Abstract
Focal cerebral ischemia is among the most common type of stroke seen in patients. Due to the clinical significance there has been a prolonged effort to develop suitable animal models to study the events that unfold during ischemic insult. These techniques include transient or permanent, focal or global ischemia models using many different animal models, with the most common being rodents.
The permanent MCA ligation method which is also referred as pMCAo in the literature is used extensively as a focal ischemia model in rodents 1-6. This method was originally described for rats by Tamura et al. in 1981 7. In this protocol a craniotomy was used to access the MCA and the proximal regions were occluded by electrocoagulation. The infarcts involve mostly cortical and sometimes striatal regions depending on the location of the occlusion. This technique is now well established and used in many laboratories 8-13. Early use of this technique led to the definition and description of “infarct core” and “penumbra” 14-16, and it is often used to evaluate potential neuroprotective compounds 10, 12, 13, 17. Although the initial studies were performed in rats, permanent MCA ligation has been used successfully in mice with slight modifications 18-20 .
This model yields reproducible infarcts and increased post-survival rates. Approximately 80% of the ischemic strokes in humans happen in the MCA area 21 and thus this is a very relevant model for stroke studies. Currently, there is a paucity of effective treatments available to stroke patients, and thus there is a need for good models to test potential pharmacological compounds and evaluate physiological outcomes. This method can also be used for studying intracellular hypoxia response mechanisms in vivo.
Here, we present the MCA ligation surgery in a C57/BL6 mouse. We describe the pre-surgical preparation, MCA ligation surgery and 2,3,5 Triphenyltetrazolium chloride (TTC) staining for quantification of infarct volumes.
Protocol
This protocol was approved by the University of Rochester committee devoted to the ethical use of animals in research (UCAR). Aseptic techniques should be followed during the protocol. The use of sterile gloves and a mask is required.
All the equipment, materials, chemicals, and tools that are used during the protocol are described in Table 1.
1. Pre-surgical Preparation
Inject mice subcutaneously with buprenorphine (0.05mg/kg) 2 h before surgery, immediately after the surgery and then every 3-5 h during the first 24 h post-operative period.
Sterilize all the surgical instruments, gauze sponges, and cotton tip applicators by autoclave. Keep surgical tools in 70% ethanol during the surgery and air dry on sterile gauze right before use. Spray the work area with 70% ethanol.
Anesthetize the mouse with a 3% isofluorane-20% oxygen gas mixture by using an anesthetic vaporizer. Adjust the oxygen amount with the flow meter. Test the level of anesthesia by toe or tail pinching (they should be unresponsive). Maintain the level of anesthesia with 2% (v/v) isofluorane.
Apply artificial tears to the mouse’s eyes and use caution to avoid damaging the eye during the surgical procedure. Place the mouse on its right side in a lateral position.
Shave the area on the left side between the left eye and the base of the left ear using the animal clippers. Cleanse the area by alternating between a betadine solution and 70% ethanol using cotton tip applicators and lightly rubbing the area. Repeat as necessary.
Place the mouse on to a heating pad connected to a rectal probe to maintain body temperature at 37°C. Insert the rectal probe by using mineral oil.
Position the mouse on its right side under the microscope and secure with tape. Cut a window in a gauze sponge and cover surgical area.
2. Surgical Procedure and MCA Ligation
Make a vertical incision between the left eye and the base of the left ear by using fine straight scissors. Use curved hemostats to keep the surgical area open.
Make a horizontal incision on the temporal muscle by using spring scissors and slightly separate the temporal muscle from the skull by slowly pulling with forceps.
Make a small subtemporal craniotomy at the junction of zygomatic arch and squamosal bone by using an 18G needle while holding the jawbone with curved forceps.
Expose the MCA by removing the small pieces of skull with bone rongeurs. The zygomatic arch and orbital contents should not be damaged during this process. If excessive drying of tissue occurs during this process, apply sterile PBS with cotton tip applicators.
Ligate the distal portion of the MCA by using a small vessel cauterizer.
Place the temporal muscle back into its original position and close the incision site with surgical 5-0 nylon sutures.
