Abstract
Over hundreds of millions of years, animals have evolved endogenous lipoprotein nanoparticles for shuttling hydrophobic molecules to different parts of the body. In the last 70 years, scientists have developed an understanding of lipoprotein function, often in relationship to lipid transport and heart disease. Such biocompatible, lipid–protein complexes are also ideal for loading and delivering cancer therapeutic and diagnostic agents, which means that lipoprotein and lipoprotein-inspired nanoparticles also offer opportunities for cancer theranostics. By mimicking the endogenous shape and structure of lipoproteins, the nanocarrier can remain in circulation for an extended period of time, while largely evading the reticuloendothelial cells in the body’s defenses. The small size (less than 30 nm) of the low-density (LDL) and high-density (HDL) classes of lipoproteins allows them to maneuver deeply into tumors. Furthermore, lipoproteins can be targeted to their endogenous receptors, when those are implicated in cancer, or to other cancer receptors.
In this Account, we review the field of lipoprotein-inspired nanoparticles related to the delivery of cancer imaging and therapy agents. LDL has innate cancer targeting potential and has been used to incorporate diverse hydrophobic molecules and deliver them to tumors. Nature’s method of rerouting LDL in atherosclerosis provides a strategy to extend the cancer targeting potential of lipoproteins beyond its narrow purview. Although LDL has shown promise as a drug nanocarrier for cancer imaging and therapy, increasing evidence indicates that HDL, the smallest lipoprotein, may also be of use for drug targeting and uptake into cancer cells. We also discuss how synthetic HDL-like nanoparticles, which do not include human or recombinant proteins, can deliver molecules directly to the cytoplasm of certain cancer cells, effectively bypassing the endosomal compartment. This strategy could allow HDL-like nanoparticles to be used to deliver drugs that have increased activity in the cytoplasm. Lipoprotein nanoparticles have evolved to be ideal delivery vehicles, and because of that specialized function, they have the potential to improve cancer theranostics.
Introduction
Current diagnosis and treatment options for many cancers must be improved, despite significant advances over the past decades. Extensive preclinical research in cancer nanotechnology holds much further potential to generate improved options for diagnosing and treating the disease. There are several features of nanoparticles that make them suited for both diagnosis and therapy. One central advantage of therapeutic nanoparticles is the concept of high payload incorporation (i.e., drug) into a nanoparticle. In carrying a large payload, nanocarriers can favorably modulate biodistribution and pharmacokinetic profiles of the drug formulations. Another advantage of most nanoparticles is their multimodal loading capability. The surface or core of the nanoparticle may be loaded with multiple agents, so that treatment and imaging of treatment can occur simultaneously. Chelators may be included for radioisotopes for PET/SPECT imaging and other metals for CT and MRI in vivo imaging. Thus, this presents a new opportunity for monitoring drug distribution. While tritiated drugs have been used to monitor biodistribution of small molecule drugs, using these for routine treatment has not been practical. Since nanoparticles have the capacity to be accurately tracked in vivo, this opens up many new avenues for nanomedicine. For instance, drug biodistribution could be monitored on a patient by patient basis to determine whether drug accumulation is sufficient for a therapeutic effect. Preclinical nanomedicine research is already firmly entrenched in combined imaging and therapy, and as imaging modalities improve, so will the monitoring methods. To aid in the realization of these goals, an ideal theranostic nanocarrier should have excellent biocompatibility, good loading efficiency, therapeutic shielding capacity, and targeting capability and be amenable to multimodal contrast agent modification. In many ways, lipoprotein-inspired nanoparticles can address these goals and are a promising platform for theranostic cancer nanomedicine.
Lipoproteins, Nature’s Nanoparticles
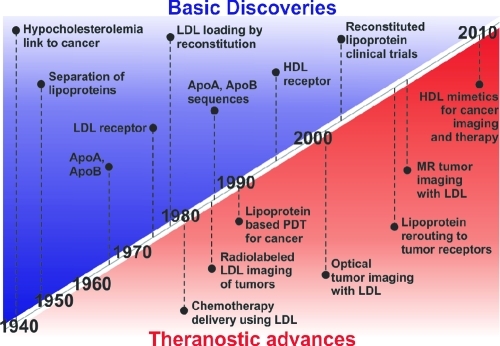
An ever-increasing arsenal of diverse nanoparticles exists for potential cancer treatment. Validating how these behave with regards to biocompatibility and loading ability is a considerable undertaking that will continue for many years to come. Although many of these nanoparticles have been developed in the past 20 years, one notable exception is the lipoprotein class of nanoparticles, which are naturally present in most metazoan species and are essential in humans to control lipid metabolism.(1) These endogenous nanoparticles are utilized in organisms to transport hydrophobic cholesterol and triglycerides to cells through the circulatory system. The human endogenous lipoprotein system consists of several types of lipoprotein nanoparticles that have varying structure and function, differentiated by their hydrodynamic size and the apolipoprotein(s) bound on their surfaces. The properties of these different lipoproteins are summarized in Table 1. The amphiphilic nature of the phospholipid helps maintain the lipid emulsion in aqueous solution, while the protein stabilizes the emulsion. The lipoprotein nanoparticle field has benefited from extensive history of basic discoveries stemming from research in cholesterol metabolism. Some of those discoveries overlap with the lipoprotein breakthroughs relevant to cancer shown in Figure 1, along with the significant theranostic advances.2−7
Table 1. Characteristics of Four Classes of Lipoproteinsa.
| surface component (mol %) |
core lipids (mol %) |
||||||
|---|---|---|---|---|---|---|---|
| diameter (nm) | protein components | protein | phospholipid | cholesterol | cholesterol ester | triglycerides | |
| chylomicron | 75–1200 | ApoB-48 | 2 | 63 | 35 | 5 | 95 |
| VLDL | 30–80 | ApoB-100 | 2 | 55 | 43 | 24 | 76 |
| LDL | 18–25 | ApoB-100 | 2 | 58 | 42 | 19 | 81 |
| HDL | 5–12 | ApoA-I, A-II, E, C | 2 | 72 | 23 | 82 | 18 |
Adapted with permission from Reference (59).
