Abstract
In rodents, bile salt-stimulated lipase (BSSL) and pancreatic lipase-related protein 2 (PLRP2) are the dominant lipases expressed in the exocrine pancreas in early life when milk is the main food. The aim of the present study was to evaluate whether BSSL and PLRP2 are also key enzymes in neonatal intestinal fat digestion. Using Caco-2 cells as a model for the small intestinal epithelium, purified human enzymes were incubated in the apical compartment with substrates, bile salt composition and concentrations physiologic to newborn infants. Both BSSL and PLRP2 hydrolyzed triglycerides (TG) to free FA and glycerol. Released FA were absorbed by the cells and reesterfied to TG. Together, BSSL and PLRP2 had a synergistic effect, increasing cellular uptake and reesterification 4-fold compared with the sum of each lipase alone. A synergistic effect was also observed with retinyl ester as a substrate. PLRP2 hydrolyzed cholesteryl ester but not as efficiently as BSSL, and the two had an additive rather than synergistic effect. We conclude the key enzymes in intestinal fat digestion are different in newborns than later in life. Further studies are needed to fully understand this difference and its implication for designing optimal neonatal nutrition.
Keywords: fat, lipase, Caco-2 cells, bile salt-stimulated lipase, pancreatic lipase-related protein 2
The fat intake of newborn infants per kg body weight is at least 3- to 5-fold higher than adults, and half of the energy content in human milk or infant formulas is derived from fat. Therefore, efficient gastrointestinal digestion and absorption of fat is important for energy utilization, optimal growth, and development of newborns. It may therefore seem a paradox that the major enzyme responsible for intestinal digestion of dietary triglycerides (TG) in adults, i.e., pancreatic triglyceride lipase (PTL), is expressed at low or undetectable levels at birth (1). The luminal bile salt concentration during digestion reported for healthy adults is in the range 2–30 mM (2), partly depending on the composition of the meal (3). In newborn infants, this range is much lower, 1–5 mM (2, 4, 5), particularly during established fat absorption (6). The critical micellar concentration is around 3.5 mM (7), and Badley et al. (8) suggested that the concentration of bile acids in the upper small intestine should exceed 4 mM, which is necessary to avoid steatorrhea in adults. Hence, there is evidence to suggest that both fat digestion and absorption are different in newborn infants compared with later in life (9).
Previous studies in humans and rodents have shown that PTL and phospholipase A2 (PLA2), the key enzyme in intestinal phospholipid digestion in adults, are not expressed at significant levels in the exocrine pancreas before weaning (1, 10). Not only is the fasting lipase activity low but the exocrine pancreas does not respond to cholecystokinin with increased secretion of lipase after a meal (11). Instead, the bile salt-stimulated lipase (BSSL) and pancreatic lipase-related protein 2 (PLRP2) are the dominant lipases expressed in the pancreas of the newborn (1, 10, 12). The exact levels of PLRP2 and BSSL in the small intestinal contents of newborns are not known, nor how these levels relate to those of adults. Both BSSL and PLRP2 have broad substrate specificities and are expressed in the exocrine pancreas in all species examined so far. In some species, including humans (13, 14) and mice (15), BSSL is also expressed in the lactating mammary gland and secreted with milk. Milk supplied BSSL is thought to compensate for the low endogenous capacity to digest dietary fat in early life (9, 16). This was confirmed in a recent study in which preterm infants were fed mother's milk, either raw or pasteurized. Pasteurization of human milk renders BSSL inactive (17); accordingly, the coefficient of fat absorption in preterm infants was lower when fed pasteurized versus raw mother's milk (18). Moreover, when fed raw milk, the infants gained more weight and the knee-heal length increased more compared with when fed pasteurized milk (18).
The importance of BSSL and PLRP2 during infancy is further supported by animal studies. Both BSSL- (19, 20) and PLRP2-(21) deficient mouse pups suffer from fat malabsorption. Moreover, weight gain is slower in PLRP2-deficient pups compared with wild-type littermates (21) and in BSSL-deficient pups, accumulation of undigested fat in the distal ileum was shown to cause disruption of the intestinal epithelium (19). Interestingly, fat malabsorption in both BSSL- and PLRP2-deficient mice is transient and is not seen after weaning; i.e., it decreases with maturation of the exocrine function of the pancreas.
The aim of this study was to evaluate the role of BSSL and PLRP2, separately and together, in the hydrolysis and uptake of lipids using cultured Caco-2 cells as a model of the small intestinal epithelium. The cells were incubated with lipid substrates, bile salt composition and concentration physiologic to the newborn infants; i.e., low bile salt concentration and emulsified lipid substrates. Purified human enzymes were added to study the effect of both BSSL and PLRP2 on hydrolysis of TG, diglycerides (DG), monoglycerides (MG), retinyl esters (RE), and cholesterol esters (CE) as well as on the uptake of released FA and reesterification to TG within the cells.
MATERIALS AND METHODS
Taurocholic acid, glycocholic acid, glycochenodeoxycholic acid, taurochenodeoxycholic acid, triolein, gum arabic, retinyl palmitate, cholesteryl oleate, and porcine colipase were all purchased from Sigma (St. Louis, MO). Labeled triolein (triolein [9,10-3H(N)], 91 CI/mmol) was from Perkin Elmer (Waltham, MA), labeled retinyl palmitate (pal-1-14C, 55 CI/mmol) from American Radiolabeled Chemicals, Inc. (St. Louis, MO), and labeled cholesteryl oleate (cholesteryl [1-14C] oleate, 50 mCI/mmol) from GE Healthcare (Stockholm, Sweden). DMEM GlutaMax II, Na-pyruvate, nonessential amino acids, and FBS were all from Gibco (Invitrogen Corporation, Paisley, UK).
