Abstract
Adipose triglyceride lipase (ATGL) catalyzes the first step of triacylglycerol hydrolysis in adipocytes. Abhydrolase domain 5 (ABHD5) increases ATGL activity by an unknown mechanism. Prior studies have suggested that the expression of ABHD5 is limiting for lipolysis in adipocytes, as addition of recombinant ABHD5 increases in vitro TAG hydrolase activity of adipocyte lysates. To test this hypothesis in vivo, we generated transgenic mice that express 6-fold higher ABHD5 in adipose tissue relative to wild-type (WT) mice. In vivo lipolysis increased to a similar extent in ABHD5 transgenic and WT mice following an overnight fast or injection of either a β-adrenergic receptor agonist or lipopolysaccharide. Similarly, basal and β-adrenergic-stimulated lipolysis was comparable in adipocytes isolated from ABHD5 transgenic and WT mice. Although ABHD5 expression was elevated in thioglycolate-elicited macrophages from ABHD5 transgenic mice, Toll-like receptor 4 (TLR4) signaling was comparable in macrophages isolated from ABHD5 transgenic and WT mice. Overexpression of ABHD5 did not prevent the development of obesity in mice fed a high-fat diet, as shown by comparison of body weight, body fat percentage, and adipocyte hypertrophy of ABHD5 transgenic to WT mice. The expression of ABHD5 in mouse adipose tissue is not limiting for either basal or stimulated lipolysis.
Keywords: adipose triglyceride lipase, triacylglycerol, macrophage, Toll-like receptor 4, abhydrolase domain 5
In vertebrates, energy is stored primarily in triacylglycerols (TAGs) in adipose tissue. During fasting and exercise, stored TAG is hydrolyzed, releasing fatty acids and glycerol for use by other tissues. The complete hydrolysis of TAG is catalyzed by several lipases, including adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and monoacylglycerol lipase (1–5). The first step, hydrolysis of TAG to diacylglycerols and fatty acids, is catalyzed by ATGL and HSL, which account for 90-95% of TAG hydrolase activity in mouse adipose tissue (3, 5). The hydrolysis of diacylglycerol to monoacylglycerol and fatty acids is catalyzed by HSL in adipose tissue (6); HSL-null mice accumulate diacylglycerol in adipose tissue, suggesting that HSL is the primary diacylglycerol lipase in that tissue (6, 7). The final step of hydrolysis of monoacylglycerol to glycerol and fatty acids is catalyzed by monoacylglycerol lipase (8).
Adipocyte lipolysis is regulated by hormones to ensure adaptation to variable nutritional and physiological conditions. Catecholamines bind to β-adrenergic receptors, increasing cAMP and activating cAMP-dependent protein kinase A, which phosphorylates HSL and perilipin (2, 9, 10). Phosphorylation of HSL minimally increases its TAG hydrolase activity while promoting its translocation from the cytosol to lipid droplets (9, 11), facilitating lipase access to lipid substrates and a major increase in lipolysis. Concurrent phosphorylation of perilipin A is required for maximal TAG hydrolysis by both HSL and non-HSL lipases, including ATGL (12–15). ATGL is not known to be phosphorylated by protein kinase A (5) and localizes to lipid droplets under both basal and hormonally stimulated conditions (5, 16).
The acute-phase response of animals to infection or inflammation also triggers increased adipose lipolysis to produce fuel for cells mediating innate immunity. The administration of bacterial endotoxin to mice induces lipolysis through activation of the Toll-like receptor 4 (TLR4) signaling pathway, resulting in increased release of fatty acids and glycerol into circulation (17–20). Moreover, activation of TLR4 induces adipocytes to secrete tumor necrosis factor α (TNFα) and other cytokines, some of which also increase lipolysis (19, 21). The Lipid A moiety of bacterial endotoxin binds to TLR4 on adipocytes, initiating a signaling cascade through MEK1/2 (MAPK/ERK kinase; mitogen-activated protein kinase/extracellular signal-regulated kinase kinase) and ERK1/2 (Extracellular signal-regulated kinase) (20); similarly, incubation of adipocytes with TNFα also activates MEK1/2 and ERK1/2 signaling (22). The consequence of increased MEK and ERK phosphorylation is increased phosphorylation of perilipin A (20, 22) and modest increases in protein levels of ATGL and HSL (20), leading to increased lipolysis.
Abhydrolase domain 5 (ABHD5, also called CGI-58) is a ubiquitously expressed protein linked to TAG metabolism. Mutations in ABHD5 were identified to be the cause of a neutral lipid storage disorder called Chanarin-Dorfman syndrome characterized by ichthyosis, hepatic steatosis, and excessive storage of TAG in multiple cells and tissues (23). ABHD5 lacks TAG hydrolase activity, yet increases the lipase activity of ATGL (24, 25) through a poorly understood mechanism. Addition of purified recombinant ABHD5 to either purified recombinant ATGL or lysates of cells overexpressing ATGL increases in vitro TAG hydrolase activity (24–28). Similarly, addition of ABHD5 to cytosolic extracts of adipose tissue from wild-type (WT) mice, but not ATGL-null mice, increases in vitro TAG hydrolase activity (3), suggesting that the effects of ABHD5 on lipolysis require ATGL. Interestingly, overexpression of ABHD5 in CHO K1, McA RH777, Cos-1, and HepG2 cells reduces TAG content (29), suggesting that low levels of endogenous CGI-58 limit TAG hydrolysis in some cells. Consistent with an important role for ABHD5 in TAG hydrolysis, reduction of ABHD5 expression causes accumulation of TAG in McA RH7777 and Hepa1 cells (28, 29) and in mouse liver (30), and it decreases lipolysis in 3T3-L1 adipocytes and Hepa1 cells (24, 28). Thus, modulating the expression of ABHD5 significantly alters lipolysis and levels of TAG; furthermore, it has been suggested that endogenous levels of ABHD5 are limiting in adipose tissue of mice (24).
We hypothesized that overexpression of ABHD5 selectively in adipose tissue will increase lipolysis while decreasing storage of TAG, and it may render mice resistant to diet-induced obesity. To test this hypothesis, we generated transgenic mice that selectively express ectopic mouse ABHD5 in adipose tissue. We studied lipolysis in the whole animal and in isolated adipocytes. Additionally, we fed the mice a high-fat diet to induce the development of obesity. Our results show that levels of endogenous ABHD5 are not limiting for lipolysis in mouse adipose tissue and that overexpression of ABHD5 does not prevent the development of diet-induced obesity.
MATERIALS AND METHODS
Generation of transgenic mice overexpressing ABHD5 in adipose tissue
Transgenic mice were generated as described (31) at the Herbert Irving Comprehensive Cancer Center Transgenic Mouse Facility at Columbia University. The cDNA of mouse ABHD5 (32) was subcloned into a plasmid that contains the fatty acid binding protein 4 (Fabp4) promoter to direct gene expression selectively in adipose tissue and the distal region, including the 3′ untranslated region, of the rabbit β-globin gene (33). Transgenic mice were generated using C57Bl/6J×CBA F2 fertilized oocytes. Four ABHD5 transgene-positive founder lines were established, and the line with the highest level of ABHD5 expression in adipose tissue was selected for further study. These transgenic mice were backcrossed to C57BL/6J mice for four to six generations before being used for experiments. Mouse genotypes were determined by PCR analysis of DNA contained in ear-clip samples (34) using the primers 5′-GCCGCTTACTCACTGAAGTACCC-3′ and 5′-CTCAAACGCTGCACTAGACTTAACC-3′, which amplify a 209 bp DNA fragment specific to DNA from the transgenic mice.
