Abstract
The main causes of familial hypercholesterolemia (FH) are mutations in LDL receptor (LDLR) gene. Functional studies are necessary to demonstrate the LDLR function impairment caused by mutations and would be useful as a diagnostic tool if they allow discrimination between FH patients and controls. In order to identify the best method to detect LDLR activity, we compared continuous Epstein-Barr virus (EBV)-transformed B-lymphocytes and mitogen stimulated T-lymphocytes. In addition, we characterized both novel and known mutations in the LDLR gene. T-lymphocytes and EBV-transformed B-lymphocytes were obtained from peripheral blood of 24 FH patients and 24 control subjects. Functional assays were performed by incubation with fluorescent LDL followed by flow cytometry analysis. Residual LDLR activity was calculated normalizing fluorescence for the mean fluorescence of controls. With stimulated T-lymphocytes we obtained a better discrimination capacity between controls and FH patients compared with EBV-transformed B-lymphocytes as demonstrated by receiver operating characteristic (ROC) curve analysis (the areas under the curve are 1.000 and 0.984 respectively; P < 0.0001 both). The characterization of LDLR activity through T-lymphocytes is more simple and faster than the use of EBV-transformed B-lymphocytes and allows a complete discrimination between controls and FH patients. Therefore the evaluation of residual LDLR activity could be helpful not only for mutation characterization but also for diagnostic purposes.
Keywords: LDL receptor, familial hypercholesterolemia, EBV-transformed B-lymphocytes, mitogen stimulated T-lymphocytes, functional activity
Familial hypercholesterolemia (FH) is an autosomal dominant disorder characterized by elevated plasma LDL cholesterol, tendon xanthomas, and premature coronary heart disease. FH is mostly caused by mutations within the low density lipoprotein receptor (LDLR; MIM# 143890) gene, or in its ligand apoB-100 (MIM# 107730), or in the proprotein convertase subtilisin/kexin type 9 (PCSK9; MIM# 607786) gene (1).
At present, more than 1,100 variants of LDLR gene have been listed in the LDLR databases underlying a high genetic heterogeneity of LDLR mutations. These are distributed across the 18 exons, introns, and the promoter region of the LDLR gene and include point mutations, insertions, deletions, and major rearrangements (2).
Although LDLR defects are primarily identified by genetic methods, it is extremely important to demonstrate a deficiency in the LDLR function of FH suspect patients through functional studies. Receptor assays reported so far include measurement of radiolabeled-LDL or fluorescently-labeled LDL binding and/or uptake in skin fibroblasts (3, 4) or leukocytes (5, 6). The Epstein-Barr virus (EBV)-transformed B-lymphocytes show high LDLR levels and allow an unlimited supply of cells without being influenced by the patient's diet and drug treatment (7). The main problem of these functional assays was the bad separation between residual LDLR activity from FH patients and controls. The separation of different leukocyte populations obtains more accurate results (8); the selection of viable lymphocytes by stimulation with a mitogen or by flow cytometry gating improves the discrimination between patients and controls although it does not allow complete discrimination of FH heterozygote patients from healthy controls (9, 10).
The aim of this study was to improve the method of T-lymphocyte stimulation for LDLR activity evaluation through the comparison of different mitogen combinations. We also aimed to compare continuous EBV-transformed-lymphocytes and mitogen stimulated T- lymphocytes obtained from peripheral blood samples in order to identify the best method to detect LDLR activity by fluorescently-labeled LDL. In addition, we characterized both novel and known mutations in the LDLR gene.
MATERIALS AND METHODS
FH patients and control subjects
Patients with clinically diagnosed FH were enrolled at the Dipartimento di Medicina Clinica e Sperimentale, Università degli Studi di Napoli Federico II and at the AORN Cardarelli, Napoli. The FH diagnosis was based on the criteria established by the Società Italiana per lo Studio della Arteriosclerosi for the identification and treatment of dyslipidemias. Twenty-four patients bearing new and known mutations were selected for functional characterization. As healthy controls, 24 unrelated subjects were selected from voluntary donors of the Centro Trasfusionale of the AORN Cardarelli, Napoli, on the basis of normal lipid levels and absence of LDLR mutations. The supplementary table reports characteristics of FH patients and controls. The study was performed according to the current version of the Helsinki Declaration. Informed consent was obtained for each patient.