Cell culture
Caco-2 cells (American Type Culture Collection, Rockville, MD) from passages 29 to 50 were used for the experiments. The cells were cultured in DMEM GlutaMaxII, which was supplemented with penicillin (100 U/ml), streptomycin (100 µg/ml), nonessential amino acids (0.1 mM), Na-pyruvate (1 mM), and 10% FBS in a 95% air, 5% CO2 atmosphere at 37°C. The medium was changed every second to third day. Cells were plated in 175 cm2 flasks and split with 0.25% trypsin, 1 mM EDTA when they reached 80–95% confluence. For experiments, 1 ×106 cells were plated on 24 mm polycarbonate Transwell filters with 0.4 µm pores (Costar, Corning Inc., Corning NY) and grown 15 days postconfluence. Transepithelial resistances were measured with a Millicell-ERS apparatus (Millipore, Bedford, MA) before and after each experiment to ensure that tight junction formation was persistent. The experimental medium (EM) contained DMEM + GlutaMaxII, 0.1 mM nonessential amino acids, and 1 mM Na-pyruvate.
BSSL was purified from human milk as described (22). After lyophilization, the purified enzyme was dissolved in PBS pH 7.2. Catalytic activity of the native enzyme was determined as described (22) using triolein emulsified with gum arabic as a substrate. A mixture of bile salts (stock solution: 84 mM taurocholic acid, 52 mM taurochenodeoxycholic acid, 44 mM glycocholic acid, and 20 mM glycochenodeoxycholic acid) physiologic to the newborn infant was prepared according to Järvenpää et al. (4). Recombinant human PLRP2 (23) was a generous gift from Dr Frédéric Carriére, Marseille, France.
Experimental design
The TG substrate contained triolein and trace amounts of 3H-triolein emulsified with 10% gum arabic in EM and the RE substrate contained 95% triolein, 5% retinyl palmitate, and a trace amount of retinyl palmitate [pal-1-14C] emulsified with gum arabic in EM. The CE substrate was prepared similar to the RE substrate but with 5% cholesteryl oleate and a trace amount of cholesteryl [1-14C] oleate. Cells grown 15 days postconfluence on a Transwell membrane were incubated with EM. Substrate, enzymes, bile salts, and colipase (0.1µg colipase was added to all experiments with no negative effect on BSSL activity) were added to the medium at the apical compartment in a total volume of 1.5 ml. The total TG concentration was 0.30 µmol/ml. Two milliliters of EM was added to the basolateral compartment. The typical incubation was 120 min at 37°C; afterwards, the apical and basolateral media were collected for radioactivity measurements, lipid extraction, and separation of lipid classes. The cells were washed three times with ice-cold EM and finally with ice-cold PBS, pH 7.4 before being scraped off using a magnetic cell scraper (3/4”, INC Biomedicals, Inc., Ohio). The cells were then subjected to lipid separation and radioactivity measurements using a Wallac, Winspectral 1414 liquid scintillation counter. The sum of radioactivity recovered from the apical, intracellular, and basolateral compartments corresponds to 100%.
Product composition in the apical compartment after hydrolysis
Caco-2 cells were incubated with TG substrate in EM medium, pH 7.4, containing 0.1 µg colipase and 2 mM bile salt mixture at 37°C and increasing amounts of BSSL or PLRP2. After 2 h, radioactivity was measured in the apical, basolateral, and cellular compartments. For acidic lipid extraction, 0.9 ml of the apical medium was mixed with 0.9 ml chloroform-methanol (2:1). The tubes were centrifuged at 1,700 g for 3 min. The upper water-soluble phase was discarded and the lower chloroform-phase was dried under nitrogen. The samples were then dissolved in 30 μl chloroform and applied to (19C)-Silica Gel TLC plates (250 μm). The plates were developed in heptane-ether-methanol-acetic acid (85:30:3:2, v/v/v/v) for separation of TG, DG, MG, and FA. Standards and samples were visualized by spraying the plate with 50% H2SO4 in ethanol and heating at 150°C until brown spots developed. The plates were scanned in a GelDoc scanner (Bio-Rad, Hercules, CA), and the amount of lipid in each spot was calculated as a proportion of the sum of density in all spots in a given lane.
Comparison of PLRP2 and PTL catalyzed TG hydrolysis in absence of cells
To study whether PLRP2 has the capacity to hydrolyze MG, we incubated increasing amounts of PLRP2, and PTL as reference, in EM medium, pH 7.4 containing 0.1 µg colipase and 2 mM bile salt mixture at 37°C, in the absence of Caco-2 cells. After 2 h, lipids were extracted and separated by TLC. Lipid extraction and quantification of hydrolysis products by density proportions were as described above.
TG hydrolysis, cellular uptake, and resynthesis
Caco-2 cells were incubated with TG substrate in the presence of 0.1 µg colipase and 2 mM or 4 mM bile salt mixture. Increasing amounts of BSSL or PLRP2 were added. TG and FA were separated by mixing 200 µl of apical medium or harvested cells with 3.25 ml methanol-chloroform-heptane (30:34:27, v/v/v) and 1.0 ml 0.1 M sodium carbonate buffer, pH 10.5 (24). The samples were centrifuged at 1,700 g for 10 min. The FA were recovered from the upper water-soluble phase and the TG from the lower lipid-soluble phase (24). Results are presented as the mean (± SD) percentage of radioactivity in each lipid fraction recovered from each compartment.