Figure 1.
Key discoveries and theranostic advances in lipoprotein cancer research.
Lipoproteins are ideal for the delivery of cancer drug and imaging agents since they are able to circulate in the bloodstream for a significant amount of time,(8) the hydrophobic core facilitates the incorporation of poorly soluble drugs or imaging agents, and they are highly amenable to bioconjugation. The structural aspects of lipoproteins may also play a critical role in their utility. Studies have shown that the extracellular matrix of some cancer tumors may impede the diffusion of nanoparticles due to the presence of collagen fibrils.(9) Electron microscopy measurements have shown the opening between these spaces to be less than 40 nm, a distance which may hinder the penetration of larger nanoparticles. This size range is difficult for phospholipid-based nanoparticles due to the destabilizing effects of increasing lipid curvature. As lipid curvature increases, so too does the hydrophobic surface exposed to the water. Lipoproteins are able to surmount this instability by intercalating amphipathic α-helical proteins between the phospholipids. Since low-density (LDL) and high-density lipoproteins (HDL) are the only lipoproteins with diameters less than 40 nm, we will focus our discussion on these two classes of lipoproteins as they relate to cancer.
Transforming Lipoproteins into Versatile Nanoparticles
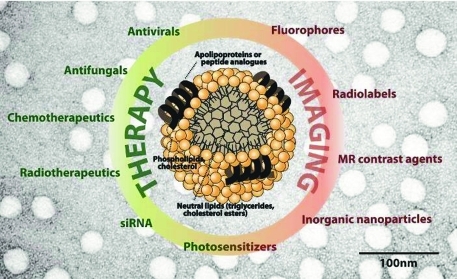
There are three strategies to modify lipoproteins with imaging or drug agents (Figure 2). These are covalent modification of the phospholipid or protein, intercalation of the agent into the phospholipid shell, and encapsulation of the agent in the nanoparticle core through the reconstitution technique. Loading an agent at a specific site on the nanoparticle is dependent on the method of incorporation as well as the chemical nature of the molecule.
Figure 2.
Examples of drugs, imaging agents, and targeting ligands incorporated into different locations of lipoprotein-like nanoparticles.
Surface Loading
Surface loading involves noncovalent intercalation of the agent within the surface of the lipoprotein. Weak interactions including van der Waals forces govern the degree of intercalation of the agent into the particle surface. Agents that intercalate have a level of amphiphilicity that allows its structure to be partially buried in the surface of the lipoprotein leaving the hydrophilic face partially exposed to the aqueous environment for hydrogen bonding or ionic interactions. The balance between these forces influences the loading efficiency and stability. This strategy has been used in used in combination with the cholate dialysis technique to make drug loading lipoprotein nanoparticles.(10)
Covalent Modification
Covalent modification of the lipoprotein involves conjugating the therapeutic or imaging agent to the surface of the apolipoprotein or onto the phospholipid headgroup. Typical amino acids that are used for conjugation include the lysine, arginine, tyrosine, and cysteine residues.(11) In addition to the surface protein or peptides, covalent modification can also be made on phospholipid headgroups.(11)
Core Loading through Reconstitution
Hydrophobic molecules can also be encapsulated into the nanoparticle core. Generally, this involves exchanging the lipoprotein core lipids with the desired hydrophobic molecule through lyophilization and organic extraction.(12) Another strategy involves synthesizing the lipoprotein-like nanoparticle from its component parts through methods such as cosonication or cholate dialysis.(13)
LDL: The Prototypic Lipoprotein for Cancer Theranostics
Cholesterol is one of several hydrophobic molecules transported by lipoproteins. In the body, it functions as an essential component of the plasma membrane and regulates the fluidity of the lipid bilayer. Cholesterol is derived through dietary sources or de novo biogenesis. As early as 1930, it was noted that patients with leukemia also had a greater chance of having hypocholesterolemia.(14) Later studies showed that the hypocholesterolemia may be due to increased LDLR presentation in the malignancy.(15) This observation has also been made for other cancer types.(16)
LDL labeled with radiotracers has been used by investigators for over 20 years to image and characterize LDL tumor accumulation within animals. Iodine-131 (131I), iodine-125 (125I), and technetium-99m (99mTc) have been the most commonly used radiolabels for in vivo imaging. Radioactive iodine monochloride or reductive coupling of [99mTc]pertechnetate using sodium dithionite was used for covalent modification of the protein. Studies with 99mTc-conjugated LDL in B16 melanoma-bearing mice showed accumulation in the tumor after 18 h (Figure 3a).(17) While the gamma camera used to capture the accumulation of the radiolabeled LDL was poor in resolution, it provided a glimpse in real-time of the distribution of LDL within the host. In addition to gamma imaging, other imaging modalities and probes have also been used to observe other pathological situations. The effect of liver LDL uptake in normal and hypocholesterolemic rabbits was imaged by PET and gamma scintigraphy using gallium-68 and indium-111, respectively.(18) These metals were attached to LDL using a bifunctional metal chelator, diethylene triamine pentaacetic acid (DTPA) anhydride, which was covalently attached to apoB-100. While cancer was not the focus of these studies, these techniques can be applied to the imaging of neoplastic malignancies.
Figure 3.
(a) Gamma scintigraphy image of mouse bearing B-16 melanoma tumor upon uptake of 99mTc-labeled LDL (ref (17)). (b) Magnetic resonance imaging of mouse xenograft (ref (20)). (c) Optical imaging of cancer tumor. (d) Demonstrated uptake of LDL loaded with CT contrast in HepG2 cancer cells (ref (25)). Panels a, b, and d are reproduced with permission from the corresponding references.
