Abstract
Pomegranate juice (PJ), a rich source of polyphenols including ellagitannins, has attracted much attention due to its reported health benefits. This has resulted in the consumption of liquid and powder pomegranate extracts as alternatives to PJ. Therefore establishing the bioavailability of polyphenols from these extract preparations is necessary. Sixteen healthy volunteers sequentially consumed, with a 1-week washout period between treatments, PJ (8 ounces, Wonderful fruit variety), a pomegranate polyphenol liquid extract (POMxl, 8 ounces), and a pomegranate polyphenol powder extract (POMxp, 1,000 mg). The three interventions provided 857, 776, and 755 mg of polyphenols as gallic acid equivalents, respectively. Plasma bioavailability, judged based on ellagic acid levels over a 6-hour period, did not show statistical differences in area under the curve for the three interventions: 0.14 ± 0.05, 0.11 ± 0.03, and 0.11 ± 0.04 μmol · hour/L for PJ, POMxl, and POMxp, respectively. The time of maximum concentration was delayed for POMxp (2.58 ± 0.42 hours) compared to PJ (0.65 ± 0.23 hours) and POMxl (0.94 ± 0.06 hours). Urolithin-A glucuronide, a urinary metabolite of ellagic acid, was not significantly different with the three interventions, reaching levels of approximately 1,000 ng/mL. This study demonstrates that ellagitannin metabolites, delivered from pomegranate fruits, as PJ, POMxl, and POMxp, reach equivalent levels with a delay in time of maximum concentration of POMxp compared to PJ and POMxl.
Key Words: • metabolites, • plasma, • polyphenols, • pomegranate extracts, • pomegranate juice, • urine
Introduction
Pomegranate (Punica granatum L.) fruits are widely consumed as pomegranate juice (PJ), and their health benefits have been studied extensively in animals and humans.1–6 The pomegranate fruit is a rich source of polyphenols, most of which are ellagitannins (ETs). Pomegranate extracts, which incorporate these major polyphenols from the pomegranate fruit, have been developed in dry and liquid forms to provide alternative convenient sources for obtaining the bioactive polyphenols found in PJ. Despite the commercial availability and wide consumption of pomegranate extracts in liquid and dry powder forms as supplements, there have been no studies on their bioavailability in comparison to PJ. We have recently reported on the pharmacokinetics and metabolism of pomegranate polyphenols, after the consumption of PJ (obtained from the Wonderful variety of pomegranate fruits), in normal healthy human volunteers.7 In humans, ETs are converted to ellagic acid (EA) in the small intestines and absorbed with a maximum plasma concentration observed at approximately 1 hour. EA disappears from plasma 6 hours after administration of PJ and is then further converted by gut microflora to urolithin-A derivatives, which are metabolized by phase II enzymes and excreted in human urine for up to 48 hours after consumption of PJ.7
Commercial PJ is obtained from the partial pressing of whole pomegranate fruits, and polyphenol-enriched extracts were prepared for food and dietary supplement use from additional pressing and water extraction to produce a liquid concentrate extract (POMxl) and then further resin purification and drying to produce a powder extract (POMxp) (POM Wonderful LLC, Los Angeles, CA). Because the pharmacokinetics and metabolism of these pomegranate polyphenol extracts have not been determined, we investigated plasma EA pharmacokinetics and urinary urolithin excretion in 16 normal healthy volunteers following adminstration of PJ, POMxl, and POMxp.
Subjects and Methods
Reagents and instruments
All solvents were high-performance liquid chromatography (HPLC) grade from Fisher Scientific Co. (Tustin, CA). EA, formic acid, and phosphoric acid were purchased from Sigma-Aldrich (St. Louis, MO). The HPLC-ultraviolet (UV) analyses were carried out on a Waters Alliance 2690 system equipped with a photo diode array detector (Waters Corp., Milford, MA), and data handling was with Waters Millenium version 3.02 software. The HPLC-mass spectrometry (MS) system consisted of an LCQ Classic Finnigan system (ThermoFinnigan, San Jose, CA), equipped with an HP 1100 series (Palo Alto, CA) HPLC system consisting of an autosampler/injector, quaternary pump, column heater, and diode array detector with Xcalibur version 1.2 software (Finnigan Corp.).