To explore how BSSL and PLRP2 operate together, we incubated the cells with TG substrate, 4 mM bile salts, and 0.5 µg of either BSSL or PLRP2 or 0.5 µg of both. After 2 h, the FA and TG were separated from the apical medium and the harvested cells and radioactivity determined as above. When using RE and CE as substrates, 1 µg of the respective enzyme was used but the conditions were otherwise the same as for TG.
RESULTS
BSSL and PLRP2 both hydrolyze TG to FA and glycerol
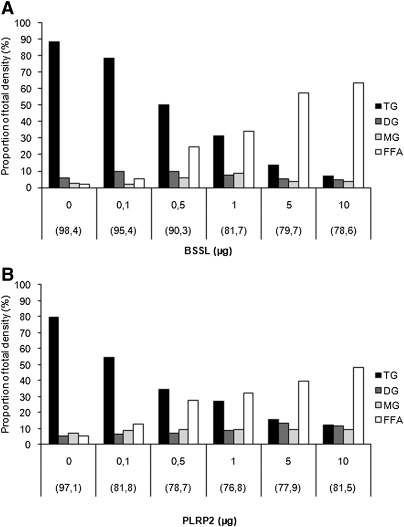
With increasing lipase concentration, both BSSL (Fig. 1A) and PLRP2 (Fig. 1B) were able to hydrolyze at least 90% of the TG emulsion mainly to FFA and glycerol. This was illustrated by the disappearance of TG and concomitant increase of FA but no accumulation of either DG or MG in the upper compartment with increasing lipase concentration. As glycerol is water soluble, it could not be quantified.
Fig. 1.
Product composition of the apical compartment after hydrolysis of triglycerides with increasing concentrations of BSSL or PLRP2. Caco-2 cells were incubated with the 3H-triolein/gum arabic emulsion as a substrate, at pH 7.4, with 0.1 µg colipase, 2 mM bile salt mixture, and increasing concentrations of BSSL (A) or PLRP2 (B) for 2 h at 37°C. Lipids from the apical compartment were extracted and separated by TLC and quantified as described in Materials and Methods. The radioactivity recorded from the apical, cellular, and basolateral compartments was set to 100%. The amount remaining in the apical compartment is indicated within brackets for each lipase concentration. The amount of lipid in each spot was calculated as a density proportion of the sum of all spots in a given lane.
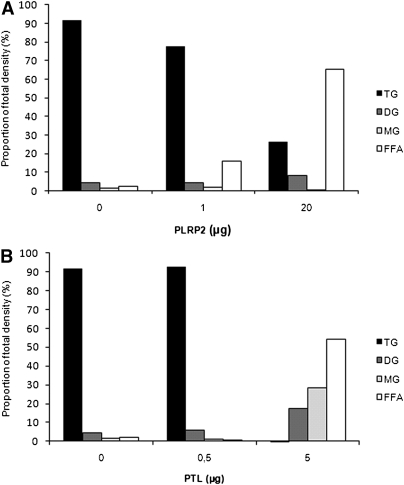
The indicated capacity of PLRP2 to hydrolyze MG was confirmed in an experiment using the same conditions as above but without Caco-2 cells present (Fig. 2A). In this experimental setting, PLRP2 again hydrolyzed the TG emulsion without accumulation of MG, whereas incubation with PTL resulted in almost 30% residual MG (Fig. 2B). This confirms that PLRP2 does not share the positional specificity for the sn-1(3) position with PTL.
Fig. 2.
Comparison of the capacities of PLRP2 and PTL to hydrolyze monoglycerides. Increasing concentration of PLRP2 (A) and PTL (B) was incubated with a triolein/gum arabic emulsion as a substrate, at pH 7.4, with 0.1 µg colipase and 2 mM bile salt mixture for 2 h at 37°C. Lipids were extracted and separated by TLC and quantified as described in Materials and Methods. The amount of lipid in each spot was calculated as a density proportion of the sum of all spots in a given lane, set to 100%.
Hydrolysis of TG and product uptake are dependent on the bile salt concentration
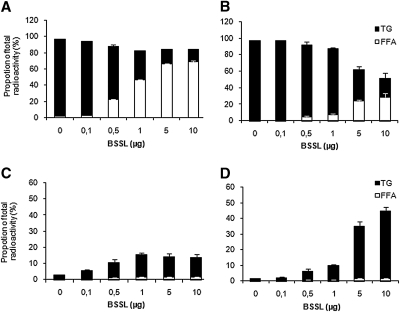
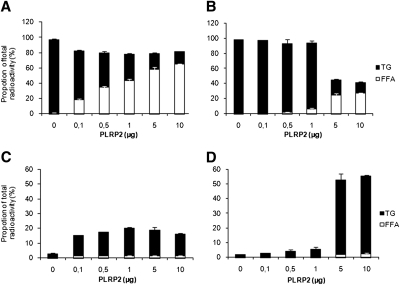
The effects of bile salt concentration on TG hydrolysis by BSSL and PLRP2 and subsequent cellular uptake and reesterification of the lipolysis products were tested. Caco-2 cells were grown as described in Materials and Methods in medium containing either 2 mM or 4 mM of the bile salt mixture. There was no visible effect on the cells morphology or change in transepithelial resistance during the 2 h incubation with 2 mM nor 4 mM of bile salt. However, hydrolysis of TG by both BSSL (Fig. 3A, B) and PLRP2 (Fig. 4A, B) was more efficient at 2 mM bile salts compared with 4 mM; this was indicated by a higher proportion of labeled FA relative to TG in the upper compartment at the lower bile salt concentration at identical enzyme concentrations.