Mutation screening
Molecular analysis for the identification of LDLR mutations was performed as previously described (11). Briefly, the promoter and the 18 exons of the LDLR gene were amplified by PCR and directly sequenced. In the case of novel mutations, we verified the absence in 150 chromosomes from normocholesterolemic individuals of the same ethnic group, the segregation of the sequence variant in the relatives available, and the conservation of substituted amino acid residues across homologous proteins by BLASTP.
Mononuclear cell isolation and generation of EBV-transformed B-lymphocytes
Peripheral blood mononuclear cells (PBMCs) were isolated from 9 ml of EDTA blood collected from each subject enrolled in this study. The sample was diluted 1 in 2 with PBS without Ca2+ and Mg2+ pH 7.4, and then layered on Ficoll-Paque (Lymphocyte Separation Medium, MP Biomedicals). After centrifugation at 1,500 rpm for 30 min at 4°C, mononuclear cells were recovered at interface, washed three times in PBS and stored in 90% FBS (Lonza) and 10% DMSO in liquid nitrogen until required for use.
EBV-transformed cell lines were generated by exposing PBMCs to free EBV particles, produced by an EBV-infected marmoset cell line (B95.8). After EBV infection, transformed PBMCs were cultured in an incubator at 37°C in a 5% CO2 atmosphere in Iscove's Modified Dulbecco's Medium (IMDM, Sigma-Aldrich) supplemented with 20% FBS and 2% Ultraglutamine (Lonza). The continuous cell lines bearing the LDLR mutations were also analyzed to verify the presence of DNA variations. In order to upregulate LDLR, the cells were incubated in a medium containing a lipoprotein deprived serum (LPDS, Sigma-Aldrich) for 48 h before being analyzed.
Peripheral blood T-lymphocytes stimulation
After two wash steps in FBS, thawed PBMC were counted and seeded in a 24-well plate (BD-Falcon) at 1 × 106 cells/ml in IMDM supplemented with 10% human LPDS to induce LDLR upregulation and 2% Ultraglutamine. Stimulation of T-lymphocytes was carried out for 48 h at 37°C and 5% CO2 with three different combinations of mitogens: 1) 5μg/ml phytohemagglutinin (PHA) (Sigma-Aldrich), 2) PHA and 10 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich), and 3) 1 μM ionomycin (Sigma-Aldrich) and 10 ng/ml PMA according to Makar et al. (12).
LDL uptake assay by fluorescent-activated cell sorter flow cytometry
Residual LDLR activity was evaluated by measuring the binding and uptake of a fluorescently labeled LDL, namely 1,19-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate-conjugated LDL (DiI-LDL, Invitrogen), in EBV-transformed B lymphocytes and mitogen-stimulated T-lymphocytes. Briefly, cells were collected and diluted at 1 × 106 cells/ml in DMEM:F12 (Sigma-Aldrich) without phenol red and LPDS. Two aliquots of cells for each sample analyzed were then incubated for 3 h at 37°C and 5% CO2 as follows: a) with 30 mg/L unlabeled LDL (Sigma-Aldrich), to measure background fluorescence; and b) with 10 mg/L DiI-LDL to estimate the maximum LDL binding and uptake. In preliminary experiments we performed an additional incubation with 30 mg/L unlabeled LDL plus 10 mg/L DiI-LDL (ratio 3:1) to evaluate the displacement of DiI-LDL and in this condition, we observed a reduction of cell fluorescence indicating the specific binding of DiI-LDL (supplementary ). After three washes in with PBS without Ca2+ and Mg2+, fluorescence intensities were measured with a FACSCanto (Becton-Dickinson) flow cytometer according to the manufacturer's instructions. Forward scatter and side scatter gates were established to exclude dead cells and cell debris. An example of flow cytometry gating is shown in supplementary that also shows the percentage of CD3+ cells (T-lymphocytes) obtained with the ionomycin plus PMA stimulation. For each experiment, 10,000 events were counted.
The results are expressed as the median of fluorescence and as the ratio between the median fluorescence intensity of cells from FH patients and the mean of the median fluorescence intensity of control cells. This ratio represents residual LDLR activity. All measurements were performed in duplicate.