Mouse experiments
Mice were conventionally housed at a temperature of 21-24°C, with a 12 h-12 h light-dark cycle. They were fed Breeder Diet (LabDiet 5021) or standard chow diet ad libitum, unless otherwise noted. All procedures were conducted in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the institutional animal care and use committees of Rutgers, Columbia, and Wake Forest Universities.
In vivo lipolysis was studied under several conditions. To study lipolysis stimulated by fasting, 26- to 29-week-old female mice were either given free access to food or fasted for 24 h. Following a 24 h fast, mice were euthanized by carbon dioxide inhalation, and blood was collected from the vena cava. To study β-adrenergic receptor-stimulated lipolysis, saline vehicle or the β3-specific agonist, CL 316243 (Sigma C5976), was administered to 10- to 11-week-old female mice at 0.1 µg/gram body weight by intraperitoneal injection, and blood was collected after 15 min from the submandibular vein. To study cytokine-stimulated lipolysis and the acute phase response, mice were acutely treated with lipopolysaccharide (LPS). Briefly, 16- to 24-week-old female ABHD5 Tg and WT mice were injected intraperitoneally with 5 μg LPS (Escherichia coli 0111:B4) or saline vehicle. Exactly 1 h post LPS injection, mice were terminally anesthetized with ketamine/xylazine, and blood was collected by heart puncture. Following blood collection, whole-body perfusion was conducted with 0.9% saline to remove residual blood. Multiple tissues, including liver, white adipose tissue, brown adipose tissue (BAT), lung, spleen, heart, and kidney, were collected and snap frozen for subsequent analysis.
For the diet-induced obesity study, 4-week-old male mice were caged individually and fed diets of defined composition in which either 10% or 60% of calories were derived from fat (Research Diets D12450B and D12492, respectively). Body weight was determined at regular intervals. Body composition was determined by Dual Energy X-Ray Absorptiometry using a PIXImus machine (GE Lunar PIXIMus) of mice anesthetized with acepromazine-ketamine-xylazine. After 120 days of treatment, the mice were euthanized by carbon dioxide inhalation. Perigonadal and subcutaneous adipose tissues and liver were dissected and weighed, and sections were prepared for histological analyses or frozen prior to solvent extraction for TAG assays.
Adipocyte isolation and incubations
Adipocytes were isolated from perigonadal fat of wild-type and ABHD5 transgenic mice according to the method of Rodbell (35) with modifications (36, 37). Adipocytes were incubated in Krebs-Ringer bicarbonate buffer containing 5 mM glucose, 4% fatty acid-free BSA, 200 nM adenosine, 8 µg/ml adenosine deaminase, and 20 nM N6-phenylisopropyladenosine, either with or without 1 µM isoproterenol (for stimulated or basal conditions, respectively). Adipocytes were incubated in tubes gassed with 5% carbon dioxide in air for 2 h at 37°C, using an oscillating water bath. Aliquots of the incubation medium were collected for analysis of glycerol content.
Peritoneal macrophage isolation and culture
Elicited peritoneal macrophages were collected four days after injection of 1 ml of 10% thioglycolate into the peritoneal cavities of 16- to 24-week-old male mice that were maintained on a standard chow diet as previously described (38). Macrophages from five WT or five ABHD5 Tg mice were pooled for signaling analyses. Following 2 h of culture, nonadherent cells were removed by washing three times with PBS, and remaining adherent macrophages were maintained in serum free RPMI-1640 for an additional 2 h to dampen basal serum-induced signaling. Thereafter, cells were treated with vehicle (PBS) or 10 ng/ml of the defined TLR4 agonist Kdo2-Lipid A for a time course of up to 2 h. TLR4 signaling was examined by immunoblotting.
Immunoblot analysis
White adipose tissue (epididymal fat pad) was homogenized with a rotor/stator type tissue homogenizer in a solution containing 50 mM HEPES, pH 7.4, 2 mM EDTA, 50 mM NaF, 100 µM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, 10 mg/l leupeptin, 500 µM benzamidine, and 5% SDS (2 ml/gram of tissue). Homogenates were incubated in a sonicating water bath at 37°C for 1 h with vortexing every 10 min. Homogenates were centrifuged at 14,000 g for 10 min at room temperature and the infranatants were collected. Solubilized proteins contained in the resulting infranatants (below visible fat layers) were resolved by SDS-PAGE and electrophoretically transferred to nitrocellulose membranes. ABHD5 protein was detected as described (32) and quantified by scanning densitometry.
For immunoblots depicted in Fig. 1C, whole tissue or cell homogenates were made in a modified RIPA buffer as previously described (38, 39). Proteins were separated by 4-12% SDS-PAGE, transferred to polyvinylidene difluoride membranes, and proteins were detected after incubation with specific antibodies. Antibodies used include anti-CGI-58 (ABHD5) mouse monoclonal (Novus Biologicals #HL00051099-M01), anti-β-actin (Sigma #A5441), anti-BIP rabbit polyclonal (Cell Signaling Technologies #3183), phospho-SAPK/JNK (Thr183/Tyr185) rabbit monoclonal (Cell Signaling Technologies #4668), phospho-p38-MAPK (Thr180/Tyr182) rabbit monoclonal (Cell Signaling Technologies # 4631), and phospho-IκBα (Ser32) rabbit monoclonal (Cell Signaling Technologies #2859).
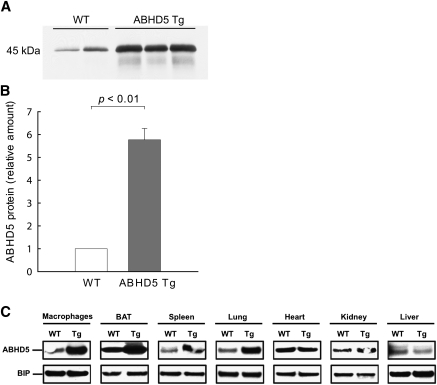
Fig. 1.
Protein levels of ABHD5 are increased in adipose tissue and macrophages of ABHD5 Tg mice. (A) Protein levels of ABHD5 in white adipose tissue from perigonadal fat pads of wild-type (WT) and ABHD5 Tg mice were analyzed by immunoblotting; equal amounts of total protein were loaded in each lane. Results from a representative experiment are shown. (B) Levels of ABHD5 protein were quantified by densitometry and expressed relative to the level of ABHD5 present in WT mice. Values represent the means ± SEM from three independent experiments, each with 2-4 mice per genotype. Statistical analysis was performed using a one-sample t-test. (C) Protein levels of ABHD5 in tissues and cells of wild-type (WT) and ABHD5 Tg mice. For macrophage protein, freshly isolated thioglycolate-elicited macrophages were pooled from n = 5 mice per genotype and cultured as described in “Materials and Methods.” For tissue distribution, protein was pooled from n = 3 mice per genotype. Samples were probed on the same blotting membrane and binding immunoglobulin protein (BIP) was used as an invariant loading control.
