Abstract
Mouse bladder wall injection is a useful technique to orthotopically study bladder phenomena, including stem cell, smooth muscle, and cancer biology. Before starting injections, the surgical area must be cleaned with soap and water and antiseptic solution. Surgical equipment must be sterilized before use and between each animal. Each mouse is placed under inhaled isoflurane anesthesia (2-5% for induction, 1-3% for maintenance) and its bladder exposed by making a midline abdominal incision with scissors. If the bladder is full, it is partially decompressed by gentle squeezing between two fingers. The cell suspension of interest is intramurally injected into the wall of the bladder dome using a 29 or 30 gauge needle and 1 cc or smaller syringe. The wound is then closed using wound clips and the mouse allowed to recover on a warming pad. Bladder wall injection is a delicate microsurgical technique that can be mastered with practice.
Protocol
1. Mouse Bladder Wall Injection
Choice of mouse strain, age, and sex is dictated by experimental needs. We use mice between 8 and 12 weeks of age, since this is a window of immunological maturity prior to senescence. As a general guideline, mice should arrive at least one week prior to experimental manipulation in order to avoid stress-induced confounding factors.
Clean the surgical table surface with soap and water.
Wipe the surgical table surface with Cide Swipes or antiseptic wipes.
Autoclave clean surgical instruments prior to use in surgery.
In addition, sterilize surgical instruments with hot bead sterilizer immediately prior to use, as well as between animals during surgery.
Clean 100 μL Hamilton syringes and 29 or 30 gauge (1/2 inch long) needles by repeated aspiration and injection with absolute alcohol before first use and at the end of the final surgical procedure.
Wash and rinse Hamilton syringes with sterilized phosphate-buffered saline between each animal.
Anesthetize mice by placing them in an isoflurane induction chamber, with the isoflurane set between 2-5%.
Once general anesthesia is achieved, remove the mouse from the chamber and place in the supine position with a warm pad underneath to help maintain normal body temperature.
Achieve maintenance anesthesia by placing the animal’s snout in a nozzle containing vaporize isoflurane (titrated from 1-3% as necessary to maintain appropriate anesthesia).
Shave the abdominal skin with clippers.
Use a disposable, sterile surgical drape to cover the anus to prevent fecal contamination during surgery.
Use a second disposable, sterile surgical drape to cover the surgical field (lower abdomen).
Prep the abdomen with three pieces of Betadine-soaked gauze. Repeat this step three times.
Using a dissecting microscope for magnification, make a lower midline abdominal incision with scissors.
Expose the bladder.
If the bladder is full, partially decompress it by gentle downward pressure at the dome.
Inject the sample solution (up to 50 μL), using a 29 or 30 gauge needle and syringe, into the wall of the bladder dome (intramural injection) with the bevel of the needle facing upwards.
Push the plunger of the syringe to inject the sample solution into the bladder wall. A well-localized bleb is an indication of successful injection of the cells into the bladder wall.
Remove the syringe, close the incision with wound clips and allow the mouse to recover on a warming pad.
2. Representative Results:
A well-localized bleb that does not leak fluid and stays stable in size is an indication of successful injection of the cells into the bladder wall (Figure 1). Histological analysis can be performed to confirm the presence of the injected cells in the bladder wall.
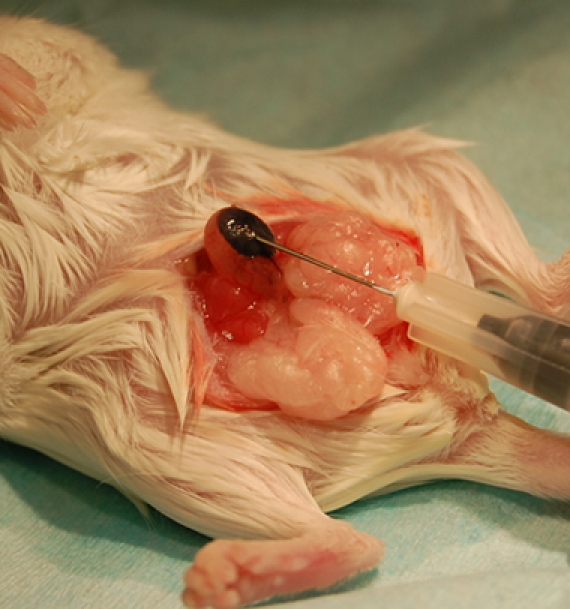 Figure 1. Example of successful bladder wall injection using India ink for illustration.