Pomegranate study materials
PJ and pomegranate polyphenol extracts, in liquid (POMxl) and powdered (POMxp) forms, made from the Wonderful fruit variety, are commercially available for human consumption and were provided by POM Wonderful LLC. The compositions of PJ and the pomegranate extracts have been previously reported.7,8 Briefly, PJ is obtained from the first-press squeezing of whole pomegranate fruits. POMxl is a liquid concentrated extract that is produced by extraction of the remaining fruit residue obtained after the first pressing for PJ. POMxp is a powdered solid obtained from a solid-phase extraction of POMxl to produce a powder with a high concentration of polyphenols. The extracts are proprietary standardized (to at least 90% pomegranate hydrolysable tannins [Jess Reed, University of Wisconsin-Madison, Madison, WI]). The pomegranate test materials were standardized for total phenolics, as gallic acid equivalents, by Covance Analytical Laboratories, Inc. (Madison). Eight ounces of PJ and POMxl beverages contain 857 and 776 mg of gallic acid equivalents, respectively. One capsule of POMxp contains 1,000 mg of dry powder and provides 755 mg of gallic acid equivalents.
Human study design
Sixteen normal healthy human subjects (five men and 11 women) with a mean age of 29.7 ± 8.3 years old and mean body mass index of 24.1 ± 3.6 kg/m2 were recruited for the study. Subjects had no clinical disease and were not on any weight-reducing regimen as determined using a medical history questionnaire. Subjects were asked to consume a “polyphenol-free” diet (no fruits, vegetables, wine, tea, etc.) and to avoid antioxidant and herbal supplements for 4 days prior to the study day. Female subjects were neither pregnant nor lactating. Each subject sequentially received PJ (8 ounces), followed by a 1-week washout, then POMxl (1 teaspoon diluted to 8 ounces with water with added non-polyphenolic flavoring and colors), followed by a 1-week washout, and then POMxp (1,000 mg of encapsulated powder of pomegranate extract). On the study day, participants, after fasting overnight, were provided with a light breakfast of toast with butter or cereal and low-fat milk. After 20 minutes, baseline EDTA-coagulated blood was drawn, the participants ingested the pomegranate test materials, and then further blood samples were collected at 0.5, 1, 2, 3, 4, 6, and 24 hours after ingestion. Participants self-collected 2 × 24-hour urine samples (in 12-hour split collection) after the administration of each test material. Participants kept urine samples refrigerated at 4°C until delivery to our laboratory. UCLA Institutional Review Board approval for studies with human subjects was obtained, which complied with the Helsinki Declaration of 1975 as revised in 1983. The protocol was fully explained to all subjects, and informed consent was obtained prior to participation.
Preparation of plasma samples
The EDTA-coagulated blood samples were centrifuged at 3,000 g for 10 minutes at 4°C, and the plasma was quickly removed and stored at −80°C until analyses.7 Plasma (1 mL) was adjusted to pH 2.5 with 1 mol/L potassium dihydrogen phosphate solution and 50% phosphoric acid, vortex-mixed with acetonitrile (1 mL) for 1 minute, and centrifuged at 250 g for 15 minutes at 5°C. After evaporation to dryness at 35°C in a SpeedVac (Savant, Sunnyvale, CA), the supernatant liquor was reconstituted in methanol (200 μL) and analyzed for EA levels by HPLC-UV.
Preparation of urine samples
The 2 × 24-hour batch urine was collected and immediately frozen at −20°C. A urine sample (1 mL) was diluted with H2O (2% formic acid)/methanol (9:1 vol/vol; 1 mL), vortex-mixed for 30 seconds, and centrifuged at 3,000 g for 15 minutes at 5°C. The supernatant was filtered and analyzed for EA metabolites by HPLC-MS.
HPLC-UV analyses
Conditions were as previously reported.7 EA was purchased from Sigma, and urolithin A-glucuronide (UAG) was isolated in our laboratory from human urine as previously reported9 with slight modification of the use of an XAD-16 instead of an XAD-2 (Amberlite® [Rohm & Haas, Philadelphia, PA] resin, Sigma) column. The standards were individually serially diluted to afford five different concentrations, which were used for construction of calibration curves. Each standard was injected in triplicate, and concentrations were determined from the peak area by using the equation for linear regression obtained from the calibration curve. Control plasma (for EA) or urine (for UAG) was spiked with individual solutions, extracted, and analyzed as previously reported.7 The calibration curve was linear (R2 = 0.9975) over a concentration range of 3.3–0.05 μmol/L, and the calculated lower limit of quantitation of EA and UAG was 0.01 μmol/L. The recoveries of EA from human plasma were 103%, 120%, 113%, and 117% for concentrations of 1.66, 0.83, 0.42, and 0.21 μmol/L, respectively.