Plasma analyses
Plasma was isolated using EDTA as an anticoagulant and stored at −70°C until analyses were conducted. Glycerol concentrations were determined using the Free Glycerol Determination Kit (Sigma F6428). Plasma nonesterified fatty acid (NEFA) concentrations were measured enzymatically (Wako NEFA C or NEFA-HR; Wako Diagnostics). TAG concentrations were measured using the Infinity Triglycerides Liquid Stable Reagent (ThermoScientific). Plasma TNFα was determined by enzyme-linked immunosorbent assay (R and D Systems #MTA00).
Adipocyte lipolysis
Adipocyte lipolysis was assessed by measuring glycerol released into the incubation medium using a fluorimetric enzyme-based assay (37, 40). Data were normalized by cell number as calculated from cell size and lipid mass (41).
Histological studies
Pieces of liver and perigonadal fat (∼0.2 g) were fixed in Z-fix (Anatech), embedded in paraffin, cut into 5 μm sections and stained with hematoxylin and eosin at the Histology Core Facilities of the Columbia University Diabetes and Endocrine Research Center. Histologic sections were viewed using a Nikon Diaphot-TMD Inverted Microscope (Nikon), and digital images were captured using a SPOT digital camera (Diagnostic Instruments). To measure sizes of adipocytes, digital images of histology sections were converted into binary format with Adobe Photoshop 7.0 (Adobe Systems), and adipocyte surface areas were quantified using the Image Processing Tool Kit (Reindeer Games) (42).
Quantitative real-time PCR
Tissue RNA extraction and quantitative real-time PCR (Q-PCR) were conducted as previously described (38, 43). Cyclophilin B was used as an invariant control for these studies, and expression levels were calculated based on the ΔΔ-CT method. Q-PCR was conducted using the Applied Biosystems 7500 Real-Time PCR System. Primers used for Q-PCR are as follows: cyclophilin B (forward, 5′-CCGTCGTCTTCCTTTTGCT-3; reverse, 5′-TCCTTGATGACACGATGGAA-3′), ABHD5 (forward, 5′-TGACAGTGATGCGGAAGAAG-3′; reverse, 5′-AGATCTGGTCGCTCAGGAAA-3′), CD-68 (forward, 5′- CCTCCACCCTCGCCTAGTC-3′; reverse, 5′- TTGGGTATAGGATTCGGATTTGA-3′), TNFα (forward, 5′- CCACCACGCTCTTCTGTCTAC-3′; reverse, 5′- AGGGTCTGGGCCATAGAACT-3′), interleukin (IL)-1β (forward, 5′-TGTGAAATGCCACCTTTTGA-3′; reverse, 5′-GGTCAAAGGTTTGGAAGCAG-3′), IL-6 (forward, 5′- TGATGCACTTGCAGAAAACA-3′; reverse, 5′- ACCAGAGGAAATTTTCAATAGGC-3′), and interferon γ (IFNγ; forward, 5′- ACAGCAAGGCGAAAAAGGAT-3′; reverse, 5′- TGAGCTCATTGAATGCTTGG-3′).
TAG assay
To measure the TAG content of the livers of mice from the diet-induced obesity study, pieces of liver were homogenized, TAG was solvent-extracted, and TAG mass was determined as described (44). The values were expressed relative to protein content, which was determined using the Coomassie Plus Reagent (Pierce).
Statistical analyses
Data correspond to either means and SD or means and SEM of replicate samples, as specified in the figure legends. Statistical analyses were performed by Student t-test when comparing two groups. To compare more than two groups, one-way or two-way ANOVA with Bonferroni posthoc test was used. GraphPad Prism or SigmaStat software was used for the analyses. Differences were considered significant when the P value was less than 0.05.
RESULTS
ABHD5 expression is increased in adipose tissue and macrophages of ABHD5 Tg mice
To study the function of ABHD5 in adipose tissue, we generated transgenic mice that express mouse ABHD5 under control of the Fabp4 promoter. The protein mass of ABHD5 increased by 6-fold in white adipose tissue from ABHD5 Tg mice compared with WT mice (Fig. 1A, B). Lysates from brown adipose tissue (BAT) showed similar elevated protein levels of ABHD5 in ABHD5 Tg mice (Fig. 1C). Expression of the transgene, as measured by Northern blot with a transgene-specific probe, was not detected in liver, kidney, heart, or testis (data not shown). Consistent with these observations, protein levels of ABHD5 were comparable in lysates of heart, kidney, and liver from ABHD5 Tg and WT mice (Fig. 1C). Endogenously, the Fabp4 promoter drives expression of FABP4 in macrophages, in addition to white and brown adipocytes. To examine whether ABHD5 was overexpressed in macrophages, thioglycolate-elicited peritoneal macrophages were collected from ABHD5 Tg and WT mice. As expected, protein levels of ABHD5 were elevated in macrophages from ABHD5 Tg mice relative to WT mice (Fig. 1C). Tissue lysates from spleen and lung also showed higher protein mass of ABHD5 in ABHD5 Tg mice, likely due to high levels of resident macrophages in these tissues; there is no evidence that elevated ABHD5 in these tissues has an impact on development or energy metabolism. Thus, ABHD5 Tg mice overexpress ABHD5 selectively in adipose tissue and also in macrophages.
Overexpression of ABHD5 in adipose tissue had no effect on body weight or body composition in mice fed a standard chow diet (Table 1). Moreover, the plasma concentrations of TAG and fatty acid were comparable in ABHD5 Tg and WT mice (Table 1).
TABLE 1.
Characteristics of ABHD5 Tg and WT mice maintained on either a standard chow diet or defined low-fat diet
| Parameter | WT | ABHD5 Tg |
| Weight (g) | ||
| Malesa | 21.8 (± 1.4) | 21.1 (± 1.3) |
| Femalesb | 23.3 (± 1.7) | 22.8 (± 2.4) |
| Body composition | ||
| Fat mass (%)a | 16.0 (±2.3) | 15.5 (±2.3) |
| Lean mass (%)a | 83.8 (±2.2) | 84.4 (±2.8) |
| Naso-anal length (cm)a | 8.8 (±0.3) | 8.7 (±0.3) |
| Plasma triacylglycerol (mg/dl)c | 49.6 (±6.5) | 48.0 (±17.5) |
| Plasma NEFA (mM)d | 0.21 (±0.10) | 0.23 (±0.82) |
Male 12-week old mice fed a low fat diet (n = 17 and 16).
Female 10- to 12-week old mice fed chow (n = 10).
Female 26- to 29-week old mice, fasted for 24 h (maintained on a chow diet) (n = 8).
Female 27- to 30-week old mice fed chow (n = 10).
