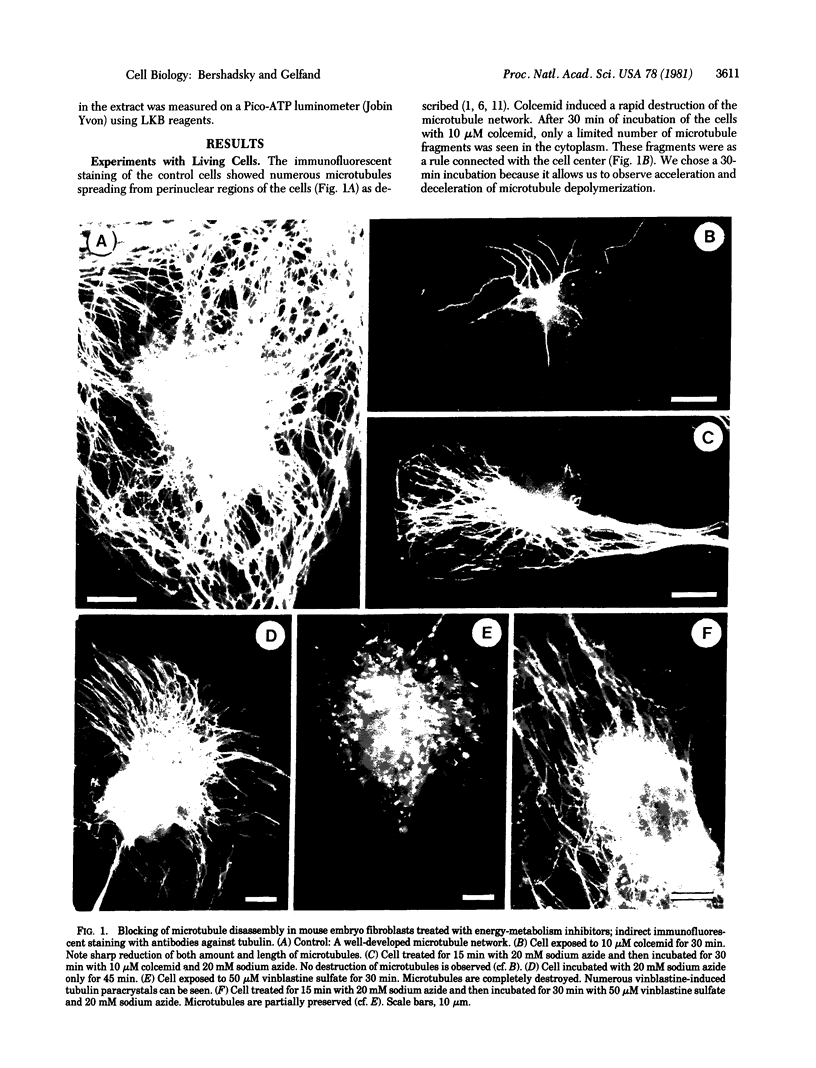
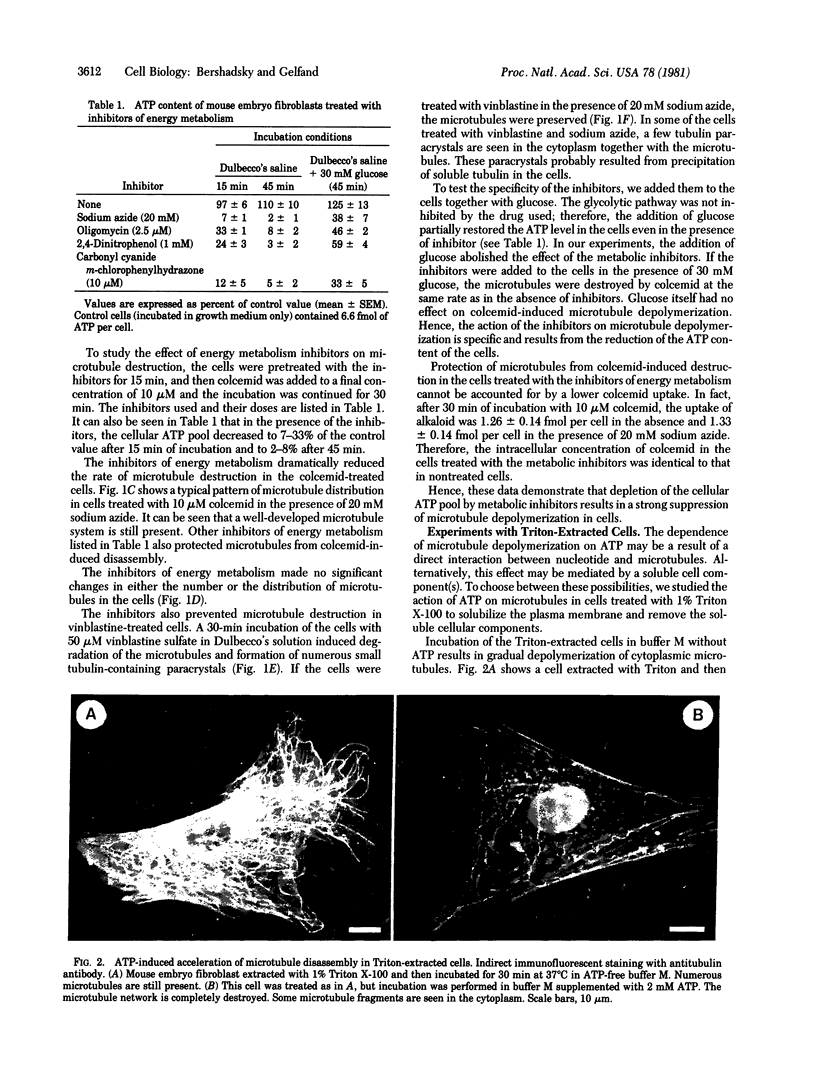
Abstract
Indirect immunofluorescent staining with an antitubulin antibody was used for studying the role of ATP in the regulation of cytoplasmic microtubule disassembly. Depletion of the cellular ATP pool in cultured mouse fibroblasts with various inhibitors of energy metabolism leads to inhibition of the microtubule disassembly induced by colcemid or vinblastine. Glucose added to the inhibitor-containing incubation medium partially restores the cellular ATP content and abolishes the inhibition of microtubule disassembly. The metabolic inhibitors did not change [3H]colcemid uptake by the cells; therefore, their action on the microtubule disassembly was not caused by the reduction in intracellular colcemid. Addition of ATP to the cytoskeleton preparations obtained by Triton X-100 treatment of the cells markedly stimulates microtubule depolymerization. This effect was specific for ATP; it was not observed in the presence of GTP, UTP, CTP, ADP, AMP, adenosine 5'-(beta, gamma-methylene)triphosphate (a nonhydrolyzable analogue of ATP), or inorganic pyrophosphate or tripolyphosphate. Therefore, depletion of the cellular ATP pool reduces the rate of microtubule disassembly whereas addition of ATP increases it. These results suggest that a certain ATP-dependent reaction [most probably, phosphorylation of some of the microtubule protein(s)] controls microtubule disassembly in the cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bershadsky A. D., Gelfand V. I., Svitkina T. M., Tint I. S. Microtubules in mouse embryo fibroblasts extracted with Triton X-100. Cell Biol Int Rep. 1978 Sep;2(5):425–432. doi: 10.1016/0309-1651(78)90093-0. [DOI] [PubMed] [Google Scholar]
- Brenner S. L., Korn E. D. Substoichiometric concentrations of cytochalasin D inhibit actin polymerization. Additional evidence for an F-actin treadmill. J Biol Chem. 1979 Oct 25;254(20):9982–9985. [PubMed] [Google Scholar]
- Brown S. S., Spudich J. A. Cytochalasin inhibits the rate of elongation of actin filament fragments. J Cell Biol. 1979 Dec;83(3):657–662. doi: 10.1083/jcb.83.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Levinson W., Spudich J. A. Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J Supramol Struct. 1976;5(2):119–130. doi: 10.1002/jss.400050203. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. Organization and energy-dependent growth of microtubules in cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2798–2802. doi: 10.1073/pnas.73.8.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson L., Frey T., Zeeberg B., Dalldorf F., Caplow M. Inhibition of microtubule assembly by phosphorylation of microtubule-associated proteins. Biochemistry. 1980 May 27;19(11):2472–2479. doi: 10.1021/bi00552a027. [DOI] [PubMed] [Google Scholar]
- Lin D. C., Tobin K. D., Grumet M., Lin S. Cytochalasins inhibit nuclei-induced actin polymerization by blocking filament elongation. J Cell Biol. 1980 Feb;84(2):455–460. doi: 10.1083/jcb.84.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin A., Richardsson A., Thore A. Continous monitoring of ATP-converting reactions by purified firefly luciferase. Anal Biochem. 1976 Oct;75(2):611–620. doi: 10.1016/0003-2697(76)90116-0. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Addition of colchicine--tubulin complex to microtubule ends: the mechanism of substoichiometric colchicine poisoning. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3466–3470. doi: 10.1073/pnas.74.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Regulation of the microtubule steady state in vitro by ATP. Cell. 1979 Nov;18(3):673–679. doi: 10.1016/0092-8674(79)90122-3. [DOI] [PubMed] [Google Scholar]
- Moskalewski S., Thyberg J., Friberg U. Cold and metabolic inhibitor effects on cytoplasmic microtubules and the Golgi complex in cultured rat epiphyseal chondrocytes. Cell Tissue Res. 1980;210(3):403–415. doi: 10.1007/BF00220198. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. The display of microtubules in transformed cells. Cell. 1977 Nov;12(3):561–571. doi: 10.1016/0092-8674(77)90257-4. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Rudolph S. A., Rosenbaum J. L., Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci U S A. 1975 Jan;72(1):177–181. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V., Celis J. E. Filament arrangements in negatively stained cultured cells: the organization of actin. Cytobiologie. 1978 Feb;16(2):308–325. [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C., Deery W. J., Dickinson P. J. Tubulin-nucleotide interactions during the polymerization and depolymerization of microtubules. Biochemistry. 1976 Sep 21;15(19):4248–4254. doi: 10.1021/bi00664a018. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]