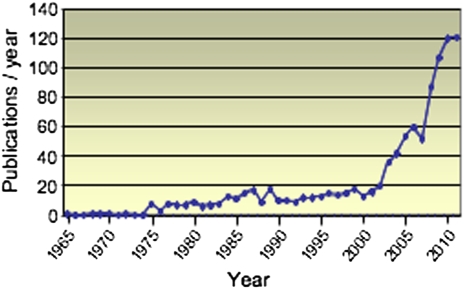
This series of Thematic Review articles concerns the biology of lipid droplets. Lipid droplets have been described in diverse cell types in histological and morphological studies for more than a century and a half. It is likely that structures referred to historically in the literature as “lipid bodies”, “lipid globules”, “lipid particles”, and “oil bodies” all represent lipid droplets. But the recognition that lipid droplets represent a bona fide organelle containing specialized proteins and having a dynamic itinerary in cells has occurred much more recently. At present, lipid droplets are under intensive study due to the increasing recognition that they have significant roles in many aspects of health and disease. A search of the scientific literature from 1965 to the present using the term “lipid droplet” yields 1,000 publications. Notably, nearly 35% of these have appeared in 2009–2011 (Fig. 1).
Fig. 1.
PubMed citations containing the compound search term “lipid droplet” for each year from 1965 to September 2011.
Due to exciting research in the lipid droplet field, several excellent reviews on lipid droplets have appeared in the last few years (1–11). In the current review series, we will present articles on lipid droplet biology and metabolism from an evolutionary point of view, in organisms ranging from yeast to invertebrates, plants, and mammals. In preparation for these interesting and informative articles, we present here a brief introduction to lipid droplet structure and function in mammalian physiology and pathophysiology.
LIPID DROPLET CHARACTERISTICS IN ADIPOCYTES AND OTHER CELL TYPES
The mammalian adipocyte is considered the “professional” lipid droplet-storing cell, but cytosolic lipid droplets occur universally in the many cell types that make up eukaryotic organisms as well as in prokaryotes (12). Lipid droplets typically consist of neutral lipids in the form of triacylglycerols, cholesteryl esters, or retinyl esters surrounded by a phospholipid monolayer. The surface of lipid droplets is adorned with proteins that serve structural and metabolic functions. The first lipid droplet-associated proteins identified were the perilipins and related proteins, which have important metabolic roles in the control of triacylglycerol storage and release from lipid droplets (2, 13). The specific repertoire of proteins varies for lipid droplets in different cell types and even among different droplets within a cell (14). Some of the most frequently associated proteins include enzymes involved in triacylglycerol and phospholipid biosynthesis (acyl-CoA:diacylglycerol acyltransferase 2, acyl-CoA synthetase; CTP:phosphocholine cytidylyltransferase), membrane-trafficking proteins (ARF1, Rab5, Rab18), and the adipose tissue triacylglycerol lipase (ATGL) (15–17). Large-scale proteomics studies have identified dozens of additional lipid droplet-associated proteins (18–20). The constellation of proteins present is likely to be a key determinant of individual lipid droplet function, but the roles of most of these proteins remain poorly understood.
Lipid droplets in mammalian white adipocytes are perhaps the most extensively characterized type of lipid droplet, and they have a defined role as the body's major stored energy supply. Unlike most cell types, white adipocytes typically contain a single, large lipid droplet ranging up to 100 μm in diameter. In most other cell types, lipid droplets are usually less than 1 μm in size, although in hepatic steatosis, lipid droplets may reach 10 μm (10). White adipocytes appear to have unique properties in the localization, mobility, and fate of lipids stored within the droplet. White adipocyte lipid droplets typically occupy the majority of the cytosol, are localized a short distance from the plasma membrane, are associated with intermediate filaments, and have very limited mobility within the cell. By contrast, the multiple, small lipid droplets present in nonadipocytes are often observed juxtaposed next to the endoplasmic reticulum, mitochondria, and peroxisomes. These small lipid droplets exhibit directional movement across long distances within the cell through interaction of lipid droplet-associated proteins with microtubules (21).
