Chris Raetz (Fig. 1) passed away on August 16, 2011 after a three-year battle with anaplastic thyroid cancer. His passing represents an extraordinary loss to the lipid community. Chris was born in East Berlin in 1946 and immigrated with his parents to the United States in 1951. He entered Yale University in 1963 and followed in both his parents’ footsteps by majoring in chemistry. He entered Harvard Medical School in 1967 in pursuit of an MD degree, but his past interests in chemistry led him to the research bench in the laboratory of Eugene Kennedy.2 He received his MD and PhD degrees from Harvard Medical School in 1973. After a year as house officer at the Peter Bent Brigham Hospital in Boston, Chris spent two years as a postdoctoral fellow with Herbert Tabor at the National Institutes of Health in Bethesda and then joined the biochemistry faculty at the University of Wisconsin in 1976 and rapidly rose through the ranks to full professor. Chris left academia from 1987 to 1993 to join Merck Research Laboratories first as executive director of biochemistry and later as executive vice president of research in biochemistry and microbiology. In 1993, he became the George Barth Geller Professor and Chair of Biochemistry at Duke University Medical Center. He served as chair until 2007 and remained on the faculty until his passing.
Fig. 1.

Chris Raetz at the 2006 ICBL meeting in The Netherlands.
Chris Raetz's most noted contributions to phospholipid and lipopolysaccharide biochemistry fall into four areas: 1) the development of general methods for the isolation of mutants in phospholipid biosynthesis; 2) the discovery of the enzymatic pathways for the assembly and modification of the Lipid A component of lipopolysaccharide; 3) the discovery of potent new antibiotics that target the Lipid A system; and 4) the elucidation of the high-resolution 3D structures of some of the enzymes of what should be coined the “Raetz Pathway for Lipid A Biosynthesis” (1).
Chris had a deep mechanistic understanding of chemistry likely instilled by his parents. His training and research interests provided a breadth of knowledge in medicine, pharmacology, biochemistry, genetics, and structural biology, which he applied to a lifetime pursuit of mechanistic understanding of lipid metabolism. In his own words [taken from a presentation at Eugene Kennedy's 90th birthday celebration (Fig. 2)], he attributed much of his success to “Lessons I Learned from Eugene Kennedy”:
Fig. 2.
Chris Raetz with Eugene Kennedy on the occasion of the latter's 90th birthday celebration in Boston, MA in November 2009.
Discovery of novel composition and function takes priority.
Development of in vitro assays in crude systems is crucial.
A clever experiment is worth more than a piece of equipment.
Curiosity driven research generates the best science.
It helps to sprinkle ideas around the lab in an informal way.
Every person has their own project and can talk about it.
Scientific criticism is not personal; everything is on the table.
People move on to their next position in a timely manner.
These principles can be seen in all his major contributions. Kennedy's earlier work (2, 3) implicated CDP-diacylglycerol as the common intermediated to the major phospholipids of Escherichia coli, but the compound had never been identified in cell extracts. While in Kennedy's lab as a graduate student, Chris isolated both CDP- and dCDP-diacylglycerol as minor lipids in E. coli extracts (4). Attention to detail when it comes to assays in crude extracts led him to establish that phosphatidylserine synthesis was not associated with membranes in crude extracts, as was generally assumed, but was associated with ribosomes (5).
While at the NIH, Chris developed a clever screening method to identify mutants in lipid biosynthetic enzymes that he first applied to E. coli (6) and later to somatic cells (7). These seminal pieces of work opened up lipid metabolism in eukaryotes and prokaryotes to rigorous genetic approaches just as major developments in molecular genetics, gene cloning, and DNA sequencing were blossoming. He published one of the first reports of using amplification of cloned genes to overproduce proteins for purification (8).
Upon arrival at Wisconsin, Chris was armed with a strong grounding in chemistry, biochemistry, and lipidology along with a powerful method to screen for mutants in lipid metabolism. These became supporting approaches to his fascination and study of minor small lipid molecules. In an early experiment, he noted that chloroform-methanol extracts of E. coli radiolabeled with 32PO4, when separated by thin-layer chromatography and subjected for a short time to film, displayed only the expected major phospholipid species. However, the longer he exposed the chromatogram to film the more individual species appeared until the film was almost black. Few of these spots had been previously recognized or characterized.
His curiosity as to the source and identity of such minor species was the beginning of his lifetime pursuit to map the biosynthetic pathway for Lipid A, the membrane anchor of lipopolysaccharide of Gram-negative bacteria. He noted two new phosphate-containing spots in lipid extracts of mutant cells deficient in the ability to make the anionic phospholipids of E. coli, phosphatidylglycerol, and cardiolipin. Chris isolated these molecules from cell extracts, characterized their structures, and verified their presence as minor lipid species in wild-type cells (9). Using the “rules of enzymology”, he deduced and then proved that these were early precursors to Lipid A biosynthesis. The rest is, of course, history.