LDL has also been used as a nanocarrier for magnetic resonance imaging contrast agents. Using a modified-DTPA molecule originally synthesized by Jasanada et al.,(19) our group chelated gadolinium (Gd) and intercalated the complex into the lipid layer of LDL.(20) Through this procedure, we were able to insert 180 molecules of Gd onto the surface of the particle. While the relaxivity of the construct on a per Gd basis was negligibly greater than free gadolinium, a clear demarcation of the tumor area could be observed upon injection into tumor-bearing mice over a period of 24–48 h (Figure 3b). Building on this work, Crich et al. substituted DTPA with another Gd chelator named AAZTAC17, which features enhanced relaxivity due to water molecule coordination to the Gd ion.(21) They found that this complex can be stably incorporated into LDL and had a 2.5-fold enhancement over the free Gd complex.
LDL nanoparticles have long been used for optical imaging. Fluorescent probes have been incorporated into lipoproteins to study their function.(12) Our laboratory has been interested in using optical techniques to image the uptake of LDL within tumors (Figure 3c). Light penetration is a concern with respect to optical imaging through tissues. To meet this challenge, we developed a series of near-infrared probes that were suitable for in vivo imaging.22−24
To extend the applicability of LDL for the diagnostic imaging of tumors, we asked whether LDL could be used as a carrier for (CT) contrast agents through the incorporation of polyiodinated triglyceride (ITG) molecules.(25) ITG-loaded LDL was taken up by HepG2 cells and showed enhanced contrast compared with the cells alone and was inhibited by the addition of LDL in excess (Figure 3d).
Endogenous LDL is able to transport an impressive number of hydrophobic molecules in each particle. Estimates of this number range from 1200 to 1300 cholesterol esters and 250–300 triglycerides.(26) This efficient loading of molecules has driven interest in discovering whether drug delivery vehicles can be constructed that mimic these properties. There have been many reports of antineoplastic drugs that have been incorporated into LDL for cancer therapy.(27) While the drugs capable of being accommodated within LDL are diverse, it is generally assumed that hydrophobic compounds partition more favorably to the LDL core and amphipathic compounds tend to remain closer to the surface of LDL through less stable interactions. Photodynamic therapy (PDT) agents have also been loaded into LDL. In studying the use of porphyrin photosensitizers for cancer therapy, it was noticed that porphyrins had a propensity to associate with serum lipoproteins.(28) Furthermore, it was concluded that the tumor localization properties of the porphyrin might also be influenced by this interaction. The incorporation of phthalocyanines,(22) benzoporphyrins,(29) and tetraphenylporphines(30) (TPP) have been reported. Our group has also focused on incorporating photosensitizers within LDL particles through core-loading reconstitution, which we predicted would improve the incorporation yield and serum stability of the photosensitizer. Using this strategy, we have successfully incorporated lipid-anchored NIR-active photosensitizers tetra-t-butyl silicon phthalocyanine,(22) naphthalocyanine,(23) and pyropheophorbide.(31)
LDL Rerouting: Expanding the Horizon of Lipoprotein Cancer Targeting
One of the challenges of using LDLR as a target for cancer theranostics is that the strategy is limited to cancers where the receptor is highly expressed. For situations where the LDLR is not highly expressed, a method for targeting LDL nanoparticles to other cancer receptors is warranted and will expand the purview of all lipoprotein-based nanoparticles. Remarkably, nature has already devised such a strategy as demonstrated through the generation of oxidized LDL in atherosclerosis. The oxidation of native LDL apolipoproteins leads to a change in uptake from LDLR to receptors involved in foam cell generation, which is a major factor in atherosclerosis. Bijsterbosch et al. showed that a similar strategy of protein modification through reductive lactosamination was able to increase the binding of LDL to Kupffer and parenchymal cells in the liver.(32) To extend this approach, our laboratory developed a general method for rerouting LDL nanoparticles to other cancer targets (Figure 4a). The principle of the technique involves modifying a portion of the 225 exposed lysine residues that exist on the highly basic domain on the apolipoprotein; 53 lysine residues have a pKa of 8.9 due to their local environment, which makes them highly amenable to modification.(33) Modification of 20% of the side chain ε-amino groups on lysine residues through alkylation abolished the binding of LDL nanoparticles to the LDLR.(33) This phenomenon removes the native targeting of the LDL nanoparticle while at the same time increasing its interaction with other cancer targets.(34) Using the folate receptor (FR) as a cancer target, we modified the lysines with folate on drug-loaded LDL nanoparticles, thereby rerouting the particle from its endogenous receptor to the FR (Figure 4b). Furthermore, this application of tumor targeting has also been shown to be extendable to HDL nanoparticles (Figure 4c).(35)
Figure 4.
(a) Schematic diagram showing the process of rerouting lipoproteins to new cancer targets. (b) Whole body fluorescence image showing folate-modified LDL uptake in FR-positive and negative cell lines (ref (24)). (c) Rerouting of HDL nanoparticles from SR-BI to the FR (ref (35)). Panels b and c are reproduced with permission from their corresponding references.