Fig. 3.
Effect of bile salt concentration on the hydrolysis of triglycerides and subsequent uptake of lipolysis products and reesterification with increasing concentrations of BSSL. Caco-2 cells were incubated for 2 h with 3H-triolein/gum arabic as a substrate, at pH 7.4, with 0.1 µg colipase, 2 mM (A, C) or 4 mM (B, D) bile salt, and increasing concentrations of BSSL for 2 h at 37°C. TG and FA were separated from the apical (A, B) and cellular (C, D) compartments, respectively. Data are expressed as the percent radioactivity in each lipid class after 2 h incubation. The sum of radioactivity in the apical, intracellular, and basolateral compartments corresponds to 100%.
Fig. 4.
Effect of bile salt concentration on hydrolysis of triglycerides and subsequent uptake of lipolysis products and reesterification with increasing concentrations of PLRP2. Caco-2 cells were incubated for 2 h with 3H-triolein/gum arabic as a substrate, at pH 7.4, with 0.1 µg colipase, 2 mM (A, C) or 4 mM (B, D) bile salt, and increasing concentrations of PLRP2 for 2 h at 37°C. TG and FFA were separated from the apical (A, B) and cellular (C, D) compartments, respectively. Data are expressed as the percent radioactivity in each lipid class after 2 h incubation. The sum of radioactivity in the apical, intracellular, and basolateral compartments corresponds to 100%.
In contrast to hydrolysis, the cellular uptake of FA and reesterification into TG was more efficient at 4 mM than at 2 mM bile salts for both lipases (Fig. 3C, D and Fig. 4C, D, respectively). During the 2 h the cells were incubated, only a small percent of the radioactivity passed through the cells to the basolateral side (data not shown).
BSSL and PLRP2 operate synergistically
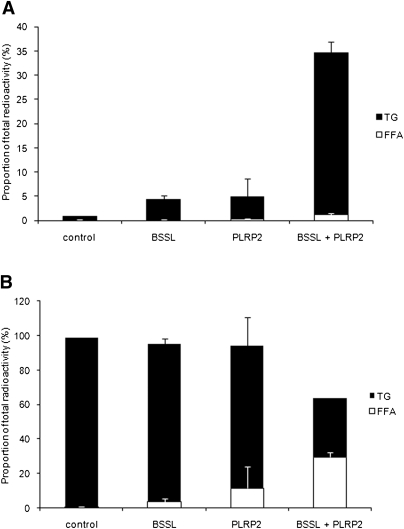
To explore whether BSSL and PLRP2, if added together, have an additive or synergistic effect upon TG hydrolysis and cellular uptake, Caco-2 cells were grown as above on Transwell membranes in medium containing 4 mM bile salts. The result confirmed that both BSSL and PLRP2 are able to promote TG hydrolysis and product absorption under these conditions; moreover, adding the two lipases simultaneously achieved a strong synergistic effect on the net uptake and reesterification. The cellular uptake of lipids increased approximately 4-fold when BSSL and PLRP2 were added simultaneously, compared with when each lipase was added alone at the same concentration (Fig. 5). A comparable effect could not be achieved by doubling the amount enzyme when each lipase operated separately.
Fig. 5.
Synergistic effect of BSSL and PLRP2 on triglyceride hydrolysis and subsequent cellular uptake and reesterification. Caco-2 cells were incubated for 2 h with 3H-triolein/gum arabic as a substrate, at pH 7.4, with 0.1 µg colipase, 4 mM bile salt mixture, and BSSL (0.5 µg) or PLRP2 (0.5 µg), separately, or 0.5 µg BSSL + 0.5 µg PLRP2 as described in Material and Methods. TG and FFA were separated and quantified in the cellular compartment (A) and apical compartment (B). Data are expressed as the percent radioactivity in each lipid class after 2 h incubation. The sum of radioactivity in the apical, intracellular, and basolateral compartments corresponds to 100%.