Statistical analysis
The normality of variable distributions was evaluated with Kolmogorov-Smirnov test. Continuous variables were indicated as mean ± SD. Differences between the continuous variables were evaluated with t-test. A value of P < 0.05 was considered statistically significant. Statistical analyses were carried out using the statistical Predictive Analytics Software version 18.0 (SPSS Inc.). Analysis of receiver operating characteristic (ROC) curves and dot diagrams was performed with MedCalc Version 11.5.1. The statistical significance of the area under the ROC curves (AUC) was calculated against the null hypothesis AUC = 0.5 as recommended by DeLong et al. (13). The threshold values were determined by the farthest point from the bisector of the ROC curve.
RESULTS
Mutation screening
We identified four new mutations absent in 150 chromosomes from normal individuals (Table 1). These alterations are novel missense mutations (c.440C>T; c.974G>A; c.1130G>T; c.1739C>T) that result in a single amino acid substitution in LDL receptor protein (p.Thr147Ile; p.Cys325Tyr; p.Cys377Phe; p.Ser580Phe). The novel mutations cause changes of amino acid residues conserved across fourteen species examined during our study.
TABLE 1.
Medians of fluorescent intensity and LDLR residual activities evaluated on EBV-transformed B-lymphocytes and stimulated T-lymphocytes from control subjects.
| Control ID | Median of fluorescent intensity on EBV-transformed B-lymphocytes | Median of fluorescent intensity on stimulated T-lymphocytes | LDLR residual activity on EBV-transformed B-lymphocytes | LDLR residual activity on stimulated T-lymphocytes |
| Control 1 | 2,173 | 1,132 | 124.0 | 111.9 |
| Control 2 | 1,654 | 1,161 | 94.4 | 114.8 |
| Control 3 | 1,754 | 1,349 | 100.1 | 133.4 |
| Control 4 | 2,304 | 931 | 131.5 | 92.0 |
| Control 5 | 1,981 | 1,106 | 113.0 | 109.3 |
| Control 6 | 2,150 | 928 | 122.7 | 91.7 |
| Control 7 | 2,103 | 1,103 | 120.0 | 109.0 |
| Control 8 | 1,524 | 948 | 86.9 | 93.7 |
| Control 9 | 1,289 | 869 | 73.5 | 85.9 |
| Control 10 | 2,056 | 952 | 117.3 | 94.1 |
| Control 11 | 1,924 | 904 | 109.8 | 89.4 |
| Control 12 | 1,257 | 799 | 71.7 | 79.0 |
| Control 13 | 1,582 | – | 90.2 | – |
| Control 14 | 1,387 | – | 79.1 | – |
| Control 15 | 1,238 | – | 70.6 | – |
| Control 16 | 1,658 | – | 94.6 | – |
| Control 17 | – | 1,048 | – | 103.6 |
| Control 18 | – | 963 | – | 95.2 |
| Control 19 | – | 905 | – | 89.5 |
| Control 20 | – | 1,231 | – | 121.7 |
| Control 21 | – | 1,101 | – | 108.9 |
| Control 22 | – | 940 | – | 92.9 |
| Control 23 | – | 882 | – | 87.2 |
| Control 24 | – | 960 | – | 94.9 |
| Mean ± SD | 1,752 ± 355 | 1,011 ± 138 | 100.0 ± 20.3 | 100.0 ± 13.6 |
| CV% | 20.3 | 13.6 | 20.3 | 13.6 |
Missing data are relative to unavailable samples. CV%, coefficient of variation.
T-lymphocytes mitogen selection
In order to select the best way to stimulate T-lymphocytes able to express LDLR, we compared three known procedures for in vitro growth stimulation of T-cells. The flow cytometry analysis showed that the amount of viable T-lymphocytes in cells treated with 1) PHA, 2) PHA plus PMA, and 3) ionomycin plus PMA was 76.4%, 83.8%, and 87.3%, respectively. As regards LDL-R expression evaluated by the uptake of DiI-LDL, in case of PHA stimulation, the uptake of DiI-LDL was lower than that observed with PHA plus PMA and ionomycin plus PMA (supplementary Fig. I). Due to the higher yield of live T-lymphocytes obtained with ionomycin plus PMA with respect to PHA plus PMA and considering the LDLR upregulation in T-lymphocytes described by Makar et al. (12) using ionomycin plus PMA, we selected this method for further experiments.