SIGNIFICANCE OF LIPID DROPLETS IN MAMMALIAN PHYSIOLOGY
Lipid droplets play significant biological roles in many cell types in addition to white adipocytes [reviewed in (12)]. In hepatocytes, triacylglycerol and cholesteryl esters are stored in lipid droplets. Hepatic lipid droplet pools provide up to 70% of the triacylglycerol substrate for the assembly of very low density lipoproteins with the remainder coming from de novo synthesis (22). Lipid droplets within retinoid stellate cells in the liver contain the majority of the body's vitamin A and its metabolites (23). In addition to retinyl esters, these lipid droplets also contain substantial triacylglycerol and cholesteryl esters, but the number and size of these lipid droplets is influenced chiefly by the amount of dietary retinoid intake. Lipid droplets in secretory epithelial cells of the mammary gland contribute to the nutritional composition of breast milk (24). Lipid droplets appear to have important functions in several cell types of the immune system. Lipid droplets in macrophages and other leukocytes participate in inflammation and the immune response (25). Interestingly, during macrophage phagocytosis, the lipid droplets tend to move to a position surrounding the phagolysosomes, suggesting an active role in this basic macrophage function (26).
Lipid droplet components are a source of substrates for hormones and other factors (12). For example, small cholesteryl ester-rich lipid droplets in adrenal cortex, testes, and ovaries are utilized in steroid hormone synthesis. Lipid droplets also provide substrates for pulmonary surfactant synthesis in maturation of the lungs. In macrophages and leukocytes, lipid droplets are involved in arachidonic acid metabolism. Lipids stored within lipid droplets may also serve as signaling molecules or ligands for transcription factors. An example is the role of lipid droplets in cardiac mitochondrial function. Triacylglycerol-rich lipid droplets in cardiomyocytes are hydrolyzed to generate lipid ligands to activate the nuclear receptor peroxisome proliferator-activated receptor α and mitochondrial function (27).
Lipid droplets have been implicated in some unexpected roles. It has been proposed that lipid droplets serve as a temporary storage site for hydrophobic proteins, which may prevent their aggregation or perhaps promote their degradation (4, 28). One example is the accumulation of apolipoprotein B on the surface of lipid droplets in hepatocytes in which degradation by proteasomes or autophagy is inhibited (29). The hydrophobic protein α-synuclein, dysfunction of which is associated with neurodegenerative disorders such as Parkinson's disease, also accumulates on lipid droplets (30). The association of these proteins with lipid droplets is thought to occur via hydrophobic interaction with lipid esters and may serve a protective role by preventing their aggregation or interactions with other proteins. Histones in the Drosophila embryo become associated with lipid droplets through electrostatic interactions during a particular stage of development, to be released for entry into the nucleus at a later point (31). Thus, it appears that lipid droplets can serve as a ‘holding cell’ for proteins with various subsequent fates.
LIPID DROPLETS AND HUMAN DISEASE
As described above, lipid droplets have important functional roles in cellular lipid homeostasis and energy metabolism. However, excessive or defective lipid droplet accumulation is a prominent feature of common diseases including diabetes, atherosclerosis, and the metabolic syndrome [reviewed in (8, 32)]. The accumulation of cholesteryl ester-rich lipid droplets has long been known as a hallmark feature of macrophage foam cell formation in atherosclerosis. The accumulation of triacylglycerol-rich lipid droplets in tissues such as liver, pancreatic β cells, and skeletal muscle—often referred to as lipotoxicity—is central to the pathogenesis of metabolic dysregulation in obesity, insulin resistance, and nonalcoholic steatohepatitis (33). Interestingly, rare mutations in PLIN1, encoding perilipin 1, or in CIDEC (also known as fat specific protein 27) cause partial lipodystrophy and insulin resistance in human patients (34, 35), illustrating the importance of normal lipid droplet function in metabolic homeostasis.
Mutations in proteins that regulate lipid droplet hydrolysis cause diseases associated with triacylglycerol accumulation in skeletal and cardiac muscle. Thus, ATGL mutations cause a form of neutral lipid disease with myopathy, whereas mutations in the ATGL activator CGI-58 cause Chanarin-Dorfman syndrome, which resembles the condition caused by ATGL deficiency with the added symptom of ichthyosis due to the accumulation of triacylglycerol-rich lipid droplets in skin (36, 37). Interestingly, mice deficient in ATGL, which are unable to hydrolyze stored adipose tissue lipid droplets, are protected from cachexia in cancer because failure to break down lipid droplets prevents the wasting of adipose tissue and muscle (38).