HDL: The Smallest Lipoprotein
Properties and Advantages of Using HDL
In contrast to the LDL, HDL forms smaller lipid nanoparticles (5–12 nm) stabilized by the interactions of surface apolipoprotein A-1 (ApoA-1) proteins. This 243 amino acid protein adopts an amphipathic α-helix structure and interacts with phospholipids to generate discoidal or spherical structures depending on the stoichiometry of the components.(36) Structural studies on each of these forms of HDL have shown that discoidal HDL is comprised of two ApoA-1 proteins per particle arranged in a belt-like structure around a bilayer of lipids. In contrast, spherical HDL has been shown to contain two to three ApoA-1 proteins arranged to form a trefoil structure around the spherical emulsion.(36)
Selective Uptake of Cholesterol Esters
Functionally, HDL is responsible for facilitating transport of cholesterol from tissues back to the liver for catabolism through a process known as reverse cholesterol transport. It also delivers cholesterol esters to steroidogenic tissues. The specific receptor involved with HDL cholesterol uptake was discovered to be the scavenger receptor class B type I (SR-BI) through molecular cloning and pharmacological techniques.(2) Transport of CE across the cell membrane was found to be mediated through a nonendocytic pathway. This mechanism, referred to as selective uptake, is thought to occur by a process in which a hydrophobic channel forms in SR-BI that allows for the transfer of core molecules across the membrane into the cell.(37)
SR-BI and Cancer
There is mounting evidence that HDL may also be a source of cholesterol in some malignancies. HDL cholesterol levels were found to be lower in cancer patients when compared with age- and sex-matched noncancer patients.(38) In humans, HDL has a role in cholesterol efflux to cells. Similar to LDL, this observed decrease in serum HDL might be a result of increased SR-BI receptor expression on cancer cells, which shifts the serum HDL cholesterol levels to a hypocholesterolemic state. In support of this view, increased SR-BI expression has been reported in cancer cell lines including prostate adenocarcinoma,(39) cervical carcinoma,(40) breast cancer (MCF-7/HBL-100),(41) lung cancer cell lines,(42) and hepatoma cell lines (HepG2 and HUH-7).(43)
HDL as Nanocarriers for Theranostic Agents
One of the first groups to systematically study the usage of HDL as a nanocarrier for therapeutics was the Van Berkel laboratory. They found that lipophilic antiviral-analogs dioleyl iododeoxyuridine and 9-(2-phosphonylmethoxyethyl)adenine could be incorporated and delivered to liver cells.(44) Other groups have also investigated the usage of HDL as a nanocarrier for chemotherapeutics. Lacko et al. studied the use of paclitaxel-loaded HDL particles on cancer cells and found the IC50 to be 5–20 times lower than that of the free drug.(13)
As a promising avenue of research, we designed a dual function theranostic probe, bacteriochlorin e6 bisoleate, which can be easily incorporated into HDL (Figure 5a).(40) This fluorescent photosensitizer can be tracked in vivo through NIR fluorescence imaging and can be activated to generate singlet oxygen upon light irradiation. Using fluorophore-loaded HDL, we were also able to monitor the uptake of the fluorescent payload over time using the dorsal skinfold window chamber technique, which allows for monitoring of nanoparticle tumor penetration with high spatial and temporal resolution (Figure 5b).
Figure 5.
(a) Optical imaging of a SR-BI expressing cancer tumor using HDL nanoparticles loaded with NIR fluorescent photosensitizer bacteriochlorin e6 bisoleate (ref (40)). (b) Fluorophore-loaded HDL (red) showing distribution to GFP-expressing tumor (green) through the bloodstream (cyan). Figure 5A is reproduced with permission from the corresponding reference.
HDL-Inspired Peptide-Phospholipid Scaffold Nanoparticles
While HDL represents a useful agent for the delivery of therapeutic and imaging agents to tumor cells, we foresee several challenges on the road to clinical application. One in particular is the source of apolipoproteins. While recombinant ApoA-1 has been made in bacterial expression systems, there are challenges relating to purity and quantity that remain to be overcome.(45) Isolation of ApoA-1 using methods such as ultracentrifugation or delipidation procedures appears to be the most common but has long processing times. Finally, there are risks of pathogen contamination in human blood-derived samples. Despite these challenges, the purification of ApoA-1 from up to 700 kg of human plasma precipitate has been reported.(46) The procedure involves several steps to ensure that the isolated protein is pathogen free. An alternative method to circumvent these problems is to utilize synthetic peptide analogs in the formulation process. One such peptide is an 18-amino acid peptide abbreviated as 18A (DWLKAFYDKVAEKLKEAF).(47) This peptide, while not having any sequence homology to ApoA-1, retains some of the characteristic features of the amphipathic protein. The peptide adopts an α-helical conformation with charged groups and hydrophobic side chains presented on opposing faces of the peptide. Our laboratory has investigated the use of amphipathic peptides as substitutes for ApoA-1 to circumvent some of the above-mentioned challenges. These synthetic HDL-mimicking peptide–phopholipid scaffold (HPPS) nanoparticles showed promising capabilities as lipoprotein-based nanoparticles through their morphological similarities to HDL-like particles prepared from apolipoproteins isolated from human sources (Figure 6a).(36) By taking advantage of their small size and modifiable surface chemistry, it is possible to conjugate epidermal growth factor (EGF) onto the surface of these particles and direct them to cancer cells expressing the EGF receptor.48,49
Figure 6.