Functional characterization of LDLR activity
The median of fluorescence intensity and the residual LDLR activity evaluated in EBV-transformed B-lymphocytes and in ionomycin plus PMA stimulated T-lymphocytes from healthy subjects with normal lipid profile and no mutations in LDLR are reported in Table 1. A representative set of data from flow cytometry analysis is shown in supplementary Fig. III. The residual LDLR activity was calculated using the mean of median values of all analyzed controls as areference (1,752 for EBV-transformed B-lymphocytes and 1,011 for stimulated T-lymphocytes). As for controls, this calculation represents a data normalization allowing the comparison between the two methods used. The minimum and maximum values of residual LDLR activity evaluated on EBV-transformed B-lymphocytes from controls were 71% and 132%, respectively, whereas the minimum and maximum values of residual LDLR activity evaluated on stimulated T-lymphocytes were 79% and 133%, respectively. The median of fluorescence intensity and the residual LDLR activity in EBV-transformed B-lymphocytes and in stimulated T-lymphocytes from FH patients with mutations in LDLR gene are reported in Table 2. The residual LDLR activities were statistically different between control subjects and FH patients analyzed by both methodologies (P < 0.0001 both). Regarding the functional characterization of new mutations, we observed low residual LDLR activities consistent with a heterozygous status of FH (Table 2).
TABLE 2.
Medians of fluorescent intensity and residual LDLR activities evaluated on EBV-transformed B-lymphocytes and stimulated T-lymphocytes from FH patients
| Nucleotide change | Effect on protein | Reference | Status | Median of fluorescent intensity on EBV-transformed B-lymphocytes | Median of fluorescent intensity on stimulated T-lymphocytes | LDLR residual activity on EBV-transformed B-lymphocytes | LDLR residual activity on stimulated T-lymphocytes |
| c.116_117delGCinsAA | p.Cys39X | 11 | heterozygote | 1,113 | 444 | 63.5 | 43.9 |
| c.367T>C and c.1478_1479delCT | p.Ser123Pro and p.Ser493CysfsX42 | 11 and 14 | compound heterozygote | 459 | – | 26.1 | – |
| c.407A>T | p.Asp136Val | 11 | heterozygote | 1,097 | – | 62.6 | – |
| c.407A>T and c.1775G>A | p.Asp136Val and p.Gly592Glu | 11 and 15 | compound heterozygote | 516 | 220 | 29.4 | 21.7 |
| c.424_430delTCCTGCC | p.Ser142ArgfsX62 | 11 | heterozygote | 1,186 | 647 | 67.6 | 64.0 |
| c.440C>T | p.Thr147Ile | new | heterozygote | 982 | – | 56.0 | – |
| c.440C>T | p.Thr147Ile | new | heterozygote | 1,241 | 562 | 70.8 | 55.6 |
| c.974G>A | p.Cys325Tyr | new | heterozygote | 949 | 572 | 54.1 | 56.6 |
| c.1130G>T | p.Cys377Phe | new | heterozygote | 750 | – | 42.8 | – |
| c.1135T>C | p.Cys379Arg | 15 | heterozygote | 1,157 | 566 | 66.0 | 56.0 |
| c.1187-10G>A | p.Gly396AspfsX19 | 16 | heterozygote | 944 | – | 53.8 | – |
| c.1187-10G>A | p.Gly396AspfsX19 | 16 | heterozygote | 1,317 | 615 | 75.1 | 60.8 |
| c.1567G>A | p.Val523Met | 17 | heterozygote | 635 | 607 | 36.2 | 60.0 |
| c.1646G>A | p.Gly549Asp | 18 | heterozygote | 979 | 354 | 55.8 | 35.0 |
| c.1646G>A | p.Gly549Asp | 18 | heterozygote | 831 | 653 | 47.4 | 64.6 |
| c.1646G>A | p.Gly549Asp | 18 | heterozygote | 1,149 | 503 | 65.5 | 49.7 |
| c.1739C>T | p.Ser580Phe | new | heterozygote | 1,152 | 646 | 65.7 | 63.9 |
| c.1739C>T and c.1646G>A | p.Ser580Phe and p.Gly549Asp | new and 18 | compound heterozygote | 741 | 306 | 42.2 | 30.2 |
| c.1775G>A | p.Gly592Glu | 15 | heterozygote | 1,284 | – | 73.2 | – |
| c.1775G>A | p.Gly592Glu | 15 | homozygote | 682 | 537 | 38.9 | 53.1 |
| c.1846-?_2311+?del | p.Asp616LeufsX17 | 17 | heterozygote | 338 | 367 | 19.2 | 36.3 |
| c.2312-3C>A | p.Ala771_796del | 19 | heterozygote | 724 | 567 | 41.3 | 56.1 |
| c.2312-3C>A | p.Ala771_796del | 19 | heterozygote | 763 | 478 | 43.5 | 47.2 |
| c.2312-3C>A | p.Ala771_796del | 19 | heterozygote | – | 653 | – | 64.6 |
Missing data are relative to unavailable samples.