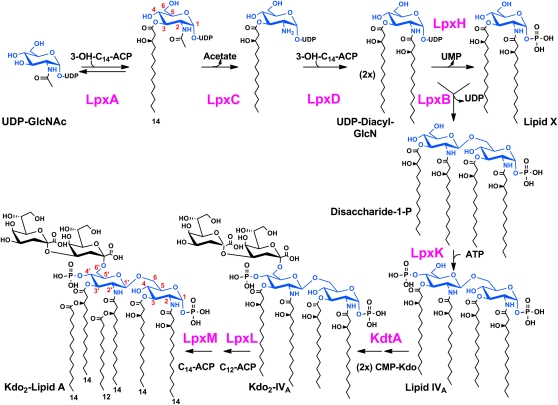
During the following years at Wisconsin, Merck and Duke, Chris's group established the Raetz Pathway (Fig. 3), identified all the genes involved, and purified the enzymes of the pathway (1). He effectively used cloned gene amplification to obtain sufficient amounts of purified enzymes for characterization. He also used his mutant screening techniques to isolate somatic cell mutants in phospholipid metabolism. While at Merck, he oversaw the development of potential antibiotics (10) directed at the Raetz Pathway, thus establishing one of the few new and novel antibiotic targets.
Fig. 3.
The Raetz Pathway for Lipid A Biosynthesis. This figure was reproduced from (1) with permission (c) Annual Reviews of Biochemistry.
The move to Duke University invigorated his research program and expanded his investigations of Lipid A structure and function over a broad spectrum of Gram-negative bacteria. He established that postsynthetic modification of Lipid A and the lipopolysaccharide core in E. coli and other Gram-negative organisms is used as a protective mechanism against external assault including a defense against antibiotics (11). He characterized the variation in Lipid A structures in a number of bacteria, identifying and characterizing the enzymes responsible for these variations along the way.
As part of the LIPID MAPS consortium, his core developed mass spectrometric methods for identifying novel phospholipids and Lipid A species (1, 12). His approach of small molecule identification in defining the Raetz Pathway for Lipid A Biosynthesis is reflected in the goals of his LIPID MAPS core:
Determine the structures of all unknown minor lipid molecules (in the wild-type, in mutants or in clone collections).
Use the rules of enzymology to postulate all plausible pathways.
Make all reasonable substrates to search for new enzymes.
Purify or expression clone all new enzymes that are found.
Sequence to identify the genes encoding these new enzymes.
In the latter years of his career, much of the focus was on determining the 3D structure of the enzymes of the Raetz Pathway (13–16). This was a particularly challenging undertaking because many of these enzymes are peripheral or integral membrane proteins. Coming full circle during the last year, he returned to several unresolved issues in E. coli phospholipid metabolism. He identified the third and final genes and enzymes for the conversion of phosphatidylglycerol phosphate to phosphatidylglycerol (17) and the latter to cardiolipin (B. Tan, M. Bogdanov, J. Zhao, W. Dowhan, C. R. Raetz, and A. Guan, in preparation).
Chris received many awards and honors, including the 1979 Camille and Henry Dreyfus Teacher-Scholar Award, the 2002 American Society for Biochemistry and Molecular Biology Avanti Award in Lipids, the 2006 L. L. M. van Deenen Medal from the University of Utrecht, the 2006 Frederik B. Bang Award from the International Endotoxin and Innate Immunity Society, and election to the National Academy of Sciences in 2006. Chris published over 300 original manuscripts and review articles with a large amount of data awaiting publication within the lab notebooks of his surviving postdocs and graduate students.
Chris Raetz exemplified the energetic, active scientist driven by curiosity to understand the molecular basis underlying biological function. Most important for the next generation of scientists, he has imparted his enthusiasm for science and his dedication to critical and precise experimentation through the mentoring of over 30 PhD students and 40 postdoctoral fellows. He was an extraordinary scientist, mentor of young scientists, and friend to many (Fig. 4). His passing at such a young age is a tremendous loss to the lipid community, his friends, and his family. Chris is survived by his wife Madeline, daughters Jackie and Elisabeth, and grandson Leo.
Fig. 4.
Chris Raetz in happier times. Pictured with Edward Dennis (left) and William Dowhan (right) at the Biochemistry chairman's meeting in the Galapagos Islands in January, 2004.