Discontinue anesthesia and remove the rectal probe. Inject the mouse with a 2nd dose of buprenorphine (0.05mg/kg) subcutaneously. Return the mouse to the cage which was kept at 37°C with a heating panel.
Closely monitor the mouse for the next 24 h for any discomfort (decreased appetite/water consumption, hunched posture, increased respiration, pilo-erected hair). Inject the mouse with buprenorphine subcutaneously every 3-5 h post-surgery up to 24h. Provide the mouse with recovery gel during this period.
3. TTC Staining and Determination of Stroke Volume
Twenty-four hours after the surgery deeply anesthetize the mouse, transcardially perfuse the mouse with a 4% TTC (w/v) in phosphate buffered saline (PBS) for 15 min and then with a 4% paraformaldehyde solution for 10 min by using a mini peristaltic pump at medium flow rate. Remove the brain and place it into the 4% paraformaldehyde solution overnight.
Position the brain into the sectioning block. Slice the brain into 1mm thick slices by using razor blades.
Place the slices next to a millimeter scale ruler. Photograph the slices by using a digital camera connected to the dissecting microscope.
Calculate the stroke area by subtracting the non-infarcted area of the ipsilateral site from the total area of the contralateral site using Image J Software (http://rsbweb.nih.gov/ij/). Calculate the stroke volume by stacking the stroke area of the slices 22.
4. Directions to use Image J software
Open the image file to be analyzed in Image J software by clicking on the file menu.
Click on the ‘straight line’ tab in the program. Draw a straight line between the two margins on the ruler. Click on ‘analyze’ menu and select ‘set scale’. Set the known distance as 1, unit of length as mm.
Click on ‘freehand circle’ tab. Draw a circle to outline the contralateral hemisphere. Click on ‘analyze’ menu and select ‘measure’. The calculation window will pop up showing the area of the circle drawn.
Draw another circle around the ipsilateral hemisphere excluding stroke (white) area. Measure the area as indicated in step 4.3. The new value will be added to the calculation window. The difference between the 1st and 2nd values represents the area of the infarct which is indicated as A in the formula below.
Calculate the stroke area in each slice by repeating steps 4.2-4.4. Take summation of the stroke area calculated in each slice (∑An). This represents stroke volume knowing the fact that the thickness of each slice is 1mm. ∑An x 1mm (thickness of each slice) = Stroke volume
5. Representative Results:
The infarcts obtained by permanent MCA ligation in the mouse are mostly cortical. However, it is possible to obtain subcortical lesions if the MCA is ligated proximal to the lenticulostriate branch. The stroke volumes after MCA ligation might vary from 10mm³ to 35mm³ 19, 23. The stroke volumes calculated with TTC staining in Moyanova et al. 19 are between 10mm³ to 22mm³ while the stroke volumes determined from MRI images in Filiano et al 23 are between 20mm³ to 35mm³. The possible reason for these differences maybe the exact location of the ligation and the different methods used to measure the stroke volumes.
In Figure 1, a TTC stained brain 24 hr post permanent MCA ligation in a wild type C57/BL6 mouse is shown. The stroke site is white in appearance (Fig. 1A, B). Figure 1A and B illustrate the infarct site from different angles. Figure 2 shows 1mm thick slices of the TTC stained stroke brain from anterior to posterior (Fig. 2A-G). Infarct volume was calculated 24 h post-surgery. The volume of the infarct is ˜23mm³.
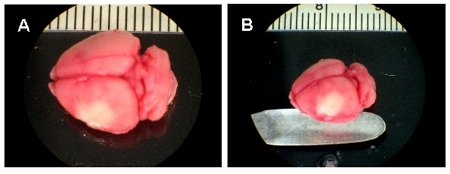 Figure 1. Representation of stroke site. A-B, TTC stained whole brain images, 24 h after MCA ligation. White area represents the infarct.
Figure 1. Representation of stroke site. A-B, TTC stained whole brain images, 24 h after MCA ligation. White area represents the infarct.