Finally, lipid droplets in host cells play a role in the pathogenesis of viruses and bacteria. For example, the hepatitis C virus, which infects about 3% of the world's population and contributes to liver damage and hepatocellular carcinoma, relies upon an intimate relationship with liver cell lipid droplets for replication and assembly of virus particles (39). After replication, the viral RNA genome is recruited to endoplasmic reticulum membranes surrounding lipid droplets and encapsulated by the viral nucleocapsid core to produce progeny virions. Dengue virus, an arthropod-borne pathogen that still causes human epidemics, and rotaviruses, which are a major cause of gastroenteritis in children, also carry out part of their life cycle in lipid droplet structures (40, 41). Importantly, inhibition of lipid droplet formation greatly reduces dengue virus replication, raising the possibility of lipid droplet-targeted therapies for these viral pathogens. Intracellular bacterial pathogens such Chlamydia also exploit cytoplasmic lipid droplets within host cells. Chlamydia proteins injected into a host cell become established within a membrane-bound inclusion that interacts with cellular organelles, including lipid droplets, which provide components for viral replication (42). Thus, the processes that govern the formation, composition, movement, and turnover of lipid droplets have major significance in both basic biology and in the development of metabolic and infectious diseases.
WHY STUDY LIPID DROPLETS IN DIVERSE ORGANISMS?
This introduction focuses primarily on characteristics of lipid droplets in various cell types of mammals. However, it is clear that many aspects of lipid droplet structure and function are evolutionarily conserved in single-celled organisms, invertebrates, and plants. The study of lipid droplets in simpler organisms and comparison with findings in mouse and humans will reveal both common and specialized properties and contribute to answering many outstanding questions regarding lipid droplet biogenesis, storage, trafficking, lipolysis, and metabolism. Experimental models such as Saccharomyces cerevisiae, Caenorhabditis elegansQ2: Please spell out genus in S. cerevisiae and C. elegans at first use., and Drosophila also offer tractable systems for large-scale genetic screens to identify novel genes and proteins with roles in lipid droplet biology [see, for example (15, 43–47)]. The mouse and mammalian cell systems are similarly invaluable for physiological studies of novel proteins and modeling human disease.
In line with this philosophy, this review series includes contributions describing recent work on various aspects of lipid droplet biology in a range of organisms. In this issue, Stephen G. Young (University of California, Los Angeles) describes the current understanding of the protein GPIHBP1 and the delivery of lipids from triglyceride-rich lipoproteins to cells for metabolism and storage. In subsequent articles, Andrew S. Greenberg (Tufts University) will focus on the mammalian lipid droplet proteins and the pathways that lead to lipid droplet formation and metabolism. Additional contributions will take an evolutionary step back to describe current studies of lipid droplet biology in key experimental systems. Joel M. Goodman (University of Texas Southwestern Medical Center) will provide insight into lipid droplet biology in yeast and new lessons in lipid droplet biogenesis. Ho Yi Mak (Stowers Institute for Medical Research) will describe lipid droplets in C. elegans and inroads to answering questions about their regulation and connections to whole animal energy homeostasis, feeding behavior, and lifespan. Ronald P. Kühnlein (Max Planck Institute for Biophysical Chemistry) will provide a current view of lipometabolism in the fruit fly Drosophila. And finally, Kent Chapman (University of North Texas) will enlighten us about lipid droplets in plants, where understanding neutral lipid storage is of both scientific and economic interest, due to the potential for influencing biofuel production and the nutritional content of crop plants.
Acknowledgments
The author expresses her gratitude to the investigators mentioned in this article for participating in this review series and contributing to our understanding of lipid droplet biology.