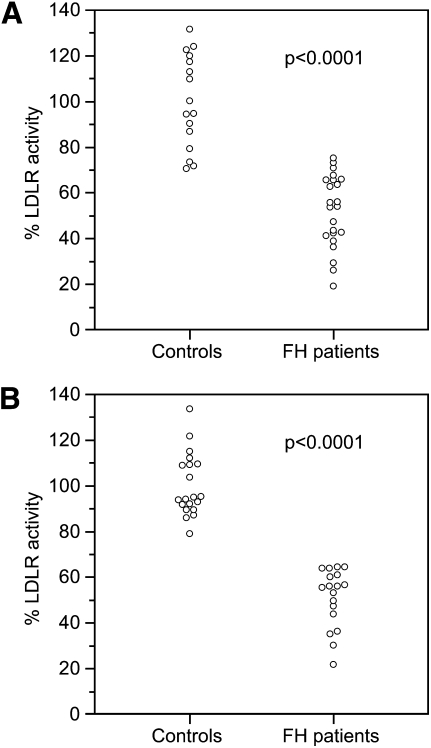
Figure 1 shows a graphical representation of residual LDLR activity of each control and FH patient evaluated by the two methods. We observed that, using EBV-transformed B-lymphocytes, there is an overlap of residual LDLR activities between controls and patients in the range 71–75%, whereas, using stimulated T-lymphocytes, no value in the range 65–79% was observed for controls nor for FH patients. The use of stimulated T-lymphocytes allows a complete discrimination between controls and FH patients.
Fig. 1.
LDLR activity in controls and FH patients evaluated in EBV-transformed B-lymphocytes and stimulated T-lymphocytes. A: EBV-transformed B-lymphocytes; B: stimulated T-lymphocytes. Data are presented as percentage of LDLR activity. The p-values are related to the comparison between controls and FH patients.
Analysis of ROC curves
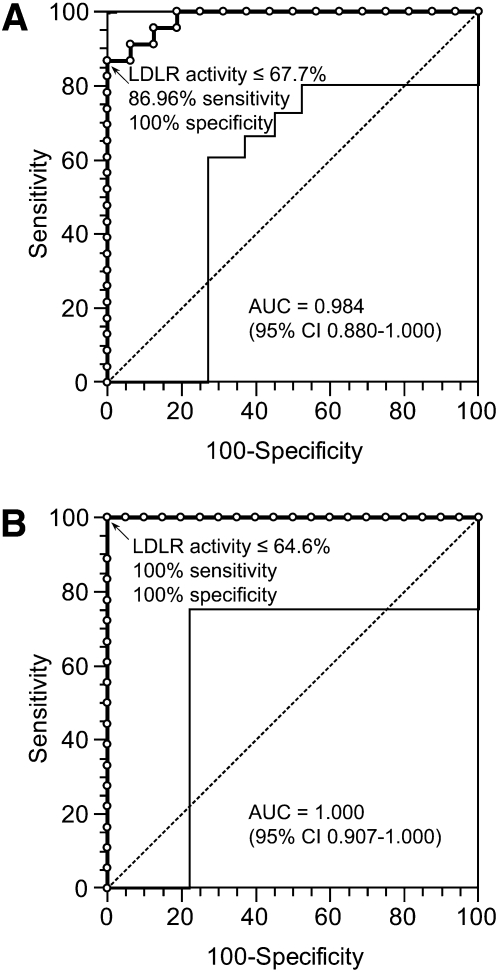
The ROC curves are constructed using the residual LDLR activity evaluated in EBV-transformed B-lymphocytes and in stimulated T-lymphocytes as a discriminator parameter between FH patients and healthy controls (Fig. 2). The AUC of the methods based on EBV-transformed B-lymphocytes and on stimulated T-lymphocytes are 0.984 [95% confidence interval (CI) 0.880–1.000] and 1.000 (95% CI 0.907–1.000), respectively (P < 0.0001 both). The comparison of the AUC of the two methods does not reveal any statistically significant difference (P = 0.41).