Figure 1. Example of successful bladder wall injection using India ink for illustration.
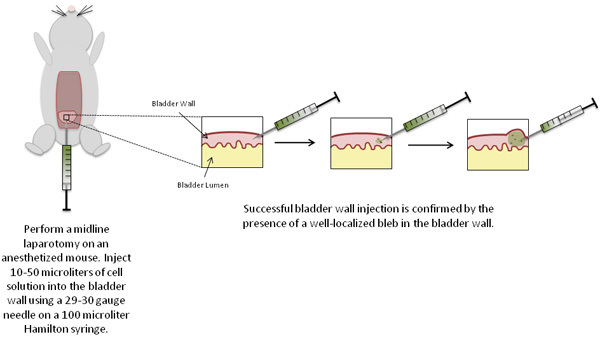 Figure 2. Schematic of experimental procedures.
Figure 2. Schematic of experimental procedures.
Discussion
Mouse bladder wall injection allows for the implantation of specific cells into defined areas of the bladder wall. This technique has wide applications for mouse models of bladder cancer, smooth muscle, and stem cell biology. By definition, mouse bladder wall injection facilitates introduction of bladder cancer or stem cells into an orthotopic location so that their growth and differentiation can be studied in a physiologically relevant anatomic context. In fact, Dinney et al. first described this technique in order to study orthotopic human bladder cancer xenografts in nude mice 1.
While the bladder wall injection technique has been utilized in a number of bladder cancer and stem cell studies 1-9, it is not the only method used. Many scientists have tried to implant bladder cancer cells into nude mice by subcutaneous, intraperitoneal, or intravenous injection, but these models have not exhibited predictable tumorigenicity and metastatic properties that allow selection of in vivo cell lines11-13. Furthermore, there are a number of bladder cancer models which rely on transurethral inoculation of mice with tumor cells 14. A leading theory of bladder carcinogenesis posits that tumor spread occurs by "seeding" of normal urothelium by cancerous cells shed into the urinary stream from elsewhere in the urinary tract. From this perspective, transurethral inoculation, which results in exposure of the urothelium to cancer cells from the luminal side of the bladder, may be more physiologically relevant than bladder wall injection. However, transurethral inoculation cannot direct where cells will implant themselves within the bladder.
In contrast, orthotopic bladder tumor implantation by bladder wall injection can consistently produce tumors located in the wall of the bladder. Thus, this method can also be applied to studies of smooth muscle and stem cell biology 3, 5, 9.
In this technique, the needle size and syringe are critical. We have found that ½ inch long, 29 and 30 gauge needles are best for bladder wall injection. Longer needles have more dead space which can contribute to wasted inocula and less accurate injection volumes. Wider needles (28 gauge or lower) are difficult to inject with, and lead to large needle tracks which easily extrude injected material. We use 100 microliter Hamilton syringes because they permit highly precise administration of desired volumes. In addition, cells and debris are easily aggregated and clumped at the end of the needle while injecting. Thus, the cell suspension should be mixed well before being drawn into syringe and the syringe and needle should be repeatedly cleaned with sterile normal saline between each injection. We have found that the minimum, accurate injectable volume is approximately 10 microliters. No more than approximately 50 microliters can be injected into the mouse bladder wall due to the small size of the injected tissue. Injection using India ink can be used by novices to help confirm they are using proper technique. The learning curve for individuals experienced with mouse procedures is short. Bladder wall injections can be performed with nearly 100% success rates after only 5-10 mice. We anticipate that even for workers unfamiliar with mouse microsurgery, very high success rates should be achievable within 10-15 mice. Injection failure mice can be used as negative controls.
In conclusion, bladder wall injection can result in highly targeted delivery of cells to defined locations in the bladder, making it a highly attractive option for studies of bladder biology. Nonetheless, this technique has an associated learning curve and requires time and practice to master.
Disclosures
No conflicts of interest declared.
Acknowledgments
We gratefully acknowledge the support of a Pilot grant from the Stanford Pediatric Research Fund and K08DK087895-01 from the NIDDK.