Overexpression of ABHD5 in adipose tissue does not alter in vivo lipolysis
In assays conducted in vitro, the addition of exogenous ABHD5 increased TAG hydrolase activity of cytosolic extracts from adipose tissue (3, 24). To evaluate the effects of overexpressing ABHD5 in vivo, we measured the products of TAG hydrolysis, glycerol, and NEFA in plasma from WT and ABHD5 Tg mice. Because lipolysis of adipose TAG stores increases during fasting (10, 45), we analyzed plasma glycerol and NEFA levels from mice that had been fasted for 24 h or had free access to food. Plasma glycerol and NEFA concentrations were equivalent in fed WT and ABHD5 Tg mice (Fig. 2A, B). Fasting increased plasma glycerol and NEFA in both groups; however, no significant differences were detected between WT and ABHD5 Tg mice (Fig. 2A, B). Moreover, no differences were observed when comparing male and female ABHD5 Tg mice. Thus, overexpression of ABHD5 had no effect on plasma glycerol or NEFA levels either in fed conditions or during fasting.
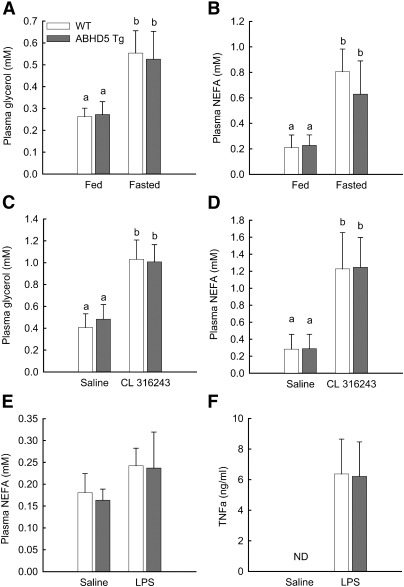
Fig. 2.
Overexpression of ABHD5 does not alter in vivo lipolysis. (A, B) WT and ABHD5 Tg mice either were allowed free access to food or fasted for 24 h. Blood was collected, and the concentrations of glycerol (A) and NEFA (B) in plasma were measured. Values represent means ± SD (n = 9-10). (C, D) WT and ABHD5 Tg mice were injected with saline solution or the β3-adrenergic receptor agonist CL316243. Blood was collected 15 min later, and the concentrations of glycerol (C) and NEFA (D) in plasma were measured. Values represent means ± SD (n = 7-11). (E, F) WT and ABHD5 Tg mice were maintained on a standard chow diet and received a single intraperitoneal injection of either saline or LPS (5 μg per mouse). Blood was collected 1 h later, and plasma concentrations of NEFA (E) and TNFα (F) were determined. Values represent means ± SEM (n = 4-5). For all panels, statistical analyses were performed using two-way ANOVA and Bonferroni posthoc test; values not sharing a common superscript differ significantly (P < 0.05). ND, levels below the limit of detection.
Catecholamines increase lipolysis by stimulating β-adrenergic receptors on adipocytes, thus activating cAMP-dependent protein kinase, which phosphorylates several key proteins in the lipolytic pathway and ultimately increases lipolysis (1, 9, 10). To investigate the effects of overexpressed ABHD5 on β-adrenergic-stimulated lipolysis, we injected CL316243, an agonist of β3-adrenergic receptors, into WT and ABHD5 Tg mice. Blood was collected after 15 min for determination of levels of glycerol and NEFA in plasma; blood collected from the same mice following an earlier injection of saline solution was used for control conditions. WT and ABHD5 Tg mice injected with saline solution had similar concentrations of glycerol and NEFA in plasma (Fig. 2C, D). Treatment with CL316243 increased plasma concentrations of glycerol and NEFA in both groups of animals; however, no significant differences were detected between WT and ABHD5 Tg mice (Fig. 2C, D). Therefore, overexpression of ABHD5 in adipose tissue does not increase plasma levels of glycerol or NEFA following administration of a β-adrenergic agonist.
The acute phase response of mice to bacterial endotoxins increases lipolysis of adipose TAG stores. To investigate the effects of ABHD5 overexpression on lipolysis triggered by the acute phase response, LPS was injected into WT and ABHD5 Tg mice. Blood was collected after 1 h for determination of levels of NEFA and TNFα in plasma; levels of TNFα were used to confirm initiation of the acute phase response. WT and ABHD5 Tg mice injected with saline solution had similar concentrations of NEFA in plasma (Fig. 2E) and undetectable levels of TNFα (Fig. 2F). Injection of LPS modestly increased plasma concentrations of NEFA in both groups of animals; however, no significant differences were detected between WT and ABHD5 Tg mice (Fig. 2E). Moreover, both groups of animals showed similarly elevated plasma TNFα (Fig. 2F), consistent with comparable induction of the acute phase response. Therefore, overexpression of ABHD5 in adipose tissue does not alter lipolysis in response to inflammation induced by LPS injection.
Overexpression of ABHD5 does not alter basal or stimulated lipolysis in isolated adipocytes
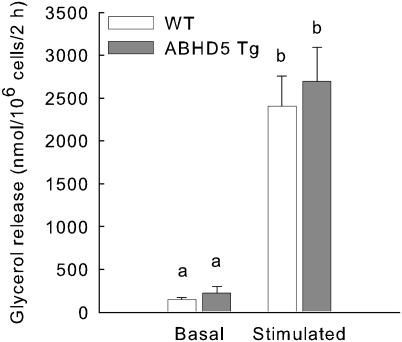
Plasma concentrations of glycerol and NEFA are commonly used as indices of lipolysis; however, rapid removal of these metabolites from circulation can limit the accuracy of this assessment. Adipocytes isolated from perigonadal fat pads of WT and ABHD5 Tg mice provided an additional experimental model. Cells were incubated either without or with isoproterenol, an agonist of β-adrenergic receptors, for basal and stimulated conditions, respectively. Additionally, media contained adenosine, adenosine deaminase, and N6-phenylisopropyladenosine to manage adenosine levels to reduce elevated lipolysis due to handling of mice prior to dissection of fat pads (46). Basal lipolysis, assessed as glycerol release in the absence of isoproterenol, was similar in adipocytes isolated from WT and ABHD5 Tg mice (Fig. 3). Incubation of cells with isoproterenol increased glycerol release 10- to 14-fold; however, no significant differences were detected in glycerol released from ABHD5 Tg adipocytes compared with WT adipocytes (Fig. 3). Hence, overexpression of ABHD5 does not increase lipolysis in isolated adipocytes.
Fig. 3.
Basal and isoproterenol-stimulated lipolysis are similar in adipocytes isolated from WT and ABHD5 Tg mice. Adipocytes were isolated from WT and ABHD5 Tg mice and incubated for two h without (basal) or with isoproterenol (stimulated) prior to measurement of glycerol released into the media. The values represent means ± SEM from four independent experiments, each with triplicate samples. Statistical analysis was performed using one-way ANOVA and Bonferroni posthoc test. Values not sharing a common superscript differ significantly (P < 0.001).