Acknowledgments
Thank you, Chris, for an exciting ride through many years as you brought lipids to the forefront of biological importance. Your scientific inspiration and guidance and your friendship will be sorely missed. William Wickner and Teresa Garrett helped to minimize any errors or oversight on my part.
REFERENCES
- 1.Raetz C. R., Reynolds C. M., Trent M. S., Bishop R. E. 2007. Lipid A modification systems in gram-negative bacteria. Annu. Rev. Biochem. 76: 295–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanfer J. N., Kennedy E. P. 1962. Synthesis of phosphatidylserine by Escherichia coli. J. Biol. Chem. 237: PC270–PC271 [PubMed] [Google Scholar]
- 3.Kiyasu J. Y., Pieringer R. A., Paulus H., Kennedy E. P. 1963. The biosynthesis of phosphatidylglycerol. J. Biol. Chem. 238: 2293–2298 [PubMed] [Google Scholar]
- 4.Raetz C. R., Kennedy E. P. 1973. Function of cytidine diphosphate-diglyceride and deoxycytidine diphosphate-diglyceride in the biogenesis of membrane lipids in Escherichia coli. J. Biol. Chem. 248: 1098–1105 [PubMed] [Google Scholar]
- 5.Raetz C. R., Kennedy E. P. 1972. The association of phosphatidylserine synthetase with ribosomes in extracts of Escherichia coli. J. Biol. Chem. 247: 2008–2014 [PubMed] [Google Scholar]
- 6.Raetz C. R. 1975. Isolation of Escherichia coli mutants defective in enzymes of membrane lipid synthesis. Proc. Natl. Acad. Sci. USA. 72: 2274–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esko J. D., Raetz C. R. 1978. Replica plating and in situ enzymatic assay of animal cell colonies established on filter paper. Proc. Natl. Acad. Sci. USA. 75: 1190–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raetz C. R., Larson T. J., Dowhan W. 1977. Gene cloning for the isolation of enzymes of membrane lipid synthesis: phosphatidylserine synthase overproduction in Escherichia coli. Proc. Natl. Acad. Sci. USA. 74: 1412–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishijima M., Raetz C. R. 1981. Characterization of two membrane-associated glycolipids from an Escherichia coli mutant deficient in phosphatidylglycerol. J. Biol. Chem. 256: 10690–10696 [PubMed] [Google Scholar]
- 10.Onishi H. R., Pelak B. A., Gerckens L. S., Silver L. L., Kahan F. M., Chen M. H., Patchett A. A., Galloway S. M., Hyland S. A., Anderson M. S., et al. 1996. Antibacterial agents that inhibit lipid A biosynthesis. Science. 274: 980–982 [DOI] [PubMed] [Google Scholar]
- 11.Trent M. S., Ribeiro A. A., Lin S., Cotter R. J., Raetz C. R. 2001. An inner membrane enzyme in Salmonella and Escherichia coli that transfers 4-amino-4-deoxy-L-arabinose to lipid A: induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. J. Biol. Chem. 276: 43122–43131 [DOI] [PubMed] [Google Scholar]
- 12.Murphy R. C., Raetz C. R., Reynolds C. M., Barkley R. M. 2005. Mass spectrometry advances in lipidomica: collision-induced decomposition of Kdo2-lipid A. Prostaglandins Other Lipid Mediat. 77: 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang P. M., Choy W. Y., Lo E. I., Chen L., Forman-Kay J. D., Raetz C. R., Prive G. G., Bishop R. E., Kay L. E. 2002. Solution structure and dynamics of the outer membrane enzyme PagP by NMR. Proc. Natl. Acad. Sci. USA. 99: 13560–13565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coggins B. E., McClerren A. L., Jiang L., Li X., Rudolph J., Hindsgaul O., Raetz C. R., Zhou P. 2005. Refined solution structure of the LpxC-TU-514 complex and pKa analysis of an active site histidine: insights into the mechanism and inhibitor design. Biochemistry. 44: 1114–1126 [DOI] [PubMed] [Google Scholar]
- 15.Bartling C. M., Raetz C. R. 2009. Crystal structure and acyl chain selectivity of Escherichia coli LpxD, the N-acyltransferase of lipid A biosynthesis. Biochemistry. 48: 8672–8683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robins L. I., Williams A. H., Raetz C. R. 2009. Structural basis for the sugar nucleotide and acyl-chain selectivity of Leptospira interrogans LpxA. Biochemistry. 48: 6191–6201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y. H., Guan Z., Zhao J., Raetz C. R. 2011. Three phosphatidylglycerol-phosphate phosphatases in the inner membrane of Escherichia coli. J. Biol. Chem. 286: 5506–5518 [DOI] [PMC free article] [PubMed] [Google Scholar]