Footnotes
The author gratefully acknowledges support from R01 HL102661, P01 HL288481, and P01 HL90553. The contents of this work are solely the responsibility of the author and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Brasaemle D. L. 2007. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48: 2547–2559 [DOI] [PubMed] [Google Scholar]
- 2.Brasaemle D. L., Subramanian V., Garcia A., Marcinkiewicz A., Rothenberg A. 2009. Perilipin A and the control of triacylglycerol metabolism. Mol. Cell. Biochem. 326: 15–21 [DOI] [PubMed] [Google Scholar]
- 3.Farese R. V., Jr, Walther T. C. 2009. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 139: 855–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimoto T., Parton R. G. 2011. Not just fat: the structure and function of the lipid droplet. Cold Spring Harb. Perspect. Biol. 3: a004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman J. M. 2008. The gregarious lipid droplet. J. Biol. Chem. 283: 28005–28009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman J. M. 2009. Demonstrated and inferred metabolism associated with cytosolic lipid droplets. J. Lipid Res. 50: 2148–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg A. S., Coleman R. A. 2011. Expanding roles for lipid droplets. Trends Endocrinol. Metab. 22: 195–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg A. S., Coleman R. A., Kraemer F. B., McManaman J. L., Obin M. S., Puri V., Yan Q. W., Miyoshi H., Mashek D. G. 2011. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Invest. 121: 2102–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kühnlein R. P. 2011. The contribution of the Drosophila model to lipid droplet research. Prog. Lipid Res. 50: 348–356 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki M., Shinohara Y., Ohsaki Y., Fujimoto T. 2011. Lipid droplets: size matters. J. Electron Microsc. (Tokyo). 60(Suppl 1): S101–S116 [DOI] [PubMed] [Google Scholar]
- 11.Walther T. C., Farese R. V., Jr 2009. The life of lipid droplets. Biochim. Biophys. Acta. 1791: 459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy D. J. 2001. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog. Lipid Res. 40: 325–438 [DOI] [PubMed] [Google Scholar]
- 13.Greenberg A. S., Egan J. J., Wek S. A., Garty N. B., Blanchette-Mackie E. J., Londos C. 1991. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 266: 11341–11346 [PubMed] [Google Scholar]
- 14.Ducharme N. A., Bickel P. E. 2008. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 149: 942–949 [DOI] [PubMed] [Google Scholar]
- 15.Guo Y., Walther T. C., Rao M., Stuurman N., Goshima G., Terayama K., Wong J. S., Vale R. D., Walter P., Farese R. V. 2008. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 453: 657–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuerschner L., Moessinger C., Thiele C. 2008. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic. 9: 338–352 [DOI] [PubMed] [Google Scholar]
- 17.Stone S. J., Levin M. C., Zhou P., Han J., Walther T. C., Farese R. V., Jr 2009. The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J. Biol. Chem. 284: 5352–5361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartz R., Zehmer J. K., Zhu M., Chen Y., Serrero G., Zhao Y., Liu P. 2007. Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation. J. Proteome Res. 6: 3256–3265 [DOI] [PubMed] [Google Scholar]
- 19.Beller M., Riedel D., Jansch L., Dieterich G., Wehland J., Jackle H., Kuhnlein R. P. 2006. Characterization of the Drosophila lipid droplet subproteome. Mol. Cell. Proteomics. 5: 1082–1094 [DOI] [PubMed] [Google Scholar]
- 20.Wu C. C., Howell K. E., Neville M. C., Yates J. R., 3rd, McManaman J. L. 2000. Proteomics reveal a link between the endoplasmic reticulum and lipid secretory mechanisms in mammary epithelial cells. Electrophoresis. 21: 3470–3482 [DOI] [PubMed] [Google Scholar]
- 21.Welte M. A. 2009. Fat on the move: intracellular motion of lipid droplets. Biochem. Soc. Trans. 37: 991–996 [DOI] [PubMed] [Google Scholar]
- 22.Lehner R., Cui Z., Vance D. E. 1999. Subcellullar localization, developmental expression and characterization of a liver triacylglycerol hydrolase. Biochem. J. 338: 761–768 [PMC free article] [PubMed] [Google Scholar]
- 23.Blaner W. S., O'Byrne S. M., Wongsiriroj N., Kluwe J., D'Ambrosio D. M., Jiang H., Schwabe R. F., Hillman E. M., Piantedosi R., Libien J. 2009. Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim. Biophys. Acta. 1791: 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heid H. W., Keenan T. W. 2005. Intracellular origin and secretion of milk fat globules. Eur. J. Cell Biol. 84: 245–258 [DOI] [PubMed] [Google Scholar]