Transgenic overexpression of ABHD5 does not alter the systemic inflammatory response to bacterial endotoxin
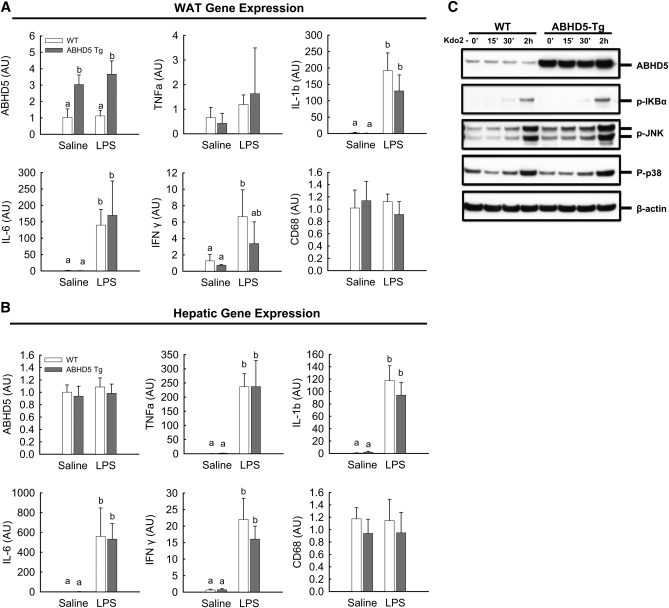
Administration of LPS to mice induces lipolysis of adipose TAG stores (47, 48) and also activates signaling pathways to increase expression and secretion of inflammatory cytokines. To determine the effect of increased ABHD5 expression on the LPS-induced inflammatory response, we assessed mRNA levels of cytokines in white adipose tissue and liver of ABHD5 Tg mice relative to WT mice following administration of LPS. As expected, mRNA levels for IL-1β, IL-6, and IFNγ were increased in both adipose tissue and liver following LPS injection; however, there were no significant differences between ABHD5 Tg and WT mice (Fig. 4A, B). Assessment of mRNA levels of CD-68 showed that macrophage infiltration into adipose tissue and liver was comparable in ABHD5 Tg and WT mice (Fig. 4A, B). Finally, because ABHD5 was overexpressed in macrophages (Fig. 1C), we investigated TLR4 signaling in thioglycolate-elicited macrophages from ABHD5 Tg and WT mice following incubation of cells with Kdo2-Lipid A, a TLR4 agonist. As expected, TLR 4 signaling increased at 30 min and 2 h, as indicated by increased phosphorylation of JNK (c-Jun N-terminal kinase), p38 MAPK (mitogen-activated protein kinase), and IκBα (Inhibitor of kappa B, alpha) (Fig. 4C); however, no differences were observed in macrophages from ABHD5 Tg mice relative to those of WT mice.
Fig. 4.
Transgenic overexpression of ABHD5 did not alter the systemic response to bacterial endotoxin. WT and ABHD5 Tg mice were maintained on a standard chow diet and received a single intraperitoneal injection of either saline or LPS (5 μg per mouse) 1 h prior to dissection of tissues. (A, B) Q-PCR analyses of epididymal white adipose tissue (WAT) (A) and hepatic (B) gene expression. TNFα, tumor necrosis factor α; IL-6, interleukin 6; IL-1β, interleukin 1β; IFNγ, interferon γ. Data in panels (A) and (B) represent the mean ± SD (n = 4); values not sharing a common superscript differ significantly (P < 0.05). (C) Freshly isolated thioglycolate-elicited macrophages were pooled from n = 5 mice per genotype and cultured as described in “Materials and Methods.” To examine TLR4-driven signal transduction, macrophages were treated with 10 ng/ml Kdo2-Lipid A (TLR4 agonist) for 2 h. Cell samples were collected at 15 and 30 min (15′, 30′) and 2 h (2h), and signaling was examined by immunoblotting to detect phospho-IκBα (Ser32), phospho-SAPK/JNK (Thr183/Tyr185), phospho-p38-MAPK (Thr180/Tyr182), and β-actin as a loading control.
Overexpression of ABHD5 in adipose tissue does not prevent diet-induced obesity
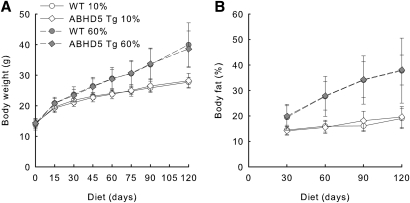
We fed WT and ABHD5 Tg mice a high-fat diet to determine whether overexpression of ABHD5 in adipose tissue can impair the development of diet-induced obesity. We used either a low- or high-fat diet of defined composition that contained 10% or 60% of calories from fat, respectively. Animals were provided with the diets starting at 4 weeks of age and continuing for 120 days; body weight and composition were determined at regular intervals. WT and ABHD5 Tg mice fed the 10% fat diet gained equal weight over 120 days (Fig. 5A). WT ABHD5 Tg mice fed the 60% fat diet gained more weight than mice fed the 10% fat diet; however, no significant differences in weight were detected between WT and ABHD5 Tg mice (Fig. 5A). Similarly, the body composition of WT and ABHD5 Tg mice fed the same diet was comparable throughout the 120-day experiment (Fig. 5B); WT and ABHD5 Tg mice displayed equivalent higher percentage of body fat when fed the 60% fat diet relative to mice fed the 10% fat diet.
Fig. 5.
Body weight and fat mass were similar in WT and ABHD5 Tg mice fed diets containing either 10% or 60% fat. Starting at the age of 4 weeks, WT and ABHD5 Tg mice were fed diets of defined composition containing either 10% or 60% of calories from fat for 120 days. The body weight (A) and percentage of fat mass (B) were determined at regular intervals. Values represent means ± SD (n = 15-19). Statistical analyses were performed using two-way ANOVA and Bonferroni posthoc test. Mice fed the 60% fat diet had greater weight and percentage of fat mass than mice fed the 10% fat diet at 15 days and later times (P < 0.001). No significant differences were observed when comparing ABHD5 Tg to WT mice fed either diet.
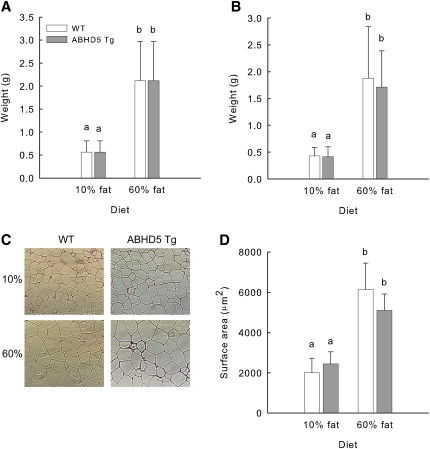
At 120 days, the mice were euthanized, and their fat pads were dissected and weighed. The weights of perigonadal and subcutaneous fat pads isolated from both WT and ABHD5 Tg mice fed the 60% fat diet were greater than those from mice fed the 10% fat diet; however, no differences were apparent between tissues from WT and ABHD5 Tg mice fed the same diet (Fig. 6A, B). Relative adipocyte size was analyzed using histological sections of perigonadal fat. Adipocytes from mice fed the 10% fat diet were smaller than adipocytes from mice fed the 60% fat diet, but no differences in adipocyte size were detected between WT and ABHD5 Tg mice that had been fed either the 10% fat diet or the 60% fat diet (Fig. 6C, D).
Fig. 6.