HPLC-MS analyses
Conditions for detection of EA metabolites were as follows: column, Symmetry C-18, 100 mm × 2.1 mm i.d., particle size 3.5 μm (Waters Corp.); solvent A, 2% formic acid in water; solvent B, 2% formic acid in methanol; gradient % A in B, initial 99%, for 30 minutes 80%, for 45 minutes 60%, for 60 minutes 5%; run time 60 minutes; flow rate 0.15 mL/minute; injection volume 20 μL. MS parameters were as follows: ionization mode, electron spray ionization in both positive and negative modes; scan range, 120–1,500 amu; scan rate 1 scan/second; cone voltage 17 eV. Peak identities of EA metabolites were obtained by matching their molecular ions (M-H+) or (M+H+) obtained by electro-spray ionization/MS and MS/MS with the expected theoretical molecular weights from literature data as follows: UAG = M-H m/z 403, MS/MS = M-H m/z 227 (corresponding to urolithin A).7,9
Statistical analysis
Pharmacokinetic curves were fitted using a pharmacokinetic mixed effects model.10 The model assumed one compartment with first-order processes. The use of a mixed effects approach allowed for the simultaneous fit of individual subject curves and estimation of population-averaged responses. This response is characterized by the area under the plasma concentration–time curve (AUC), the peak plasma concentration, and the time to peak concentration. Models were constructed using WinBUGS.10 Data in the text are mean ± SD values.
Results
The appearance and disappearance of EA in plasma samples was equivalent for PJ, POMxl, and POMxp based on the pharmacokinetic parameters detailed in Table 1. EA increased in the plasma of all subjects following administration of PJ or the pomegranate extract preparations and cleared over a 6-hour period. The pharmacokinetics of POMxp differed from those of PJ and POMxl in that plasma EA concentration reached a maximum at either 2 or 3 hours in different individuals compared to approximately 1 hour for the beverages. Nonetheless, when the AUC was calculated, there were no statistical differences in the AUC for EA after consumption of PJ, POMxl, or POMxp.
Table 1.
Pharmacokinetic Parameter Estimates for EA Detected in Human Plasma after Consumption of a Single Dose of PJ (8 Ounces) Followed by Pomegranate Polyphenol Liquid Extract (POMxl, 8 Ounces), Followed by Pomegranate Polyphenol Powder Extract (POMxp, 1,000 mg) with a 1-Week Washout Period between Treatments
| Treatment, parameter | Mean ± SDa | 2.5% quantileb | 97.5% quantileb |
|---|---|---|---|
| PJ | |||
| Ke | 0.68 ± 0.25 | 0.39 | 1.20 |
| Ka | 3.99 ± 6.22 | 1.44 | 10.66 |
| Tmax (hours) | 0.65 ± 0.23 | 0.09 | 0.96 |
| Cmax (μmol/L) | 0.06 ± 0.01 | 0.05 | 0.07 |
| AUC (μmol · hour/L) | 0.14 ± 0.05 | 0.11 | 0.18 |
| t½E (hours) | 1.14 ± 0.34 | 0.57 | 1.74 |
| POMx1 | |||
| Ke | 1.06 ± 0.06 | 0.94 | 1.19 |
| Ka | 1.06 ± 0.06 | 0.94 | 1.19 |
| Tmax (hours) | 0.94 ± 0.06 | 0.84 | 1.06 |
| Cmax (μmol/L) | 0.04 ± 0.01 | 0.04 | 0.05 |
| AUC (μmol · hour/L) | 0.11 ± 0.03 | 0.09 | 0.13 |
| t½E (hours) | 0.65 ± 0.04 | 0.58 | 0.74 |
| POMxp | |||
| Ke | 0.39 ± 0.06 | 0.28 | 0.52 |
| Ka | 0.39 ± 0.06 | 0.28 | 0.52 |
| Tmax (hours) | 2.58 ± 0.42 | 1.93 | 3.54 |
| Cmax (μmol/L) | 0.02 ± 0.01 | 0.02 | 0.03 |
| AUC (μmol · hour/L) | 0.11 ± 0.04 | 0.10 | 0.21 |
| t½E (hours) | 1.79 ± 0.29 | 1.34 | 2.46 |
AUC, integrated area under the curve from t = 0 to infinity; Cmax, maximum concentration; Ke, rate constant for elimination; Ka, rate constant for absorption; Tmax, time point at which maximum plasma concentration occurs; t½E, elimination half-life.