Fig. 2.
Receiver operating characteristic (ROC) curves of LDLR activity evaluated in EBV-transformed B-lymphocytes and stimulated T-lymphocytes. A: EBV-transformed B-lymphocytes; B: stimulated T-lymphocytes. The ROC curve is indicated with bold line and open circles represent the criterion points. Light line indicate the 95% confidence interval (CI). Dotted line indicate the bisector. AUC, area under the curve.
By the analysis of ROC curves, the suggested threshold of residual LDLR activity for discrimination between controls and patients is 67.7% for EBV-transformed B-lymphocytes (86.96% sensitivity and 100% specificity) and 64.6% for stimulated T-lymphocytes (100% sensitivity and 100% specificity).
DISCUSSION
The functional characterization of LDLR mutations by use of EBV-transformed B-lymphocytes provides the advantages of continuous cell lines that undergo rapid proliferation combined with a long lifespan. These are easily reestablished in culture after being frozen and are useful in the creation of cell banks for future molecular studies. These advantages enable a reliable characterization of novel and known mutations in LDLR gene and other genetic disorders (20). However, EBV-transformed B-lymphocytes must be used carefully because of decreased genetic stability and possible changes in cellular processes induced by viral transformation (20). The generation of EBV-transformed B-lymphocytes is wasteful, time-consuming, and often results tare unproductive depending on the quantity of leukocytes isolated from each subject.
In order to overcome these problems, we have improved an assay to evaluate LDLR activity in peripheral-blood lymphocytes using ionomycin plus PMA-induced proliferating T-lymphocytes. We selected this mitogen combination because in our experimental conditions, it gives the best results. In other studies, functional assays were conducted on T-lymphocytes using only PHA (21, 22) although the comparison between different mitogens was not performed. For the first time, we compared two cell culture techniques useful in detecting residual LDLR activity in FH patients: EBV-transformed B-lymphocytes and mitogen stimulated T-lymphocytes both treated with fluorescently-labeled LDL. In controls, we observed a higher median of fluorescent intensity in EBV-transformed B-lymphocytes than in stimulated T-lymphocytes, suggesting the highest LDLR expression in the first cell type. The residual LDLR activity evaluated on stimulated T-lymphocytes of controls shows a more limited range of values, suggesting a minor LDLR expression variability.
The minimum value of residual LDLR activity in controls is higher for stimulated T-lymphocytes than for EBV-transformed B-lymphocytes (79% and 71%, respectively) suggesting that the T-lymphocytes based method has a better capacity to detect functional activity than the other one. With ionomycin plus PMA stimulated T-lymphocytes, no value was observed in the range 65–79% (discriminating range) for controls nor for FH patients, whereas using EBV-transformed B-lymphocytes, we observed an overlap in residual LDLR activities between controls and patients in the range 71–75% (absence of a discriminating range). Then, using stimulated T-lymphocytes, we obtained a complete discrimination between FH patients and normocholesterolemic subjects, although we observed reduced LDLR activities in FH patients with both methods, which is in agreement with the presence of LDLR mutations. The technical advancement reached with our method consists in the complete discrimination between FH patients and controls, a feature that could allow use of this functional assay for screening purposes.
The analysis of ROC curves in stimulated T-lymphocytes suggests a threshold value of residual LDLR activity equal to 64.6% with a 100% sensitivity and a 100% specificity, although the use of a higher threshold in the discriminating range (65–79%) could improve the diagnostic sensitivity of the method.
To date, many techniques have been used to diagnose FH disease, including serum lipid profile and molecular methods such as DNA sequencing for the screening of point mutations, multiplex ligation-dependent probe amplification to identify large rearrangements, and RNA analysis for splicing variants (2, 23–25). The residual LDLR activity evaluated on stimulated T-lymphocytes (ionomycin plus PMA used as mitogen stimulators) may be used as a screening method for identification of LDLR defects in patients with a clinical suspect of FH. In addition, our method is relatively easy to handle and appears less laborious and time-consuming than other described techniques such as the use of EBV-transformed B-lymphocytes whose generation requires to work in class 1 biosafety level laboratories.