Fat pad weight and average adipocyte size were similar in WT and ABHD5 Tg mice fed diets containing either 10% or 60% fat. WT and ABHD5 Tg mice were fed defined diets containing either 10% or 60% of calories from fat. At 120 days, perigonadal (A) and subcutaneous (B) fat pads were dissected and weighed. Histological preparations were made from perigonadal fat pads (C) and used to determine the average sizes of adipocytes (D). Values represent means ± SD (n = 6-10). Statistical analyses were performed using one-way ANOVA and Bonferroni posthoc test. Values not sharing a common superscript differ significantly (P < 0.05).
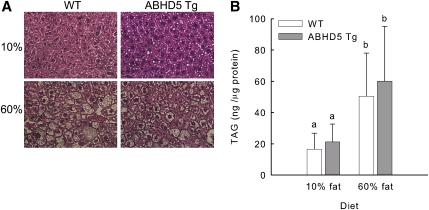
In obesity, excessive TAG is deposited not only in adipose tissue but also in other tissues, including the liver, leading to fatty liver (49). To evaluate the effects of adipose-specific overexpression of ABHD5 on fat deposition in other tissues, we assessed TAG content of liver. Mice fed the 60% fat diet showed evident lipid infiltration in histological sections of liver (Fig. 7A); however, no significant differences were detected between the livers of WT and ABHD5 Tg mice. WT and ABHD5 Tg mice fed the 10% fat diet had equal liver content of TAG (Fig. 7B). Mice fed the 60% fat diet had higher liver content of TAG than those fed the 10% fat diet; however, no differences were detected between WT and ABHD5 Tg mice (Fig. 7B).
Fig. 7.
Liver lipid infiltration and TAG content were similar in WT and ABHD5 Tg mice fed diets containing either 10% or 60% fat. WT and ABHD5 Tg mice were fed defined diets containing either 10% or 60% of calories from fat for 120 days. (A) Histological sections of liver were prepared, (B) lipids were extracted from liver samples, and the content of TAG was determined. Values represent means ± SD (n = 13-18). Statistical analyses were performed using one-way ANOVA and Bonferroni posthoc test. Values not sharing a common superscript differ significantly (P < 0.05).
DISCUSSION
The major findings of this study are that overexpression of ABHD5 in adipose tissue does not increase lipolysis of TAG stored in adipocytes, alter aspects of the acute phase response to bacterial endotoxins, or render transgenic mice resistant to diet-induced obesity. These conclusions are supported by the following results obtained from the study of ABHD5 Tg mice relative to WT mice: (i) circulating levels of glycerol and NEFA in fed ABHD5 Tg and WT mice were similar; (ii) a 24 h fast or brief treatment with a β3-adrenergic agonist increased plasma concentrations of glycerol and NEFA in ABHD5 Tg and WT mice to similar levels; (iii) basal and stimulated lipolysis were equivalent in adipocytes isolated from ABHD5 Tg and WT mice; (iv) expression of inflammatory cytokines was comparable in adipose tissue and liver of ABHD5 Tg and WT mice injected with LPS; (v) overexpression of ABHD5 did not alter TLR4 signaling in macrophages; and (vi) ABHD5 Tg mice and WT mice fed a high-fat diet developed comparable obesity. Although overexpression of ABHD5 was also observed in brown adipose tissue of ABHD5 Tg mice, given our findings, we anticipate no effect of excess ABHD5 on lipolysis required for adaptive thermogenesis.
ABHD5 is highly expressed in adipose tissue (24, 29, 32, 50) where the majority of TAG is stored, and its expression is induced during the differentiation of 3T3-L1 adipocytes (32, 50). Additionally, mutations in human ABHD5 cause excessive accumulation of TAG in multiple cells and tissues (23). Thus, ABHD5 plays an important role in TAG metabolism in adipocytes and other cells. It is now well established that ABHD5 functions as a coactivator of ATGL (3, 24, 26, 27), although the mechanism for this activity has not yet been elucidated. Previous studies have shown that addition of exogenous ABHD5 increases in vitro TAG hydrolase activity of tissue lysates from a variety of tissues, including white adipose tissue, suggesting that endogenous levels of ABHD5 are limiting for lipolysis in adipose and other tissues (24). Furthermore, this coactivation function was not observed when ABHD5 was added to tissue lysates from ATGL-null mice (3), suggesting that ABHD5 has specificity for coactivation of ATGL. Our results obtained from in vivo lipolysis experiments suggest that endogenous protein levels of ABHD5 in mouse adipose tissue are sufficient for the function of ABHD5 in basal lipolysis and multiple conditions of stimulated lipolysis. This study is the first to examine the effects of ABHD5 overexpression in vivo; the previously hypothesized tissue deficit in ABHD5 coactivation function is likely due to the procedure used to prepare cell lysates during which lipid droplets, which harbor the majority of ABHD5, may have been removed. Moreover, it is likely that adipocytes have additional compensatory mechanisms for initiation of TAG hydrolysis, as humans with inactivating mutations in ABHD5 are not obese (51, 52) and ablation of ABHD5 in mouse adipose tissue using ABHD5 anti-sense oligonucleotides (ASO) leads to reduced adipose tissue mass and resistance to high-fat diet-induced obesity (30).
Recent studies have suggested that ABHD5 is required for intracellular signaling pathways that affect lipid homeostasis and insulin sensitivity of tissues. When ABHD5 expression was reduced in mice following injection of specific ASOs, the near ablation of protein levels of ABHD5 in white adipose tissue and liver led to reduced TAG storage in adipose tissue and extreme hepatic steatosis (30). When these mice were fed a high-fat diet, the ABHD5 ASO mice were protected against the development of obesity, and despite severe hepatic steatosis accompanied by elevated hepatic diacylglycerols and ceramides, they showed normal glucose tolerance and maintained the insulin sensitivity characteristic of mice on a chow diet (30). These data suggest that ABHD5 contributes intracellular signals that couple TAG storage to insulin signaling and the development of insulin resistance. In our current study, we found that 6-fold overexpression of ABHD5 in adipose tissue does not have any effect on altering TAG stores in either adipocytes or hepatocytes or recruitment of macrophages into adipose tissue or liver. Similarly, we did not observe any effects of ABHD5 overexpression on whole-body lipid homeostasis, so if ABHD5 contributes to a mechanism by which lipid signals dampen insulin signaling in tissues, these signals are not enhanced in an environment of excess ABHD5.
Although the acute phase response changes the expression of genes for inflammatory cytokines, we found no added effect of ABHD5 overexpression on changes in gene expression in liver or adipose tissue following LPS administration. Moreover, TLR4 signaling was unchanged in macrophages from ABHD5 Tg mice, despite elevated macrophage ABHD5 expression. ABHD5 has been demonstrated to have enzyme activity as a lysophosphatidic acid acyltransferase (53, 54); hence, ABHD5 may participate in intracellular signaling pathways through the generation of the signaling lipid phosphatidic acid. Alteration of intracellular signaling pathways in ABHD5 ASO-treated mice may be due to reduced production of this lipid second messenger; however, overexpression of ABHD5 does not have the opposite effect on the same pathways.