Both BSSL and PLRP2 hydrolyze RE and CE
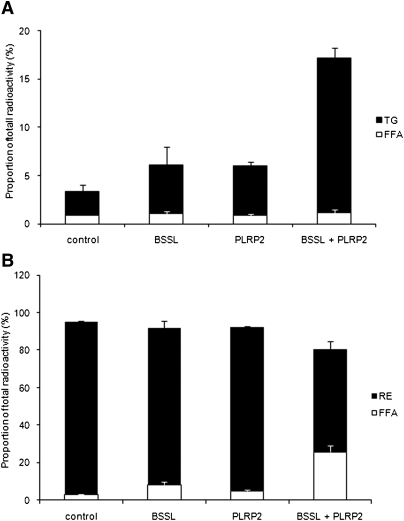
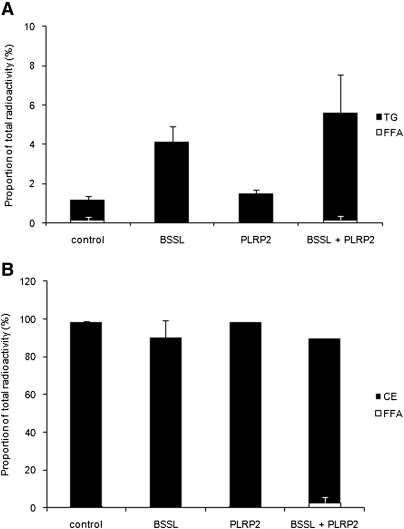
The capacity of BSSL and PLRP2 to hydrolyze RE and CE and, thereby, affect the cellular uptake of their lipolysis products was investigated. Caco-2 cells were grown as above on Transwell membranes in medium supplemented with 4 mM bile salts and RE- or CE-substrate. Both BSSL and PLRP2 were able to hydrolyze RE and CE and facilitate cellular uptake of FA, which rapidly reesterfied to TG. Although the activity against CE was low for PLRP2, it increased with increasing enzyme concentration (data not shown). Adding the two lipases simultaneously achieved a synergistic effect on hydrolysis and uptake of RE (Fig. 6), whereas the effect on CE was additive rather than synergistic (Fig. 7). Neither during hydrolysis of RE (Fig. 6B) nor of CE (Fig. 7B) was there accumulation of released FA in the apical compartment. However, the degree of hydrolysis was much less than for TG.
Fig. 6.
BSSL and PLRP2 catalyzed hydrolysis of retinyl esters and subsequent cellular uptake and reesterification. Caco-2 cells were incubated for 2 h with emulsified TG/retinyl ester as a substrate, at pH 7.4, with 0.1 µg colipase, 4 mM bile salts, and 1 µg BSSL, 1 µg PLRP2, or 1 µg BSSL + 1 µg PLRP2 as described in Materials and Methods. TG and FFA were separated and quantified in the cellular compartment (A) and the apical compartment (B). Data are expressed as the percent radioactivity in each lipid class after 2 h incubation. The sum of radioactivity in the apical, intracellular, and basolateral compartments corresponds to 100%.
Fig. 7.
BSSL and PLRP2 catalyzed hydrolysis of cholesteryl esters and subsequent cellular uptake and reesterification. Caco-2 cells were incubated for 2 h with emulsified TG/cholesteryl ester as a substrate, at pH 7.4, with 0.1 µg colipase, 4 mM bile salts, and 1 µg BSSL, 1 µg PLRP2, or 1 µg BSSL + 1 µg PLRP2 as described in Materials and Methods. TG and FFA were separated and quantified in the cellular compartment (A) and in the apical compartment (B). Data are expressed as the percent radioactivity in each lipid class after 2 h incubation. The sum of radioactivity in the apical, intracellular, and basolateral compartments corresponds to 100%.
DISCUSSION
Our previous study on enzyme expression in the exocrine pancreas of the rat and mouse suggested that BSSL and PLRP2 are key enzymes in fat digestion during infancy or as long as milk is the main food (10). In the present study, using in vitro conditions aiming at resembling physiologic conditions of the newborn infant with respect to pH, bile salt composition and concentration, lipid substrates, enzyme concentrations, and uptake of cellular lipolysis products, we demonstrated both BSSL and PLRP2 hydrolyze TG, RE, and CE. Moreover, we showed that the two lipases have a synergistic effect on TG and RE digestion and absorption. We also provided evidence that neither of the two lipases share the recognized positional specificity of PTL for the sn-1(3)-position of TG. Thus, both have the potential to hydrolyze each TG into three FA and one glycerol rather than two FA and one sn2-MG, which is a characteristic of PTL.
There are virtually no studies on the ontogeny of lipase expression and secretion in the human pancreas. However, one study by Yang et al. (1) showed PLRP 1 and 2 mRNAs are expressed from the 16th week of gestation in the human fetal pancreas whereas PTL begins to be expressed after birth. In another study, Carrère et al. (12), using an immunostaining technique, found that BSSL/CEH protein is detectable earlier than lipase protein in the human exocrine fetal pancreas. This study most likely did not discriminate between PTL and the related proteins, PLRP1 and 2, as they share a high degree of homology and the antibodies used were not necessarily specific for PTL. Hence, both studies lend support to the hypothesis that the temporal pattern of lipase mRNA expression and lipase protein secretion is similar in rodents and humans.