Pharmacokinetic data are mean ± SD values (n = 16) over a 6-hour period as quantified by HPLC-UV.
95% Bayesian credible intervals (2.5th, 97.5th quantile).
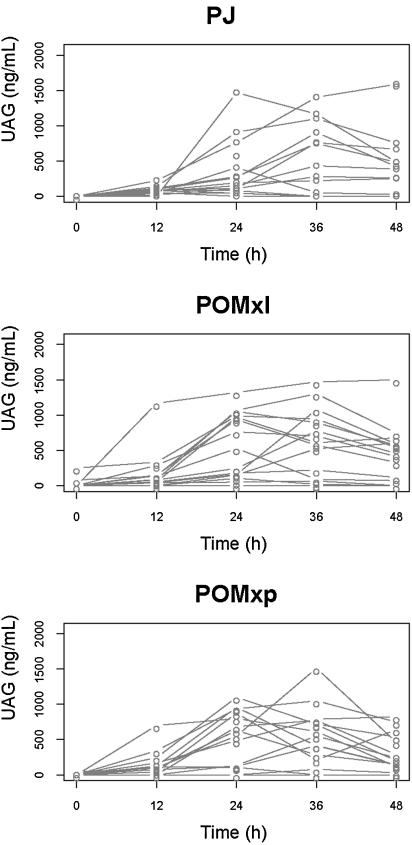
UAG, a metabolite of EA, was detected in urine samples, reaching maximum concentrations of approximately 1,000 ng/mL and remained elevated for over 48 hours after consumption of pomegranate polyphenols (Fig. 1). In the majority of participants the excretion of urinary UAG was not completed at 48 hours after the intervention. Future studies will need to collect urine for an extended time period, beyond 48 hours after ingestion of pomegranate materials, to evaluate for completion of urinary urolithin excretion.
FIG. 1.
Pharmacokinetic profile of UAG detected in urine samples collected up to 48 hours after ingestion of PJ, POMxl, and POMxp. Values are for 13 volunteers.
Discussion
PJ contains multiple phenolic compounds such as anthocyanins and ETs. However, ETs comprise most of the polyphenols found in commercial PJ, POMxp, and POMxl. Therefore the metabolism of these compounds, i.e., ETs, were the focus of this study.7,11 In our prior study, we demonstrated that EA appears in plasma after consumption of PJ.7 PJ consumed daily can have significant effects on cardiovascular biomarkers3,4 and on circulating prostate-specific antigen in prostate cancer patients,6 which is assumed to be due to the actions of EA derived from ETs and of EA metabolites, including UAG. In the present study, we compared the absorption of EA from PJ, POMxl, and POMxp and the excretion of urinary UAG.
The present study confirmed the rapid absorption of EA from PJ, POMxl, and POMxp and demonstrated that UAG was excreted in the urine for up to 48 hours after administration of PJ, POMxl, and POMxp. EA metabolites have been previously reported to be present in human urine up to 56 hours after PJ intake.9 Urinary EA metabolites, such as urolithins, arise from biotransformation of EA by the intestinal microflora, which then undergo conjugation with methyl, glucuronyl, and sulfate groups and are excreted in the urine.7,9 In the present study, UAG was the most commonly found urinary metabolite of EA. Other urinary metabolites, including sulfated and methylated forms of urolithin-A, were also detected in this study (data not shown) but with considerable interindividual variability as previously reported.7,9 Urolithin metabolism may be dependent on bowel transit time or intestinal flora as has been defined for soy isoflavones,12 and this deserves further study. Nevertheless, this research provides valuable knowledge about pomegranates and metabolomics (the integrated study of molecules produced by metabolism). It is known that the large-bowel microflora produce many of the food or dietary supplement compounds absorbed, metabolized, and passed through the blood and secreted in urine and saliva.13
In summary, we were able to demonstrate that the consumption of pomegranate polyphenols delivered by PJ, POMxl, and POMxp resulted in similar absorption of EA. However, the time course of POMxp absorption was different from that of the beverages, PJ and POMxl, and clustered around two different delayed peaks of 2 hours and 3 hours compared to approximately 1 hour for PJ and POMxl. However, despite the delayed appearance, the AUC of plasma EA was not statistically different with PJ, POMxl, and POMxp treatment. The delayed plasma appearance of EA observed by our group in this study is similar to a finding from our prior study comparing a green tea extract supplement versus tea beverages.14 In that study, polyphenol absorption following consumption of a green tea extract supplement was delayed by greater than 1 hour when compared to tea beverages with similar amounts of total tea polyphenols.14 POMxp was administered in a gel capsule, and it is possible that the delayed absorption was due to the time necessary to dissolve the capsule material and release the contents. There are a number of possible explanations for the delayed absorption, but the clustering among different individuals of plasma EA at time to peak concentration of either 2 or 3 hours is interesting. There may be differences in gut motility, in gut bacteria, or in the hydrolysis of ETs within the stirred layer of intestinal epithelial cells.