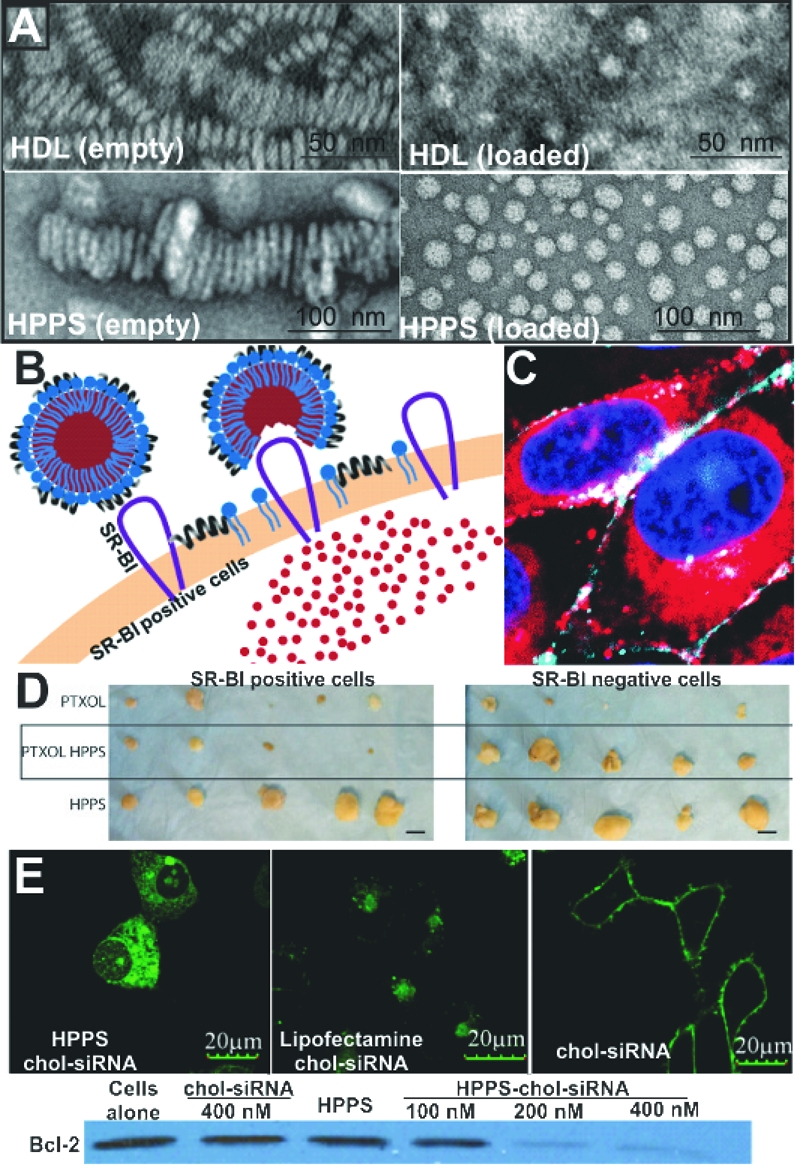
(a) Structural comparison between discoidal and spherical structure generated using ApoA-1 (first row) (ref (36)) or 4F phospholipid (second row) (ref (49)). (b) Schematic diagram illustrating transfer of HPPS core components to cells through a nonendocytic pathway (ref (49)). (c) Cytosolic delivery of HPPS (ref (50)). (d) Drug shielding and targeting of paclitaxel oleate-loaded HPPS. (e) Delivery of bcl-2 targeting siRNA to cancer cells imaged using confocal microscopy along with analysis of protein expression in treated versus untreated groups (ref (53)). Panels a, b, c and e are reproduced with permission from their corresponding references.
Using Nature’s Key To Open the Door for Intracellular Delivery
As discussed in the previous section, SR-BI has been found to be expressed in various types of cancer cells. Targeting the SR-BI receptor for delivery of cancer theranostics warrants further research. While the level of SR-BI expression on cancer cells will factor into the utility of this receptor for drug targeting, it is the mode of uptake that may have implications for lipoprotein-mediated delivery of theranostic agents. Although the exact mechanism of how hydrophobic molecules encapsulated in HDL are internalized via the SR-BI is still a subject of continued research, the general consensus is that this transport is mediated through a nonendocytic mechanism. Our group demonstrated that this mechanism of delivery can also be achieved with HPPS nanoparticles.(50) By incorporation of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide bisoleate (DIRBOA) or dioleyl fluorescein (FluoBOA) into the core of HPPS, uptake was observed to be distributed homogeneously throughout the cell interior, an indication of a nonendocytic uptake mechanism (Figure 6b,c). Further evidence for this was provided as incorporation of fluorescently labeled phospholipid into HPPS led to an uncoupling of the surface and the core fluorescence upon cell uptake. In contrast to the pattern of DIRBOA distribution, the fluorophore-labeled phospholipid remained on the cell membrane, indicating that internalization of the entire particle did not occur. A similar pattern of phospholipid–core dissociation was also observed in a Förster resonance energy transfer study using quantum dot core-loaded HDL.(51) This ability to bypass the endosome may show improved therapeutic efficacy in drugs that are active within the cytosol. Recently, we studied the utility of this nanocarrier in delivering two different types of therapeutics to cancer cells.(52) Paclitaxel oleate, a mitotic inhibitor, has been delivered to tumors using HPPS. Administration of the nanoparticle to SR-BI expressing cancer tumors led to a decrease in tumor size when compared with the vehicle control. Furthermore, toxicity was not observed in tumors that do not express the SR-BI. This indicated that HPPS is successful in limiting the toxicity to cells that express SR-BI (Figure 6d). While this formulation would only be useful in tumors where the SR-BI expression is higher than in normal tissues, the significance of these findings lies in the delivery of agents that have an additional level of specificity and may benefit from escaping endosomal trapping. Examples of such agents include siRNA, which only targets genes expressed in cancer cells, and photosensitizers, which are known to be more efficacious against intracellular organelles. Using HPPS loaded with cholesterol–siRNA, we found that delivery of this agent to the cell appeared to be through a nonendocytic mechanism (Figure 6e).(53) This uptake pattern was visibly different from the uptake of cholesterol–siRNA alone or loaded within a common transfection agent such as lipofectamine. Proteins targeted by the siRNA showed a decrease in the expression levels in HPPS chol–siRNA treated cells versus free chol–siRNA. While the exact mechanism of delivery of cholesterol–siRNA from HPPS is not fully understood, we speculate that this mechanism also involves SR-BI.
Future Outlook
Lipoprotein-based nanoparticles have been loaded with a diverse range of therapeutic and imaging agents for cancer. Application of lipoprotein-based nanoparticles should not simply be limited by this disease state. In fact, lipoprotein-mediated delivery of antifungals(54) and antivirals(55) has also been reported. Another vein of research is the application of lipoproteins to imaging atherosclerotic plaques. The creation of nanocrystal-core lipoprotein nanoparticles using inorganic molecules has extended the application of lipoprotein nanoparticles in diagnostic imaging.(56) This trend will continue as more imaging techniques such as surface-enhanced Raman spectroscopy are applied.(57) In addition, novel discoveries at the interface between lipoproteins and DNA molecular beacons have also been made, allowing for the programmable aggregation of lipoprotein nanoparticles.(58) Finally, over the last several years the landscape of ApoA-1 clinical testing has changed dramatically. In the past decade, there have been several clinical studies determining the safety and efficacy of wild-type ApoA-1 and its derivatives for cardiovascular applications. The proven safety of such compounds and the rest of the components comprising the lipoprotein nanoparticle sets the stage for clinical testing of similar nanocarriers for cancer treatment.