- 25.Melo R. C., D'Avila H., Wan H. C., Bozza P. T., Dvorak A. M., Weller P. F. 2011. Lipid bodies in inflammatory cells: structure, function, and current imaging techniques. J. Histochem. Cytochem. 59: 540–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dvorak A. M., Dvorak H. F., Peters S. P., Shulman E. S., MacGlashan D. W., Jr, Pyne K., Harvey V. S., Galli S. J., Lichtenstein L. M. 1983. Lipid bodies: cytoplasmic organelles important to arachidonate metabolism in macrophages and mast cells. J. Immunol. 131: 2965–2976 [PubMed] [Google Scholar]
- 27.Haemmerle G., Moustafa T., Woelkart G., Buttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P. C., Zierler K., et al. 2011. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat. Med. 17: 1076–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welte M. A. 2007. Proteins under new management: lipid droplets deliver. Trends Cell Biol. 17: 363–369 [DOI] [PubMed] [Google Scholar]
- 29.Ohsaki Y., Cheng J., Fujita A., Tokumoto T., Fujimoto T. 2006. Cytoplasmic lipid droplets are sites of convergence of proteasomal and autophagic degradation of apolipoprotein B. Mol. Biol. Cell. 17: 2674–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole N. B., Murphy D. D., Grider T., Rueter S., Brasaemle D., Nussbaum R. L. 2002. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J. Biol. Chem. 277: 6344–6352 [DOI] [PubMed] [Google Scholar]
- 31.Cermelli S., Guo Y., Gross S. P., Welte M. A. 2006. The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr. Biol. 16: 1783–1795 [DOI] [PubMed] [Google Scholar]
- 32.Lusis A. J., Attie A. D., Reue K. 2008. Metabolic syndrome: from epidemiology to systems biology. Nat. Rev. Genet. 9: 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unger R. H., Scherer P. E. 2010. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol. Metab. 21: 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandotra S., Le Dour C., Bottomley W., Cervera P., Giral P., Reznik Y., Charpentier G., Auclair M., Delepine M., Barroso I., et al. 2011. Perilipin deficiency and autosomal dominant partial lipodystrophy. N. Engl. J. Med. 364: 740–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubio-Cabezas O., Puri V., Murano I., Saudek V., Semple R. K., Dash S., Hyden C. S., Bottomley W., Vigouroux C., Magre J., et al. 2009. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med. 1: 280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer J., Lefevre C., Morava E., Mussini J. M., Laforet P., Negre-Salvayre A., Lathrop M., Salvayre R. 2007. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat. Genet. 39: 28–30 [DOI] [PubMed] [Google Scholar]
- 37.Schweiger M., Lass A., Zimmermann R., Eichmann T. O., Zechner R. 2009. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am. J. Physiol. Endocrinol. Metab. 297: E289–E296 [DOI] [PubMed] [Google Scholar]
- 38.Das S. K., Eder S., Schauer S., Diwoky C., Temmel H., Guertl B., Gorkiewicz G., Tamilarasan K. P., Kumari P., Trauner M., et al. 2011. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 333: 233–238 [DOI] [PubMed] [Google Scholar]
- 39.Herker E., Ott M. 2011. Unique ties between hepatitis C virus replication and intracellular lipids. Trends Endocrinol. Metab. 22: 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung W., Gill M., Esposito A., Kaminski C. F., Courousse N., Chwetzoff S., Trugnan G., Keshavan N., Lever A., Desselberger U. 2010. Rotaviruses associate with cellular lipid droplet components to replicate in viroplasms, and compounds disrupting or blocking lipid droplets inhibit viroplasm formation and viral replication. J. Virol. 84: 6782–6798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samsa M. M., Mondotte J. A., Iglesias N. G., Assuncao-Miranda I., Barbosa-Lima G., Da Poian A. T., Bozza P. T., Gamarnik A. V. 2009. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 5: e1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cocchiaro J. L., Valdivia R. H. 2009. New insights into Chlamydia intracellular survival mechanisms. Cell. Microbiol. 11: 1571–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashrafi K., Chang F. Y., Watts J. L., Fraser A. G., Kamath R. S., Ahringer J., Ruvkun G. 2003. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 421: 268–272 [DOI] [PubMed] [Google Scholar]
- 44.Beller M., Sztalryd C., Southall N., Bell M., Jackle H., Auld D. S., Oliver B. 2008. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 6: e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eastmond P. J. 2006. SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell. 18: 665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fei W., Shui G., Gaeta B., Du X., Kuerschner L., Li P., Brown A. J., Wenk M. R., Parton R. G., Yang H. 2008. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J. Cell Biol. 180: 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szymanski K. M., Binns D., Bartz R., Grishin N. V., Li W. P., Agarwal A. K., Garg A., Anderson R. G., Goodman J. M. 2007. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc. Natl. Acad. Sci. USA. 104: 20890–20895 [DOI] [PMC free article] [PubMed] [Google Scholar]