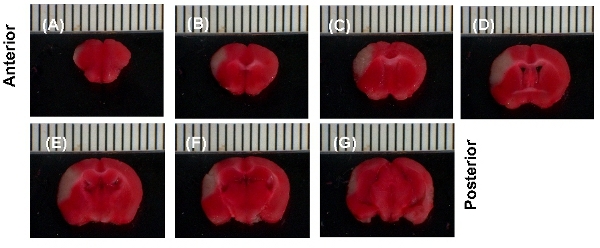 Figure 2. Representative results. (A>G), TTC stained 1mm brain sections, 24 h after MCAL. Slices are aligned from anterior to posterior. White area represents the infarct.
Figure 2. Representative results. (A>G), TTC stained 1mm brain sections, 24 h after MCAL. Slices are aligned from anterior to posterior. White area represents the infarct.
Discussion
The permanent MCA ligation method gives highly reproducible infarct volumes and increased post-operative survival rates compared to other methods available. The ease and the short duration (˜30 min) of the procedure make it even more practical. The method is widely used in both mice and rats.
This technique requires an invasive surgery under a stereomicroscope. Therefore, experience in operating under a microscope and perfecting a successful craniotomy is essential. It is best to establish consistent infarcts in the lab before experiments are performed. To attain reproducible results it is important to ligate the MCA at the same exact location each time. The MCA should be ligated proximal to lenticulostriate branches if subcortical infarcts are desired. The artery is fully ligated and the occlusion is permanent. Therefore this model does not allow reperfusion via the MCA. Although not included in this demonstration, it is best if a doppler probe is used for measuring the blood flow in the affected area to verify complete ligation of the artery.
The operator should be very careful not to damage or puncture MCA while exposing and coagulating the artery. Craniotomy should be performed with extensive care to prevent any damage to the zygomatic bone. Because the surgical area is very close to the infarct area, any damage to the cortical surface should be avoided during craniotomy or cauterization. FST 18015-00 cateurizer unit or a bipolar cateurizer can be used instead of the FST 18000-00 used in this demonstration. FST 18015-00 allows the user to adjust the temperature of the cauterizer tip to low heat which might prevent any damage to the cortical tissue. FST 18015-00 also avoids the temperature fluctuations seen in battery operated cauterization tools. During cauterization, the user should be very careful to keep the cauterizer out of the oxygen flow and can momentarily shut down the O2/isofluorane to avoid risk of ignition. This will not affect the anesthesia state of the mouse. To prevent this risk, we use a ventilation pipe extended to the surgical area which increases the air circulation sufficiently, as well as draws off any excess O2/isofluorane in the air.
The permanent MCA ligation model is extremely useful in studying ischemic stroke. It can be used to test neuroprotectants or study molecular mechanisms of ischemia in vivo on transgenic mouse models. The obvious benefit for using this in vivo approach is that this allows for the study of ischemic insult on intact neuronal networks and the behavioral response post-insult, in addition to the neuroinflammatory processes that are present after ischemic damage. Therefore, this in vivo stroke model provides a critical complementary approach to in situ models that are used to mimic ischemia in cell culture.
Disclosures
No conflicts of interest declared.
Acknowledgments
The surgical technique was originally acquired in the lab of Dr. William D. Hill at the Medical College of Georgia. The authors would also like to thank Dr. David A. Rempe and Landa Prifti for the use of the dissection camera. This research was supported by NIH NS041744, NS051279, F31 NS064700 and AHA 30815697D.