Acknowledgments
Financial support was from the Canadian Institutes of Health Research, the Ontario Institute for Cancer Research, the Natural Sciences and Engineering Research Council of Canada, the Canadian Cancer Society, and the Joey and Toby Tanenbaum/Brazilian Ball Chair in Prostate Cancer Research. We would also like to thank Dr. Trevor McKee for his assistance with the window chamber studies.
Biographies
Kenneth Ng is a Ph.D. student with Professor Gang Zheng. His research interest lies in studying the application of lipoprotein-based nanoparticles as carriers for cancer therapeutics.
Jonathan Lovell is a Ph.D. student with Professor Gang Zheng. His research interests include biophotonic applications of lipid nanoparticles.
Gang Zheng is an Associate Professor of Medical Biophysics at the University of Toronto and the Joey and Toby Tanenbaum/Brazilian Ball Chair in Prostate Cancer Research at the Ontario Cancer Institute. His research interest is to develop translatable theranostic platforms to combat cancer.
References
- Chapman M. J. Animal lipoproteins: Chemistry, structure, and comparative aspects. J. Lipid Res. 1980, 21, 789–853. [PubMed] [Google Scholar]
- Acton S.; Rigotti A.; Landschulz K. T.; Xu S.; Hobbs H. H.; Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996, 271, 518–520. [DOI] [PubMed] [Google Scholar]
- Gal D.; Ohashi M.; MacDonald P. C.; Buchsbaum H. J.; Simpson E. R. Low-density lipoprotein as a potential vehicle for chemotherapeutic agents and radionucleotides in the management of gynecologic neoplasms. Am. J. Obstet. Gynecol. 1981, 139, 877–885. [DOI] [PubMed] [Google Scholar]
- Cohn E. J.; Strong L. E.; Hughes W. L.; Mulford D. J.; Ashworth J. N.; Melin M.; Taylor H. L. Preparation and properties of serum and plasma proteins. IV. A system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. J. Am. Chem. Soc. 1946, 68, 459–475. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L.; Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. J. Biol. Chem. 1974, 249, 5153–5162. [PubMed] [Google Scholar]
- Lees R. S.; Garabedian H. D.; Lees A. M.; Schumacher D. J.; Miller A.; Isaacsohn J. L.; Derksen A.; Strauss H. W. Technitium-99m low density lipoproteins: Preparation and biodistribution. J. Nucl. Med. 1985, 26, 1056–1062. [PubMed] [Google Scholar]
- Nissen S. E.; Tsunoda T.; Tuzcu E. M.; Schoenhagen P.; Cooper C. J.; Yasin M.; Eaton G. M.; Lauer M. A.; Sheldon W. S.; Grines C. L.; Halpern S.; Crowe T.; Blankenship J. C.; Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes. J. Am. Med. Assoc. 2003, 290, 2292–2300. [DOI] [PubMed] [Google Scholar]
- Eisenberg S.; Windmueller H. G.; Levy R. I. Metabolic fate of rat and human lipoprotein apoproteins in the rat. J. Lipid Res. 1973, 14, 446–458. [PubMed] [Google Scholar]
- Pluen A.; Boucher Y.; Ramanujan S.; McKee T. D.; Gohongi T.; di Tomaso E.; Brown E. B.; Izumi Y.; Campbell R. B.; Berk D. A.; Jain R. K. Role of tumor–host interactions in interstitial diffusion of macromolecules: Cranial vs. subcutaneous tumors. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 4628–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricarello D. A.; Smilowitz J. T.; Zivkovic A. M.; German J. B.; Parikh A. N. Reconstituted lipoprotein: A versatile class of biologically-inspired nanostructures. ACS Nano 2010, 5, 42–57. [DOI] [PubMed] [Google Scholar]
- Hermanson G.Bioconjugate Techniques; Academic Press: San Diego, CA, 1996. [Google Scholar]
- Krieger M.; Anderson R. G. W.; Goldstein J. L.; Brown M. S.; Smith L. C.; Kao Y. J.; Pownall H. J.; Gotto A. M. Reconstituted low density lipoprotein: A vehicle for the delivery of hydrophobic fluorescent probes to cells. J. Supramol. Struct. 1979, 10, 467–478. [DOI] [PubMed] [Google Scholar]
- McConathy W. J.; Nair M. P.; Paranjape S.; Mooberry L.; Lacko A. G. Evaluation of synthetic/reconstituted high-density lipoproteins as delivery vehicles for paclitaxel. Anti-Cancer Drugs 2008, 19, 183–188. [DOI] [PubMed] [Google Scholar]
- Muller G. The cholesterol metabolism in health and in anemia. Medicine 1930, 9, 119–174. [Google Scholar]
- Vitols S.; Björkholm M.; Gahrton G.; Peterson C. Hypocholesterolaemia in malignancy due to elevated low-density-lipoprotein-receptor activity in tumour cells: Evidence from studies in patients with leukaemia. Lancet 1985, 326, 1150–1154. [DOI] [PubMed] [Google Scholar]
- Firestone R. A. Low-density lipoprotein as a vehicle for targeting antitumor compounds to cancer cells. Bioconjugate Chem. 1994, 5, 105–113. [DOI] [PubMed] [Google Scholar]
- Ponty E.; Favre G.; Benaniba R.; Boneu A.; Lucot H.; Carton M.; Soula G. Biodistribution study of 99mTc-labeled LDL in B16-melanoma-bearing mice. Visualization of a preferential uptake by the tumor. Int. J. Cancer 1993, 54, 411–417. [DOI] [PubMed] [Google Scholar]
- Moerlein S. M.; Daugherty A.; Sobel B. E.; Welch M. J. Metabolic imaging with gallium-68- and indium-111-labeled low-density lipoprotein. J. Nucl. Med. 1991, 32, 300–307. [PubMed] [Google Scholar]