Both BSSL and PLRP2 have broad substrate specificities. BSSL hydrolyzes TG, DG, MG, PL, RE, CE [reviewed in (9, 25)], ceramides (26), and galactolipids (27), whereas PLRP2 hydrolyzes TG, DG, PL (28), RE (29), and galactolipids (30). In the human adult, BSSL is the enzyme primarily responsible for CE hydrolysis in the digestive tract. Hence, it sometimes still is referred to as pancreatic cholesterol esterase (31). On the other hand, PLRP2 is considered mainly a galactolipase in adults (9, 32). The activity of BSSL against TG, DG, MG, RE, and CE was confirmed here under conditions aiming at resembling the small intestinal milieu of newborn infants during established fat digestion and absorption using Caco-2 cells as a model of the small intestinal absorptive endothelium. We also confirmed PLRP2 is active against TG, DG, and RE (28, 29). Under the conditions used, TG hydrolysis for both lipases was more efficient at 2 mM than 4 mM bile salt mixture, which both are within the range found in newborn infants (4). This was also true for PLRP2 in the presence of colipase and is in agreement with previous studies showing that PLRP2 is sensitive to bile salts and inhibited at higher concentrations (28). This finding also supports that when liver functions mature and the bile salt secretion increases, PLRP2 will become less important to TG digestion, probably coinciding with PTL becoming the dominant TG lipase. For BSSL, lower activity at higher bile salt concentration was not expected and is not in agreement with previous in vitro experiments. However, in this context, BSSL and PLRP2 have the capacity by themselves to hydrolyze most dietary lipids and, as we showed here, they operate more efficiently together than alone; i.e., they have a synergistic effect on TG and RE digestion. We chose incubation conditions aiming at resembling the physiological conditions of the newborn infant, i.e., 4 mM bile salt and 0.5 µg purified lipase. As the activity for each was lower at 4 mM bile salt than 2 mM, these conditions seemed suboptimal for each lipase alone. Under these conditions, the two lipases had a striking synergistic effect when operating together with net uptake and reesterification at least 4-fold higher than for each lipase alone. Hence, when operating together, the negative effect of the higher bile salt concentration seemed to be ameliorated.
A similar synergistic effect was recently shown for PLRP2 and PTL when digesting TG presented as bovine milk fat globules (33) and for PTL and PLRP2 when digesting RE (29). The molecular or physical-chemical explanation behind these synergisms are presently not understood; however, a synergistic effect between PLRP2 and BSSL should be important in neonatal lipid digestion as the capacity to digest fat is often a limiting factor in energy utilization, particularly when it is from infant formula or pasteurized human milk, as neither contains active BSSL (18).
Previously, it was suggested that the activity of PLRP2 against RE is dependent on the physical-chemical state of the substrate, as the lipase used hydrolyzed micellar RE but not emulsified RE (29). In the present study, we used emulsified RE as a substrate. As bile salts were present, we cannot determine whether only micellar RE was hydrolyzed, although this seems unlikely as the activity did not differ between 2 and 4 mM bile salt (data not shown). To our knowledge, it has not been shown that PLRP2 hydrolyzes CE, although it is not unexpected given its low substrate specificity and its activity against RE.
We showed previously that BSSL lacks positional specificity (34, 35); interestingly, we demonstrated here that PLRP2 also lacks the positional specificity characteristic of PTL; i.e., both BSSL and PLRP2 have the capacity to also hydrolyze sn2-MG. A generally held view is that efficient absorption of TG from human milk depends on palmitic acid (two-thirds of which is located at the sn-2-postion in human milk TG) escaping release as FA and thereby forming insoluble calcium soaps due to the positional specificity of PTL (36). There are clinical studies that lend support to this view (37). Although it is not known to what extent PLRP2 and BSSL achieve complete TG digestion in vivo, the importance of palmitic acid being absorbed as MG must at least be questioned. Theoretically, a beneficial effect of sn-2 hydrolysis may be dependent on the bile salt concentration in neonatal intestinal content during fat digestion. As expected, the hydrolyzed FA were more easily taken up by cells at the higher bile salt concentration. The micellar capacity is greater at 4 mM compared with 2 mM, which is below the critical concentration at which micelles begin to form (7). At low bile salt concentrations and, thus, low micellar capacity, a larger fraction of sn2-MG would remain in the oil phase and be available for digestion for a longer time compared with a high micellar capacity, which shifts the distribution of MG from the oil phase to the micellar phase and faster absorption. It is hard to predict if absorption is promoted by hydrolysis, yielding FA rather than sn2-MG, when the micellar capacity is low. Under such conditions, it is likely that absorption occurs from unilamellar vesicles to a larger extent than from mixed micelles (38–40), which would favor solubilization and, thus, absorption of FA soaps rather than sn-2 MG. A larger part of the small intestine is used for absorption when the intraluminal bile salt concentration is low compared to conditions with higher bile salt concentrations and higher micellar transport capacity (9). One might speculate that when the exocrine pancreatic and liver functions mature and luminal bile salt concentrations rise, the benefit of absorbing sn-2 MG coincides with PTL becoming the important lipase in TG digestion. However, more studies are needed to explore this possibility.
In conclusion, we have shown that under in vitro conditions similar to the milieu of the intestine of newborn infants, BSSL and PLRP2 can efficiently digest dietary TG, RE, and CE. We have further shown that the two lipases operate in a synergistic rather than additive fashion when the first two substrates are hydrolyzed, making fat digestion particularly efficient. It is noteworthy that both lipases have the potential to hydrolyze TG to completion. To fully understand the principles of why fat digestion and absorption are different in early life, or as long as milk is the main food, further studies are needed. Confirmation of the results from the present study in clinical studies would impact the design of early feeding products for infants, i.e., infant formulas.
Acknowledgments
We are grateful to Dr Frédéric Carriére, Marseille, France for his generous supply of PLRP2.
Footnotes
- BSSL
- bile salt-stimulated lipase
- CE
- cholesterol ester
- DG
- diglyceride
- EM
- experimental medium containing DMEM + GlutaMaxII, 0.1 mM non-essential amino acids, and 1 mM Na-pyruvate
- MG
- monoglyceride
- PLA2
- phospholipase A2
- PLRP2
- pancreatic lipase-related protein 2
- PTL
- pancreatic triglyceride lipase
- RE
- retinyl ester
- TG
- triglyceride
Financial support was provided by the Swedish Research Council/Medicine, Swedish Orphan Biovitrum AB, Västerbotten County Council (ALF), the Oscar Foundation, and Her Royal Highness the Crown Princess Lovisa's/Axel Tielman's Foundation.