Conflict of Interest Statement
There are no financial or contractual agreements that might cause conflict of interest for any of the co-authors involved in this study.
Acknowledgments
This work was supported by the Stewart and Lynda Resnick Revocable Trust and by NIH/NCI grant P50AT00151.
References
- 1.Aviram M. Dornfeld L. Rosenblat M. Volkova N. Kaplan M. Hayek T. Presser D. Fuhrman B. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in the atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 2000;71:1062–1076. doi: 10.1093/ajcn/71.5.1062. [DOI] [PubMed] [Google Scholar]
- 2.Aviram M. Dornfield L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis. 2001;158:195–198. doi: 10.1016/s0021-9150(01)00412-9. [DOI] [PubMed] [Google Scholar]
- 3.Aviram M. Rosenblat M. Gaitini D. Nitecki S. Hoffman A. Dornfeld L. Volkova N. Presser D. Attias J. Liker H. Hayek T. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr. 2004;3:423–433. doi: 10.1016/j.clnu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Sumner MD. Elliott-Eller M. Weidner G. Daubenmier JJ. Chew MH. Marlin R. Raisin CJ. Ornish D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am J Cardiol. 2005;96:810–814. doi: 10.1016/j.amjcard.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Rosenblat M. Hayek T. Aviram M. Anti-oxidative effects of pomegranate juice consumption by diabetic patients on serum and on macrophages. Atherosclerosis. 2006;187:363–371. doi: 10.1016/j.atherosclerosis.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Pantuck AJ. Leppert JT. Zomorodian N. Aronson W. Hong J. Barnard RJ. Seeram NP. Liker H. Wang H-E. Elashoff R. Heber D. Aviram M. Ignarro L. Belldegrun A. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12:4018–4026. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 7.Seeram NP. Henning SM. Zhang Y. Suchard M. Li Z. Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006;136:2481–2485. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 8.Heber D. Seeram NP. Wyatt H. Henning SM. Zhang Y. Ogden LG. Dreher M. Hill JO. Safety and antioxidant activity of a pomegranate ellagitannin-enriched polyphenol extract dietary supplement in overweight individuals with increased waist size. J Agric Food Chem. 2007;55:10050–10054. doi: 10.1021/jf071689v. [DOI] [PubMed] [Google Scholar]
- 9.Cerda B. Espin JC. Parra S. Martinez P. Tomas-Barberan FA. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr. 2004;43:205–220. doi: 10.1007/s00394-004-0461-7. [DOI] [PubMed] [Google Scholar]
- 10.Racine-Poon A. Wakefield JC. Statistical methods for population pharmacokinetic modelling. Stat Methods Med Res. 1998:63–84. doi: 10.1177/096228029800700106. [DOI] [PubMed] [Google Scholar]
- 11.Gil MI. Tomas-Barberan FA. Hess-Pierce B. Holcroft DM. Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson C. Frankenfield CL. Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med. 2005;230:155–170. doi: 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]
- 13.Zeisel SH. Nutrigenomics and metabolomics will change clinical nutrition and public health practice: insights from studies on dietary requirements for choline. Am J Clin Nutr. 2007;86:542–548. doi: 10.1093/ajcn/86.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henning SM. Niu Y. Lee NH. Thames GD. Minutti RR. Wang H. Go VLW. Heber D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am J Clin Nutr. 2004;80:1558–1564. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]