References
- Dinney CP, Fishbeck R, Singh RK, Eve B, Pathak S, Brown N, Xie B, Fan D, Bucana C, Fidler IJ, Killion JJ. Isolation and Characterization of Metastatic Variants from Human Transitional Cell Carcinoma Passaged by Orthotopic Implantation in Athymic Nude Mice. The Journal of Urology. 1995;154:1532–1538. [PubMed] [Google Scholar]
- Singh AV, Franke AA, Blackburn GL, Zhou J. Soy Phytochemicals Prevent Orthotopic Growth and Metastasis of Bladder Cancer in Mice by Alterations of Cancer Cell Proliferation and Apoptosis and Tumor Angiogenesis. Cancer Res. 2006;66:1851–1858. doi: 10.1158/0008-5472.CAN-05-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagiuchi A, Miyake H, Nomi M, Takenaka A, Fujisawa M. Modulation of the Microenvironment by Growth Factors Regulates the In Vivo Growth of Skeletal Myoblasts. BJU International. 2009;103:1569–1573. doi: 10.1111/j.1464-410x.2008.08318.x. [DOI] [PubMed] [Google Scholar]
- Miyake H, Hara I, Yamanaka K, Gohji K, Arakawa S, Kamidono S. Overexpression of Bcl-2 Enhances Metastatic Potential of Human Bladder Cancer Cells. British Journal of Cancer. 1999;79:1651–1656. doi: 10.1038/sj.bjc.6690264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancellor MB, Yokoyama T, Tirney S, Mattes CE, Ozawa H, Yoshimura N, Groat WCde, Huard J. Preliminary Results of Myoblast Injection into the Urethra and Bladder Wall: a Possible method for the Treatment of Stress Urinary Incontinence and Impaired Detrusor Contractility. Neurourol Urodyn. 2000;19:279–287. doi: 10.1002/(sici)1520-6777(2000)19:3<279::aid-nau9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Dinney CP, Tanguay S, Bucana CD, Eve BY, Fidler IJ. Intravesical Liposomal Muramyl Tripeptide Phosphatidylethanolamine Treatment of Human Bladder Carcinoma Growing in Nude Mice. J Interferon Cytokine Res. 1995;15:585–592. [PubMed] [Google Scholar]
- Mohamedali KA, Kedary D, Sweeney P, Kamaty A, Davisy DW, Evey BY, Huangy S, Thorpez PE, Dinney CP, Rosenblum MG. The Vascular-targeting Fusion toxin VEGF121/rGel Inhibits the Growth of Orthotopic Human Bladder Carcinoma Tumors. Neoplasia. 2005;7:912–920. doi: 10.1593/neo.05292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaton JW, Perrotte P, Inoue K, Dinney CP, Fidler IJ. Interferon-α-mediated Down-Regulation of Angiogenesis-related Genes and Therapy of Bladder Cancer Are Dependent on Optimization of Biological Dose and Schedule. Clinical Cancer Research. 1999;5:2726–2734. [PubMed] [Google Scholar]
- Yokoyama T, Huard J, Pruchnic R, Yoshimura N, Qu Z, Cao B, De Groat WC, Kumon H, Chancellor MB. Muscle-Derived Cell Transplantation and Differentiation into Lower Urinary Tract Smooth Muscle. Urology. 2001;57:826–831. doi: 10.1016/s0090-4295(00)01083-9. [DOI] [PubMed] [Google Scholar]
- Chan E, Patel A, Heston W, Larchian W. Mouse Orthotopic Models for Bladder Cancer Research. BJU International. 1988;104:1286–1291. doi: 10.1111/j.1464-410X.2009.08577.x. [DOI] [PubMed] [Google Scholar]
- Russell PJ, Jelbart M, Wills E, Singh S, Wass J, Witherspoon J, Raghavan D. Establishment and characterization of a new human bladder cancer cell line showing features of squamous and glandular differentiation. Int. J. Cancer. 1988;41:74–74. doi: 10.1002/ijc.2910410115. [DOI] [PubMed] [Google Scholar]
- Ahlering TE, Dubeau L, Jones PA. A new in vivo model to study invasion and metastasis of human bladder carcinoma. Cancer Res. 1987;41:6660–6660. [PubMed] [Google Scholar]
- Heaney JA, Omellas EP, Daly JJ, Lin JC, Rout GR. In vivo growth of human bladder cancer cell lines. Invest. Urol. 1978;15:380–380. [PubMed] [Google Scholar]
- Hadaschik B, Black P, Sea J, Metwalli A, Fazli L, Dinney C, Gleave M, So A. A validated mouse model for orthotopic bladder cancer using transurethral tumor inoculation and bioluminescence imaging. BJU International. 2007;100:1377–1384. doi: 10.1111/j.1464-410X.2007.07165.x. [DOI] [PubMed] [Google Scholar]