REFERENCES
- 1.Yang Y. Q., Sanchez D., Figarella C., Lowe M. E. 2000. Discoordinate expression of pancreatic lipase and two related proteins in the human fetal pancreas. Pediatr. Res. 47: 184–188 [DOI] [PubMed] [Google Scholar]
- 2.Watkins J. B. 1974. Bile acid metabolism and fat absorption in newborn infants. Pediatr. Clin. North Am. 21: 501–512 [DOI] [PubMed] [Google Scholar]
- 3.Clarysse S., Tack J., Lammert F., Duchateau G., Reppas C., Augustijns P. 2009. Postprandial evolution in composition and characteristics of human duodenal fluids in different nutritional states. J. Pharm. Sci. 98: 1177–1192 [DOI] [PubMed] [Google Scholar]
- 4.Järvenpää A. L., Rassin D. K., Kuitunen P., Gaull G. E., Räihä N. C. R. 1983. Feeding the low-birth-weight infant. 3. Diet influences bile-acid metabolism. Pediatrics. 72: 677–683 [PubMed] [Google Scholar]
- 5.Signer E., Murphy G. M., Edkins S., Anderson C. M. 1974. Role of bile salts in fat malabsorption of premature infants. Arch. Dis. Child. 49: 174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norman A., Ojamae O., Strandvi B. 1972. Bile-acids and pancreatic enzymes during absorption in newborn. Acta Paediatr. Scand. 61: 571–576 [DOI] [PubMed] [Google Scholar]
- 7.Järvenpää A. L. 1983. Feeding the low-birth-weight infant. 4. Fat absorption as a function of diet and duodenal bile-acids. Pediatrics. 72: 684–689 [PubMed] [Google Scholar]
- 8.Badley B. W. D., Murphy G. M., Bouchier I. A. 1969. Intraluminal bile salt deficiency in pathogenesis of steatorrhoea. Lancet. 2: 400–402 [DOI] [PubMed] [Google Scholar]
- 9.Lindquist S., Hernell O. 2010. Lipid digestion and absorption in early life: an update. Curr. Opin. Clin. Nutr. Metab. Care. 13: 314–320 [DOI] [PubMed] [Google Scholar]
- 10.Li X., Lindquist S., Lowe M., Noppa L., Hernell O. 2007. Bile salt-stimulated lipase and pancreatic lipase-related protein 2 are the dominating lipases in neonatal fat digestion in mice and rats. Pediatr. Res. 62: 537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebenthal E., Lee P. C. 1980. Development of functional response in human exocrine pancreas. Pediatrics. 66: 556–560 [PubMed] [Google Scholar]
- 12.Carrère J., Figarella-Branger D., Senegas-Balas F., Figarella C., Guy-Crotte O. 1992. Immunohistochemical study of secretory proteins in the developing human exocrine pancreas. Differentiation. 51: 55–60 [DOI] [PubMed] [Google Scholar]
- 13.Hernell O., Olivecrona T. 1974. Human milk lipases 2. Bile salt-stimulated lipase. Biochim. Biophys. Acta. 369: 234–244 [DOI] [PubMed] [Google Scholar]
- 14.Nilsson J., Bläckberg L., Carlsson P., Enerbäck S., Hernell O., Bjursell G. 1990. cDNA cloning of human-milk bile salt-stimulated lipase and evidence for its identity to pancreatic carboxylic ester hydrolase. Eur. J. Biochem. 192: 543–550 [DOI] [PubMed] [Google Scholar]
- 15.Strömqvist M., Tornell J., Edlund M., Edlund A., Johansson T., Lindgren K., Lundberg L., Hansson L. 1996. Recombinant human bile salt-stimulated lipase: an example of defective O-glycosylation of a protein produced in milk of transgenic mice. Transgenic Res. 5: 475–485 [DOI] [PubMed] [Google Scholar]
- 16.Hernell O., Bläckberg L. 1997. Digestion and absorption of human milk lipids. Encyclopedia of Human Biology. 2nd ed. Dulbecco R., editor Academic Press, New York: 319–328 [Google Scholar]
- 17.Björkstén B., Burman L. G., Dechateau P., Fredrikzon B., Gothefors L., Hernell O. 1980. Collecting and banking human milk - to heat or not to heat. BMJ. 281: 765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson Y., Sävman K., Bläckberg L., Hernell O. 2007. Pasteurization of mother's own milk reduces fat absorption and growth in preterm infants. Acta Paediatr. 96: 1445–1449 [DOI] [PubMed] [Google Scholar]
- 19.Howles P. N., Stemmerman G. N., Fenoglio-Preiser C. M., Hui D. Y. 1999. Carboxyl ester lipase activity in milk prevents fat-derived intestinal injury in neonatal mice. Am. J. Physiol. 277: G653–G661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller R., Lowe M. E. 2008. Carboxyl ester lipase from either mother's milk or the pancreas is required for efficient dietary triglyceride digestion in suckling mice. J. Nutr. 138: 927–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowe M. E., Kaplan M. H., Jackson-Grusby L., D'Agostino D., Grusby M. J. 1998. Decreased neonatal dietary fat absorption and T cell cytotoxicity in pancreatic lipase-related protein 2-deficient mice. J. Biol. Chem. 273: 31215–31221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bläckberg L., Hernell O. 1981. The bile salt-stimulated lipase in human milk - purification and characterization. Eur. J. Biochem. 116: 221–225 [DOI] [PubMed] [Google Scholar]