- Jasanada F.; Urizzi P.; Souchard J.-P.; Le Gaillard F.; Favre G.; Nepveu F. Indium-111 labeling of low density lipoproteins with the DTPA–bis(stearylamide): Evaluation as a potential radiopharmaceutical for tumor localization. Bioconjugate Chem. 1996, 7, 72–81. [DOI] [PubMed] [Google Scholar]
- Corbin I. R.; Li H.; Chen J.; Lund-Katz S.; Zhou R.; Glickson J. D.; Zheng G. Low-density lipoprotein nanoparticles as magnetic resonance imaging contrast agents. Neoplasia 2006, 8, 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crich S. G.; Lanzardo S.; Alberti D.; Belfiore S.; Ciampa A.; Giovenzana G. B.; Lovazzano C.; Pagliarin R.; Aime S. Magnetic resonance imaging detection of tumor cells by targeting low-density lipoprotein receptors with Gd-loaded low-density lipoprotein particles. Neoplasia 2007, 9, 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Marotta D. E.; Kim S.; Busch T. M.; Wileyto E. P.; Zheng G. High payload delivery of optical imaging and photodynamic therapy agents to tumors using phthalocyanine-reconstituted low-density lipoprotein nanoparticles. J. Biomed. Opt. 2005, 10, 041203. [DOI] [PubMed] [Google Scholar]
- Song L.; Li H.; Sunar U.; Chen J.; Corbin I.; Yodh A. G.; Zheng G. Naphthalocyanine-reconstituted LDL nanoparticles for in vivo cancer imaging and treatment. Int. J. Nanomed. 2007, 2, 767–774. [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Corbin I. R.; Li H.; Cao W.; Glickson J. D.; Zheng G. Ligand conjugated low-density lipoprotein nanoparticles for enhanced optical cancer imaging in vivo. J. Am. Chem. Soc. 2007, 129, 5798–5799. [DOI] [PubMed] [Google Scholar]
- Hill M. L.; Corbin I. R.; Levitin R. B.; Cao W.; Mainprize J. G.; Yaffe M. J.; Zheng G. In vitro assessment of poly-iodinated triglyceride reconstituted low-density lipoprotein: initial steps toward CT molecular imaging. Acad. Radiol. 2010, 17, 1359–1365. [DOI] [PubMed] [Google Scholar]
- Mateu L.; Tardieu A.; Luzzati V.; Aggerbeck L.; Scanu A. M. On the structure of human serum low density lipoprotein. J. Mol. Biol. 1972, 70, 105–114. [DOI] [PubMed] [Google Scholar]
- Rensen P. C.; de Vrueh R. L.; Kuiper J.; Bijsterbosch M. K.; Biessen E. A.; van Berkel T. J. Recombinant lipoproteins: lipoprotein-like lipid particles for drug targeting. Adv. Drug Delivery Rev. 2001, 47, 251–276. [DOI] [PubMed] [Google Scholar]
- Kessel D. Porphyrin-lipoprotein association as a factor in porphyrin localization. Cancer Lett. 1986, 33, 183–188. [DOI] [PubMed] [Google Scholar]
- Allison B. A.; Waterfield E.; Richter A. M.; Levy J. G. The effects of plasma lipoproteins on in vitro tumor cell killing and in vivo tumor photosensitization with benzoporphyrin derivative. J. Photochem. Photobiol. Sci. 1991, 54, 709–715. [DOI] [PubMed] [Google Scholar]
- De Smidt P. C.; Versluis A. J.; Van Berkel T. J. C. Properties of incorporation, redistribution, and integrity of porphyrin-low-density lipoprotein complexes. Biochemistry 1993, 32, 2916–2922. [DOI] [PubMed] [Google Scholar]
- Zheng G.; Li H.; Zhang M.; Lund-Katz S.; Chance B.; Glickson J. D. Low-density lipoprotein reconstituted by pyropheophorbide cholesteryl oleate as target-specific photosensitizer. Bioconjugate Chem. 2002, 13, 392–396. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch M. K.; Van Berkel T. J. Uptake of lactosylated low-density lipoprotein by galactose-specific receptors in rat liver. Biochem. J. 1990, 270, 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund-Katz S.; Ibdah J. A.; Letizia J. Y.; Thomas M. T.; Phillips M. C. A 13C NMR characterization of lysine residues in apolipoprotein B and their role in binding to the low density lipoprotein receptor. J. Biol. Chem. 1988, 263, 13831–13838. [PubMed] [Google Scholar]
- Zheng G.; Chen J.; Li H.; Glickson J. D. Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 17757–17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin I. R.; Chen J.; Cao W.; Li H.; Lund-Katz S.; Zheng G. Enhanced cancer-targeted delivery using engineered high-density lipoprotein-based nanocarriers. J. Biomed. Nanotechnol. 2007, 3, 367. [Google Scholar]
- Silva G.; Huang R.; Morris J.; Fang J.; Gracheva E. O.; Ren G.; Kontush A.; Jerome W. G.; Rye K.-A.; Davidson W. S. Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 12176–12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigueza W. V.; Thuahnai S. T.; Temel R. E.; Lund-Katz S.; Phillips M. C.; Williams D. L. Mechanism of scavenger receptor class B type I-mediated selective uptake of cholesteryl esters from high density lipoprotein to adrenal cells. J. Biol. Chem. 1999, 274, 20344–20350. [DOI] [PubMed] [Google Scholar]
- Fiorenza A. M.; Branchi A.; Sommariva D. Serum lipoprotein profile in patients with cancer. A comparison with non-cancer subjects. Int. J. Clin. Lab. Res. 2000, 30, 141–145. [DOI] [PubMed] [Google Scholar]
- Mooberry L. K.; Nair M.; Paranjape S.; McConathy W. J.; Lacko A. G. Receptor mediated uptake of paclitaxel from a synthetic high density lipoprotein nanocarrier. J. Drug Targeting 2010, 18, 53–58. [DOI] [PubMed] [Google Scholar]