References
- Britton M, Rafols J, Alousi S, Dunbar JC. The effects of middle cerebral artery occlusion on central nervous system apoptotic events in normal and diabetic rats. Int J Exp Diabesity Res. 2003;4:13–20. doi: 10.1080/15438600303727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri P, Rabuffetti M, Monopoli A, Nicosia S. Production of leukotrienes in a model of focal cerebral ischaemia in the rat. Br J Pharmacol. 2001;133:1323–1329. doi: 10.1038/sj.bjp.0704189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K. Delayed transplantation of human neural precursor cells improves outcome from focal cerebral ischemia in aged rats. Aging Cell. 2010;9:1076–1083. doi: 10.1111/j.1474-9726.2010.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig D. Evolution of GADD34 expression after focal cerebral ischaemia. Brain Res. 2005;1034:51–61. doi: 10.1016/j.brainres.2004.11.058. [DOI] [PubMed] [Google Scholar]
- Shirotani T, Shima K, Chigasaki H. In vivo studies of extracellular metabolites in the striatum after distal middle cerebral artery occlusion in stroke-prone spontaneously hypertensive rats. Stroke. 1995;26:878–884. doi: 10.1161/01.str.26.5.878. [DOI] [PubMed] [Google Scholar]
- Wu YP, Tan CK, Ling EA. Expression of Fos-like immunoreactivity in the brain and spinal cord of rats following middle cerebral artery occlusion. Exp Brain Res. 1997;115:129–136. doi: 10.1007/pl00005672. [DOI] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- Bederson JB. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Carswell HV. Genetic and gender influences on sensitivity to focal cerebral ischemia in the stroke-prone spontaneously hypertensive rat. Hypertension. 1999;33:681–685. doi: 10.1161/01.hyp.33.2.681. [DOI] [PubMed] [Google Scholar]
- Mary V, Wahl F, Uzan A, Stutzmann JM. Enoxaparin in experimental stroke: neuroprotection and therapeutic window of opportunity. Stroke. 2001;32:993–999. doi: 10.1161/01.str.32.4.993. [DOI] [PubMed] [Google Scholar]
- Menzies SA, Hoff JT, Betz AL. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1992;31:100–107. doi: 10.1227/00006123-199207000-00014. [DOI] [PubMed] [Google Scholar]
- Iaci JF. Glial growth factor 2 promotes functional recovery with treatment initiated up to 7 days after permanent focal ischemic stroke. Neuropharmacology. 2010;59:640–649. doi: 10.1016/j.neuropharm.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Richard MJP, Khan BVCBJ, Saleh TM. Cellular mechanisms by which lipoic acid confers protection during the early stages of cerebral ischemia: A possible role for calcium. Neuroscience Research. 2011 doi: 10.1016/j.neures.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Heiss WD. Progressive derangement of periinfarct viable tissue in ischemic stroke. J Cereb Blood Flow Metab. 1992;12:193–203. doi: 10.1038/jcbfm.1992.29. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Gjedde A, Diemer NH. Focal ischemia of the rat brain: autoradiographic determination of cerebral glucose utilization, glucose content, and blood flow. J Cereb Blood Flow Metab. 1986;6:414–424. doi: 10.1038/jcbfm.1986.74. [DOI] [PubMed] [Google Scholar]
- Nowicki JP, Assumel-Lurdin C, Duverger D, MacKenzie ET. Temporal evolution of regional energy metabolism following focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1988;8:462–473. doi: 10.1038/jcbfm.1988.87. [DOI] [PubMed] [Google Scholar]
- Butcher SP, Bullock R, Graham DI, McCulloch J. Correlation between amino acid release and neuropathologic outcome in rat brain following middle cerebral artery occlusion. Stroke. 1990;21:1727–1733. doi: 10.1161/01.str.21.12.1727. [DOI] [PubMed] [Google Scholar]
- Arlicot N. Detection and quantification of remote microglial activation in rodent models of focal ischaemia using the TSPO radioligand CLINDE. Eur J Nucl Med Mol Imaging. 2010;37:2371–2380. doi: 10.1007/s00259-010-1598-7. [DOI] [PubMed] [Google Scholar]
- Moyanova SG. Protective role for type 4 metabotropic glutamate receptors against ischemic brain damage. J Cereb Blood Flow Metab. 2010 doi: 10.1038/jcbfm.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortolano F. Advances in imaging of new targets for pharmacological intervention in stroke: real-time tracking of T-cells in the ischaemic brain. Br J Pharmacol. 2010;159:808–811. doi: 10.1111/j.1476-5381.2009.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill MJ, A CJ. Rodent models of focal cerebral ischemia. Current Protocols in Neuroscience. 2000:9.6.1–9.6.32. doi: 10.1002/0471142301.ns0906s12. [DOI] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- Filiano AJ, Tucholski J, Dolan PJ, Colak G, Johnson GV. Transglutaminase 2 protects against ischemic stroke. Neurobiol Dis. 2010;39:334–343. doi: 10.1016/j.nbd.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