- 23.Eydoux C., De Caro J., Ferrato F., Boullanger P., Lafont D., Laugier R., Carriere F., De Caro A. 2007. Further biochemical characterization of human pancreatic lipase-related protein 2 expressed in yeast cells. J. Lipid Res. 48: 1539–1549 [DOI] [PubMed] [Google Scholar]
- 24.Belfrage P., Vaughan M. 1969. Simple liquid-liquid partition system for isolation of labeled oleic acid from mixtures with glycerides. J. Lipid Res. 10: 341–344 [PubMed] [Google Scholar]
- 25.Hernell O. 1985. Specificity of human-milk bile salt-stimulated lipase. J. Pediatr. Gastroenterol. Nutr. 4: 517–519 [DOI] [PubMed] [Google Scholar]
- 26.Nyberg L., Farooqi A., Bläckberg L., Duan R. D., Nilsson A., Hernell O. 1998. Digestion of ceramide by human milk bile salt-stimulated lipase. J. Pediatr. Gastroenterol. Nutr. 27: 560–567 [DOI] [PubMed] [Google Scholar]
- 27.Andersson L., Bratt C., Arnoldsson K. C., Herslöf B., Olsson N. U., Sternby B., Nilsson A. 1995. Hydrolysis of galactolipids by human pancreatic lipolytic enzymes and duodenal contents. J. Lipid Res. 36: 1392–1400 [PubMed] [Google Scholar]
- 28.De Caro J., Sias B., Grandval P., Ferrato F., Halimi H., Carriere F., De Caro A. 2004. Characterization of pancreatic lipase-related protein 2 isolated from human pancreatic juice. Biochim. Biophys. Acta. 1701: 89–99 [DOI] [PubMed] [Google Scholar]
- 29.Reboul E., Berton A., Moussa M., Kreuzer C., Crenon I., Borel P. 2006. Pancreatic lipase and pancreatic lipase-related protein 2, but not pancreatic lipase-related protein 1, hydrolyze retinyl palmitate in physiological conditions. Biochim. Biophys. Acta. 1761: 4–10 [DOI] [PubMed] [Google Scholar]
- 30.Andersson L., Carriere F., Lowe M. E., Nilsson A., Verger R. 1996. Pancreatic lipase-related protein 2 but not classical pancreatic lipase hydrolyzes galactolipids. Biochim. Biophys. Acta. 1302: 236–240 [DOI] [PubMed] [Google Scholar]
- 31.Brown A. W., Hang J. L., Dussault P. H., Carr T. P. 2010. Plant sterol and stanol substrate specificity of pancreatic cholesterol esterase. J. Nutr. Biochem. 21: 736–740 [DOI] [PubMed] [Google Scholar]
- 32.Sias B., Ferrato F., Grandval P., Lafont D., Boullanger P., De Caro A., Leboeuf B., Verger R., Carriere F. 2004. Human pancreatic lipase-related protein 2 is a galactolipase. Biochemistry. 43: 10138–10148 [DOI] [PubMed] [Google Scholar]
- 33.Berton A., Sebban-Kreuzer C., Rouvellac S., Lopez C., Crenon I. 2009. Individual and combined action of pancreatic lipase and pancreatic lipase-related proteins 1 and 2 on native versus homogenized milk fat globules. Mol. Nutr. Food Res. 53: 1592–1602 [DOI] [PubMed] [Google Scholar]
- 34.Hernell O., Bläckberg L. 1982. Digestion of human-milk lipids: physiologic significance of sn-2 monoacylglycerol hydrolysis by bile salt-stimulated lipase. Pediatr. Res. 16: 882–885 [DOI] [PubMed] [Google Scholar]
- 35.Bernbäck S., Bläckberg L., Hernell O. 1990. The complete digestion of human-milk triacylglycerol in vitro requires gastric lipase, pancreatic colipase-dependent lipase, and bile salt-stimulated lipase. J. Clin. Invest. 85: 1221–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lien E. L. 1994. The role of fatty-acid composition and positional distribution in fat-absorption in infants. J. Pediatr. 125: S62–S68 [DOI] [PubMed] [Google Scholar]
- 37.Carnielli V. P., Luijendijk I. H. T., Van Goudoever J. B., Sulkers E. J., Boerlage A. A., Degenhart H. J., Sauer P. J. J. 1996. Structural position and amount of palmitic acid in infant formulas: effects on fat, fatty acid, and mineral balance. J. Pediatr. Gastroenterol. Nutr. 23: 553–560 [DOI] [PubMed] [Google Scholar]
- 38.Hernell O., Staggers J. E., Carey M. C. 1990. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 2. Phase-analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adults human beings. Biochemistry. 29: 2041–2056 [DOI] [PubMed] [Google Scholar]
- 39.Carey M. C., Hernell O. 1992. Digestion and absorption of fat. Semin. Gastrointest. Dis. 3: 189–208 [Google Scholar]
- 40.Staggers J. E., Hernell O., Stafford R. J., Carey M. C. 1990. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 1. Phase-behavior and aggregation states of model lipid systems patternes after aqueous duodenal contents of healthy adult human beings. Biochemistry. 29: 2028–2040 [DOI] [PubMed] [Google Scholar]