nov;29(11):2056–2074. [PubMed] [Google Scholar]
- Miskin R., Easton T. G., Maelicke A., Reich E. Metabolism of acetylcholine receptor in chick embryo muscle cells: effects of RSV and PMA. Cell. 1978 Dec;15(4):1287–1300. doi: 10.1016/0092-8674(78)90054-5. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Chi D., Gazzolo L., Moscovici M. G. A study of plaque formation with avian RNA tumor viruses. Virology. 1976 Aug;73(1):181–189. doi: 10.1016/0042-6822(76)90072-6. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Gazzolo L., Moscovici M. G. Focus assay and defectiveness of avian myeloblastosis virus. Virology. 1975 Nov;68(1):173–181. doi: 10.1016/0042-6822(75)90159-2. [DOI] [PubMed] [Google Scholar]
- Pacifici M., Boettiger D., Roby K., Holtzer H. Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell. 1977 Aug;11(4):891–899. doi: 10.1016/0092-8674(77)90300-2. [DOI] [PubMed] [Google Scholar]
- Schwarz R. I., Bissell M. J. Dependence of the differentiated state on the cellular environment: modulation of collagen synthesis in tendon cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4453–4457. doi: 10.1073/pnas.74.10.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Bishop J. M. DNA and RNA from uninfected vertebrate cells contain nucleotide sequences related to the putative transforming gene of avian myelocytomatosis virus. J Virol. 1979 Aug;31(2):514–521. doi: 10.1128/jvi.31.2.514-521.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Van Eldik L. J. Characterization of the immunosuppression accompanying virus-induced avian osteopetrosis. Infect Immun. 1978 Nov;22(2):452–461. doi: 10.1128/iai.22.2.452-461.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L. M., Komaromy M. C., Baluda M. A. Identification of a proviral genome associated with avian myeloblastic leukemia. Proc Natl Acad Sci U S A. 1980 May;77(5):3004–3008. doi: 10.1073/pnas.77.5.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stéhelin D., Graf T. Avian myelocytomatosis and erythroblastosis viruses lack the transforming gene src of avian sarcoma viruses. Cell. 1978 Apr;13(4):745–750. doi: 10.1016/0092-8674(78)90224-6. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M., RUBIN H. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology. 1958 Dec;6(3):669–688. doi: 10.1016/0042-6822(58)90114-4. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Friis R. R. Dissociation of transformation parameters using temperature-conditional mutants of Rous sarcoma virus. Cell. 1979 Jan;16(1):25–32. doi: 10.1016/0092-8674(79)90184-3. [DOI] [PubMed] [Google Scholar]