- Cao W.; Ng K. K.; Corbin I.; Zhang Z.; Ding L.; Chen J.; Zheng G. Synthesis and evaluation of a stable bacteriochlorophyll-analog and its incorporation into high-density lipoprotein nanoparticles for tumor imaging. Bioconjugate Chem. 2009, 20, 2023–2031. [DOI] [PubMed] [Google Scholar]
- Pussinen P. J.; Karten B.; Wintersperger A.; Reicher H.; McLean M.; Malle E.; Sattler W. The human breast carcinoma cell line HBL-100 acquires exogenous cholesterol from high-density lipoprotein via CLA-1 (CD-36 and LIMPII analogous 1)-mediated selective cholesteryl ester uptake. Biochem. J. 2000, 349, 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrzenjak A.; Reicher H.; Wintersperger A.; Steinecker-Frohnwieser B.; Sedlmayr P.; Schmidt H.; Nakamura T.; Malle E.; Sattler W. Inhibition of lung carcinoma cell growth by high density lipoprotein-associated α-tocopheryl-succinate. Cell. Mol. Life Sci. 2004, 61, 1520–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsack C.; Hirschmugl B.; Hammer A.; Levak-Frank S.; Kozarsky K. F.; Sattler W.; Malle E. Scavenger receptor class B, type I on non-malignant and malignant human epithelial cells mediates cholesteryl ester-uptake from high density lipoproteins. Int. J. Biochem. Cell Biol. 2003, 35, 441–454. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch M. K.; Van Berkel T. J. Lactosylated high density lipoprotein: A potential carrier for the site-specific delivery of drugs to parenchymal liver cells. Mol. Pharmacol. 1992, 41, 404–411. [PubMed] [Google Scholar]
- Nykiforuk C. L.; Shen Y.; Murray E. W.; Boothe J. G.; Busseuil D.; Rhéaume E.; Tardif J.-C.; Reid A.; Moloney M. M. Expression and recovery of biologically active recombinant Apolipoprotein AIMilano from transgenic safflower (Carthamus tinctorius) seeds. Plant Biotechnol. J. 2011, 9, 250–263. [DOI] [PubMed] [Google Scholar]
- Lerch P. G.; Förtsch V.; Hodler G.; Bolli R. Production and characterization of a reconstituted high density lipoprotein for therapeutic applications. Vox Sang. 1996, 71, 155–164. [DOI] [PubMed] [Google Scholar]
- Anantharamaiah G. M.; Jones J. L.; Brouillette C. G.; Schmidt C. F.; Chung B. H.; Hughes T. A.; Bhown A. S.; Segrest J. P. Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J. Biol. Chem. 1985, 260, 10248–10255. [PubMed] [Google Scholar]
- Zhang Z.; Chen J.; Ding L.; Jin H.; Lovell J. F.; Corbin I. R.; Cao W.; Lo P.-C.; Yang M.; Tsao M.-S.; Luo Q.; Zheng G. HDL-mimicking peptide-lipid nanoparticles with improved tumor targeting. Small 2010, 6, 430–437. [DOI] [PubMed] [Google Scholar]
- Jin H.; Lovell J.; Chen J.; Ng K.; Cao W.; Ding L.; Zhang Z.; Zheng G. Investigating the specific uptake of EGF-conjugated nanoparticles in lung cancer cells using fluorescence imaging. Cancer Nanotechnol. 2010, 1, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.; Cao W.; Jin H.; Lovell J. F.; Yang M.; Ding L.; Chen J.; Corbin I.; Luo Q.; Zheng G. Biomimetic nanocarrier for direct cytosolic drug delivery. Angew. Chem., Int. Ed. 2009, 48, 9171–9175. [DOI] [PubMed] [Google Scholar]
- Skajaa T.; Zhao Y.; van den Heuvel D. J.; Gerritsen H. C.; Cormode D. P.; Koole R.; van Schooneveld M. M.; Post J. A.; Fisher E. A.; Fayad Z. A.; de Mello Donega C.; Meijerink A.; Mulder W. J. M. Quantum dot and Cy5.5 labeled nanoparticles to investigate lipoprotein biointeractions via Förster resonance energy transfer. ACS Nano 2010, 10, 5131–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.; Chen J.; Cao W.; Ding L.; Ng K. K.; Jin H.; Zhang Z.; Zheng G.. Attenuation of non-targeted cell-kill using a HDL-mimicking peptide-phospholipid nanoscaffold. Nanomedicine 2011, in press. [DOI] [PubMed]
- Yang M.; Jin H.; Chen J.; Ding L.; Ng K. K.; Lin Q.; Lovell J. F.; Zhang Z.; Zheng G. Efficient cytosolic delivery of siRNA using HDL-mimicking nanoparticles. Small 2011, 7, 568–573. [DOI] [PubMed] [Google Scholar]
- Oda M. N.; Hargreaves P. L.; Beckstead J. A.; Redmond K. A.; van Antwerpen R.; Ryan R. O. Reconstituted high density lipoprotein enriched with the polyene antibiotic amphotericin B. J. Lipid Res. 2006, 47, 260–267. [DOI] [PubMed] [Google Scholar]
- Hu J.; Liu H.; Wang L. Enhanced delivery of AZT to macrophages via acetylated LDL. J. Controlled Release 2000, 69, 327–335. [DOI] [PubMed] [Google Scholar]
- Mulder W. J. M.; Strijkers G. J.; van Tilborg G. A. F.; Cormode D. P.; Fayad Z. A.; Nicolay K. Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc. Chem. Res. 2009, 42, 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam N. C. M.; Scott B. M. T.; Voicu D.; Wilson B. C.; Zheng G. Facile synthesis of raman active phospholipid gold nanoparticles. Bioconjugate Chem. 2010, 21, 2178–2182. [DOI] [PubMed] [Google Scholar]
- Lovell J. F.; Jin H.; Ng K. K.; Zheng G. Programmed nanoparticle aggregation using molecular beacons. Angew. Chem., Int. Ed. 2010, 49, 7917–7919. [DOI] [PubMed] [Google Scholar]
- Gotto A. M.; Pownall H. J.; Havel R. J. Introduction to the plasma lipoproteins. Methods Enzymol. 1986, 128, 3–41. [DOI] [PubMed] [Google Scholar]