In conclusion, our method based on the DiI-LDL uptake in ionomycin plus PMA-stimulated T-lymphocytes is simple and fast and improves the detection of LDL receptor activity defects, allowing complete discrimination between controls and FH patients. Furthermore, the evaluation of residual LDLR activity could be helpful not only for mutation characterization but also for diagnostic purposes.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contribution of the Biobank of CEINGE for isolation of peripheral blood mononuclear cells and generation of EBV-transformed cell lines.
Footnotes
Abbreviations:
- AUC
- area under the receiver operating characteristic curves
- CI
- confidence interval
- CV%
- coefficient of variation
- DiI-LDL
- 1,19-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate-conjugated LDL
- EBV
- Epstein-Barr virus
- FH
- familial hypercholesterolemia
- IMDM
- Iscove's Modified Dulbecco's Medium
- LDLR
- LDL receptor
- LPDS
- lipoprotein deprived serum
- PBMC
- peripheral blood mononuclear cell
- PCSK9
- proprotein convertase subtilisin/kexin type 9
- PHA
- phytohemagglutinin
- PMA
- phorbol 12-myristate 13-acetate
- ROC
- receiver operating characteristic
This work was supported by grants from CEINGE Convenzione Regione Campania, DGRC 1901/2009 and from IRCCS Fondazione SDN.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one tables and three figures.
REFERENCES
- 1.Soutar A. K. 2010. Rare genetic causes of autosomal dominant or recessive hypercholesterolaemia. IUBMB Life. 62: 125–131 [DOI] [PubMed] [Google Scholar]
- 2.Varret M., Abifadel M., Rabès J. P., Boileau C. 2008. Genetic heterogeneity of autosomal dominant hypercholesterolemia. Clin. Genet. 73: 1–13 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein J. L., Brown M. S. 1974. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J. Biol. Chem. 249: 5153–5162 [PubMed] [Google Scholar]
- 4.Leitersdorf E., Hobbs H. H., Fourie A. M., Jacobs M., van der Westhuyzen D. R., Coetzee G. A. 1988. Deletion in the first cysteine-rich repeat of low density lipoprotein receptor impairs its transport but not lipoprotein binding in fibroblasts from a subject with familial hypercholesterolemia. Proc. Natl. Acad. Sci. USA. 85: 7912–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitz G., Brüning T., Kovacs E., Barlage S. 1993. Fluorescence flow cytometry of human leukocytes in the detection of LDL receptor defects in the differential diagnosis of hypercholesterolemia. Arterioscler. Thromb. 13: 1053–1065 [DOI] [PubMed] [Google Scholar]
- 6.Løhne K., Urdal P., Leren T. P., Tonstad S., Ose L. 1995. Standardization of a flow cytometric method for measurement of low-density lipoprotein receptor activity on blood mononuclear cells. Cytometry. 20: 290–295 [DOI] [PubMed] [Google Scholar]
- 7.Chan P., Jones C., Lafrenière R., Parsons H. G. 1997. Surface expression of low density lipoprotein receptor in EBV-transformed lymphocytes: characterization and use for studying familial hypercholesterolemia. Atherosclerosis. 131: 149–160 [DOI] [PubMed] [Google Scholar]
- 8.Raungaard B., Heath F., Brorholt-Petersen J. U., Jensen H. K., Faergeman O. 1999. Flow cytometric assessment of LDL receptor activity in peripheral blood mononuclear cells compared to gene mutation detection in diagnosis of heterozygous familial hypercholesterolemia. Cytometry. 36: 52–59 [DOI] [PubMed] [Google Scholar]
- 9.Tada H., Kawashiri M., Noguchi T., Mori M., Tsuchida M., Takata M., Nohara A., Inazu A., Kobayashi J., Yachie A., et al. 2009. A novel method for determining functional LDL receptor activity in familial hypercholesterolemia: application of the CD3/CD28 assay in lymphocytes. Clin. Chim. Acta. 400: 42–47 [DOI] [PubMed] [Google Scholar]
- 10.Urdal P., Leren T. P., Tonstad S., Lund P. K., Ose L. 1997. Flow cytometric measurement of low density lipoprotein receptor activity validated by DNA analysis in diagnosing heterozygous familial hypercholesterolemia. Cytometry. 30: 264–268 [PubMed] [Google Scholar]