In summary, 6-fold overexpression of ABHD5 in adipose tissue of mice does not increase lipolysis, suggesting that the expression of endogenous ABHD5 is not limiting for either basal or stimulated lipolysis. Elucidation of the mechanism by which ABHD5 promotes ATGL TAG hydrolase activity requires additional study. Moreover, signaling pathways in macrophages and adipose tissue that are significantly affected by ablation of ABHD5 are unchanged by overexpression of ABHD5. Thus, although ABHD5 plays a critical role in maintenance of whole-body TAG homeostasis, increasing the expression of ABHD5 neither helps nor hinders the mechanisms by which ABHD5 contributes to these processes.
Acknowledgments
The authors thank Laboratory Animal Services at Rutgers University, in particular, Dr. David Reimer, Linda Haberl and Nancy Rossi, for training of laboratory personnel and excellent care of the animals. We thank Dr. Loredana Quadro for advice on animal breeding; Dr. Susan K. Fried and Dr. Yanxin Wang for advice on adipocyte isolation; and Dr. Vidya Subramanian, Dr. Robert V. Farese, Jr., and Dr. Hubert C. Chen for advice on adipocyte cell sizing. We thank Dr. Rudolph Leibel, Dr. Philipp Scherer, and Dr. Yiying Zhang for helpful discussions.
Footnotes
Abbreviations:
- ABHD5
- abhydrolase domain 5
- ASO
- anti-sense oligonucleotide
- ATGL
- adipose triglyceride lipase
- BAT
- brown adipose tissue
- FABP4
- fatty acid binding protein 4
- HSL
- hormone-sensitive lipase
- IL
- interleukin
- LPS
- lipopolysaccharide
- Q-PCR
- quantitative real-time PCR
- TAG
- triacylglycerol
- Tg
- transgenic
- TLR4
- Toll-like receptor 4
- TNFα
- tumor necrosis factor α
- WT
- wild-type
This work was supported by grants from the American Heart Association (Established Investigator Award to D.L.B., a Postdoctoral Fellowship from the Heritage Affiliate to J.M.C., and Beginning Grant-in-Aid 7840072 to J.M.B.), by grants from the National Institute of Diabetes, Digestive, and Kidney Diseases (1R01DK-054797 to D.L.B. and 1F32DK-084582 to J.L.B.), and by the Department of Pathology at Wake Forest University (J.M.B.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. All authors report that they have no conflicts of interest.
References
- 1.Duncan R. E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H. S. 2007. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 27: 79–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langin D. 2006. Control of fatty acid and glycerol release in adipose tissue lipolysis. C. R. Biol. 329: 598–607 [DOI] [PubMed] [Google Scholar]
- 3.Schweiger M., Schreiber R., Haemmerle G., Lass A., Fledelius C., Jacobsen P., Tornqvist H., Zechner R., Zimmermann R. 2006. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 281: 40236–40241 [DOI] [PubMed] [Google Scholar]
- 4.Zechner R., Strauss J. G., Haemmerle G., Lass A., Zimmermann R. 2005. Lipolysis: pathway under construction. Curr. Opin. Lipidol. 16: 333–340 [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 306: 1383–1386 [DOI] [PubMed] [Google Scholar]
- 6.Mulder H., Sorhede-Winzell M., Contreras J. A., Fex M., Strom K., Ploug T., Galbo H., Arner P., Lundberg C., Sundler F., et al. 2003. Hormone-sensitive lipase null mice exhibit signs of impaired insulin sensitivity whereas insulin secretion is intact. J. Biol. Chem. 278: 36380–36388 [DOI] [PubMed] [Google Scholar]
- 7.Haemmerle G., Zimmermann R., Hayn M., Theussl C., Waeg G., Wagner E., Sattler W., Magin T. M., Wagner E. F., Zechner R. 2002. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 277: 4806–4815 [DOI] [PubMed] [Google Scholar]
- 8.Fredrikson G., Tornqvist H., Belfrage P. 1986. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim. Biophys. Acta. 876: 288–293 [DOI] [PubMed] [Google Scholar]
- 9.Brasaemle D. L. 2007. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48: 2547–2559 [DOI] [PubMed] [Google Scholar]
- 10.Jaworski K., Sarkadi-Nagy E., Duncan R. E., Ahmadian M., Sul H. S. 2007. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am. J. Physiol. Gastrointest. Liver Physiol. 293: G1–G4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holm C. 2003. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 31: 1120–1124 [DOI] [PubMed] [Google Scholar]
- 12.Miyoshi H., Perfield J. W., Souza S. C., Shen W. J., Zhang H. H., Stancheva Z. S., Kraemer F. B., Obin M. S., Greenberg A. S. 2007. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J. Biol. Chem. 282: 996–1002 [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi H., Souza S. C., Zhang H. H., Strissel K. J., Christoffolete M. A., Kovsan J., Rudich A., Kraemer F. B., Bianco A. C., Obin M. S., et al. 2006. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J. Biol. Chem. 281: 15837–15844 [DOI] [PubMed] [Google Scholar]
- 14.Sztalryd C., Xu G., Dorward H., Tansey J. T., Contreras J. A., Kimmel A. R., Londos C. 2003. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J. Cell Biol. 161: 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tansey J. T., Huml A. M., Vogt R., Davis K. E., Jones J. M., Fraser K. A., Brasaemle D. L., Kimmel A. R., Londos C. 2003. Functional studies on native and mutated forms of perilipins. A role in protein kinase a-mediated lipolysis of triacylglycerols in Chinese hamster ovary cells. J. Biol. Chem. 278: 8401–8406 [DOI] [PubMed] [Google Scholar]
- 16.Yang X., Lu X., Lombes M., Rha G. B., Chi Y. I., Guerin T. M., Smart E. J., Liu J. 2010. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 11: 194–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fessler M. B., Rudel L. L., Brown J. M. 2009. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr. Opin. Lipidol. 20: 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khovidhunkit W., Kim M. S., Memon R. A., Shigenaga J. K., Moser A. H., Feingold K. R., Grunfeld C. 2004. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 45: 1169–1196 [DOI] [PubMed] [Google Scholar]
- 19.Schäffler A., Schölmerich J. 2010. Innate immunity and adipose tissue biology. Trends Immunol. 31: 228–235 [DOI] [PubMed] [Google Scholar]
- 20.Zu L., He J., Jiang H., Xu C., Pu S., Xu G. 2009. Bacterial endotoxin stimulates adipose lipolysis via toll-like receptor 4 and extracellular signal-regulated kinase pathway. J. Biol. Chem. 284: 5915–5926 [DOI] [PubMed] [Google Scholar]
- 21.Guilherme A., Virbasius J. V., Puri V., Czech M. P. 2008. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9: 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H. H., Halbleib M., Ahmad F., Manganiello V. C., Greenberg A. S. 2002. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes. 51: 2929–2935 [DOI] [PubMed] [Google Scholar]
- 23.Lefèvre C., Jobard F., Caux F., Bouadjar B., Karaduman A., Heilig R., Lakhdar H., Wollenberg A., Verret J. L., Weissenbach J., et al. 2001. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am. J. Hum. Genet. 69: 1002–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J. G., Gorkiewicz G., Zechner R. 2006. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 3: 309–319 [DOI] [PubMed] [Google Scholar]