- 11.Romano M., Di Taranto M. D., D'Agostino M. N., Marotta G., Gentile M., Abate G., Mirabelli P., Di Noto R., Del Vecchio L., Rubba P., et al. 2010. Identification and functional characterization of LDLR mutations in familial hypercholesterolemia patients from Southern Italy. Atherosclerosis. 210: 493–496 [DOI] [PubMed] [Google Scholar]
- 12.Makar R. S., Lipsky P. E., Cuthbert J. A. 1994. Non-sterol regulation of low density lipoprotein receptor gene expression in T cells. J. Lipid Res. 35: 1888–1895 [PubMed] [Google Scholar]
- 13.DeLong E. R., DeLong D. M., Clarke-Pearson D. L. 1988. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 44: 837–845 [PubMed] [Google Scholar]
- 14.Cavanaugh J. A., Easteal S., Simons L. A., Thomas D. W., Serjeantson S. W. 1994. FH-Sydney 1 and 2: two novel frameshift mutations in exon 10 of the low-density lipoprotein receptor gene detected by heteroduplex formation. Hum. Mutat. 4: 276–280 [DOI] [PubMed] [Google Scholar]
- 15.Hobbs H. H., Brown M. S., Goldstein J. L. 1992. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum. Mutat. 1: 445–466 [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Huff E., Janecka L., Hegele R. A. 2001. Low density lipoprotein receptor (LDLR) gene mutations in Canadian subjects with familial hypercholesterolemia, but not of French descent. Hum. Mutat. 18: 359. [DOI] [PubMed] [Google Scholar]
- 17.Hobbs H. H., Russell D. W., Brown M. S., Goldstein J. L. 1990. The LDL Receptor locus in familial hypercholesterolemia: mutational analysis of a membrane protein. Annu. Rev. Genet. 24: 133–170 [DOI] [PubMed] [Google Scholar]
- 18.Bertolini S., Cassanelli S., Garuti R., Ghisellini M., Simone M. L., Rolleri M., Masturzo P., Calandra S. 1999. Analysis of LDL receptor gene mutations in Italian patients with homozygous familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 19: 408–418 [DOI] [PubMed] [Google Scholar]
- 19.Liguori R., Bianco A. M., Argiriou A., Pauciullo P., Giannino A., Rubba P., De Simone V. 2001. LDL receptor cDNA sequence analysis in familial hypercholesterolemia patients: 5 novel mutations with high prevalence in families originating from southern Italy. Hum. Mutat. 17: 433. [DOI] [PubMed] [Google Scholar]
- 20.Sie L., Loong S., Tan E. K. 2009. Utility of lymphoblastoid cell lines. J. Neurosci. Res. 87: 1953–1959 [DOI] [PubMed] [Google Scholar]
- 21.Chan P. C., Edwards A., Lafrenière R., Parsons H. G. 1998. Improved detection of familial hypercholesterolemia by determining low density lipoprotein receptor expression in mitogen-induced proliferating lymphocytes. J. Lipid Res. 39: 2261–2270 [PubMed] [Google Scholar]
- 22.Cuthbert J. A., East C. A., Bilheimer D. W., Lipsky P. E. 1986. Detection of familial hypercholesterolemia by assaying functional low-density-lipoprotein receptors on lymphocytes. N. Engl. J. Med. 314: 879–883 [DOI] [PubMed] [Google Scholar]
- 23.Minhas R., Humphries S. E., Qureshi N., Neil H. A.; NICE Guideline Development Group 2009. Controversies in familial hypercholesterolaemia: recommendations of the NICE Guideline Development Group for the identification and management of familial hypercholesterolaemia. Heart. 95: 584–587 [DOI] [PubMed] [Google Scholar]
- 24.Taylor A., Wang D., Patel K., Whittall R., Wood G., Farrer M., Neely R. D., Fairgrieve S., Nair D., Barbir M., et al. 2010. Mutation detection rate and spectrum in familial hypercholesterolaemia patients in the UK pilot cascade project. Clin. Genet. 77: 572–580 [DOI] [PubMed] [Google Scholar]
- 25.Holla Ø. L., Nakken S., Mattingsdal M., Ranheim T., Berge K. E., Defesche J. C., Leren T. P. 2009. Effects of intronic mutations in the LDLR gene on pre-mRNA splicing: comparison of wet-lab and bioinformatics analyses. Mol. Genet. Metab. 96: 245–252 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.