- 25.Radner F. P., Streith I. E., Schoiswohl G., Schweiger M., Kumari M., Eichmann T. O., Rechberger G., Koefeler H. C., Eder S., Schauer S., et al. 2010. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58). J. Biol. Chem. 285: 7300–7311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruber A., Cornaciu I., Lass A., Schweiger M., Poeschl M., Eder C., Kumari M., Schoiswohl G., Wolinski H., Kohlwein S. D., et al. 2010. The N-terminal region of comparative gene identification-58 (CGI-58) is important for lipid droplet binding and activation of adipose triglyceride lipase. J. Biol. Chem. 285: 12289–12298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schweiger M., Schoiswohl G., Lass A., Radner F. P. W., Haemmerle G., Malli R., Graier W., Cornaciu I., Oberer M., Salvayre R., et al. 2008. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J. Biol. Chem. 283: 17211–17220 [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi T., Omatsu N., Morimoto E., Nakashima H., Ueno K., Tanaka T., Satouchi K., Hirose F., Osumi T. 2007. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J. Lipid Res. 48: 1078–1089 [DOI] [PubMed] [Google Scholar]
- 29.Brown J. M., Chung S., Das A., Shelness G. S., Rudel L. L., Yu L. 2007. CGI-58 facilitates the mobilization of cytoplasmic triglyceride for lipoprotein secretion in hepatoma cells. J. Lipid Res. 48: 2295–2305 [DOI] [PubMed] [Google Scholar]
- 30.Brown J. M., Betters J. L., Lord C., Ma Y., Han X., Yang K., Alger H. M., Melchior J., Sawyer J., Shah R., et al. 2010. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J. Lipid Res. 51: 3306–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowalski T. J., Liu S. M., Leibel R. L., Chua S. C., Jr 2001. Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes. 50: 425–435 [DOI] [PubMed] [Google Scholar]
- 32.Subramanian V., Rothenberg A., Gomez C., Cohen A. W., Garcia A., Bhattacharyya S., Shapiro L., Dolios G., Wang R., Lisanti M. P., et al. 2004. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3–L1 adipocytes. J. Biol. Chem. 279: 42062–42071 [DOI] [PubMed] [Google Scholar]
- 33.Pajvani U. B., Trujillo M. E., Combs T. P., Iyengar P., Jelicks L., Roth K. A., Kitsis R. N., Scherer P. E. 2005. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat. Med. 11: 797–803 [DOI] [PubMed] [Google Scholar]
- 34.Nagy A., Gertsenstein M., Vintersten K., Behringer R. 2003. Manipulating the Mouse Embryo: A Laboratory Manual. 3rd edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 35.Rodbell M. 1964. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J. Biol. Chem. 239: 375–380 [PubMed] [Google Scholar]
- 36.Honnor R. C., Dhillon G. S., Londos C. 1985. cAMP-dependent protein kinase and lipolysis in rat adipocytes. I. Cell preparation, manipulation, and predictability in behavior. J. Biol. Chem. 260: 15122–15129 [PubMed] [Google Scholar]
- 37.Wang Y., Fried S. K., Petersen R. N., Schoknecht P. A. 1999. Somatotropin regulates adipose tissue metabolism in neonatal swine. J. Nutr. 129: 139–145 [DOI] [PubMed] [Google Scholar]
- 38.Brown J. M., Chung S., Sawyer J. K., Degirolamo C., Alger H. M., Nguyen T., Zhu X., Duong M. N., Wibley A. L., Shah R., et al. 2008. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 118: 1467–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown J. M., Boysen M. S., Chung S., Fabiyi O., Morrison R. F., Mandrup S., McIntosh M. K. 2004. Conjugated linoleic acid induces human adipocyte delipidation: autocrine/paracrine regulation of MEK/ERK signaling by adipocytokines. J. Biol. Chem. 279: 26735–26747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurell S., Tibbling G. 1966. An enzymatic fluorometric micromethod for the determination of glycerol. Clin. Chim. Acta. 13: 317–322 [DOI] [PubMed] [Google Scholar]
- 41.Di Girolamo M., Mendlinger S., Fertig J. W. 1971. A simple method to determine fat cell size and number in four mammalian species. Am J Physiol. 221: 850–858 [DOI] [PubMed] [Google Scholar]
- 42.Chen H. C., Farese R. V., Jr 2002. Determination of adipocyte size by computer image analysis. J. Lipid Res. 43: 986–989 [PubMed] [Google Scholar]
- 43.Brown J. M., Bell T. A., III, Alger H. M., Sawyer J. K., Smith T. L., Kelley K., Shah R., Wilson M. D., Davis M. A., Lee R. G., et al. 2008. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J. Biol. Chem. 283: 10522–10534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz D. M., Wolins N. E. 2007. A simple and rapid method to assay triacylglycerol in cells and tissues. J. Lipid Res. 48: 2514–2520 [DOI] [PubMed] [Google Scholar]
- 45.MacDonald I. A., Webber J. 1995. Feeding, fasting and starvation: factors affecting fuel utilization. Proc. Nutr. Soc. 54: 267–274 [DOI] [PubMed] [Google Scholar]
- 46.Viswanadha S., Londos C. 2006. Optimized conditions for measuring lipolysis in murine primary adipocytes. J. Lipid Res. 47: 1859–1864 [DOI] [PubMed] [Google Scholar]
- 47.Feingold K. R., Staprans I., Memon R. A., Moser A. H., Shigenaga J. K., Doerrler W., Dinarello C. A., Grunfeld C. 1992. Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J. Lipid Res. 33: 1765–1776 [PubMed] [Google Scholar]
- 48.Fong Y. M., Marano M. A., Moldawer L. L., Wei H., Calvano S. E., Kenney J. S., Allison A. C., Cerami A., Shires G. T., Lowry S. F. 1990. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J. Clin. Invest. 85: 1896–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Browning J. D., Horton J. D. 2004. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi T., Omatsu N., Matsushita S., Osumi T. 2004. CGI-58 Interacts with perilipin and is localized to lipid droplets: possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. J. Biol. Chem. 279: 30490–30497 [DOI] [PubMed] [Google Scholar]
- 51.Igal R. A., Rhoads J. M., Coleman R. A. 1997. Neutral lipid storage disease with fatty liver and cholestasis. J. Pediatr. Gastroenterol. Nutr. 25: 541–547 [DOI] [PubMed] [Google Scholar]
- 52.Peña-Penabad C., Almagro M., Martinez W., Garcia-Silva J., Del Pozo J., Yebra M. T., Sanchez-Manzano C., Fonseca E. 2001. Dorfman-Chanarin syndrome (neutral lipid storage disease): new clinical features. Br. J. Dermatol. 144: 430–432 [DOI] [PubMed] [Google Scholar]
- 53.Ghosh A. K., Ramakrishnan G., Chandramohan C., Rajasekharan R. 2008. CGI-58, the causative gene for Chanarin-Dorfman syndrome, mediates acylation of lysophosphatidic acid. J. Biol. Chem. 283: 24525–24533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montero-Moran G., Caviglia J. M., McMahon D., Rothenberg A., Subramanian V., Xu Z., Lara-Gonzalez S., Storch J., Carman G. M., Brasaemle D. L. 2010. CGI-58/ABHD5 is a coenzyme A-dependent lysophosphatidic acid acyltransferase. J. Lipid Res. 51: 709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]