Abstract
Controlled trials provide critical tests of hypotheses generated by meta-analyses. Two recent meta-analyses have reported that gray matter volumes of schizophrenia and bipolar I patients differ in the amygdala, hippocampus, or perigenual anterior cingulate. The present magnetic resonance imaging study tested these hypotheses in a cross-sectional voxel-based morphometry (VBM) design of 17 chronic schizophrenia and 15 chronic bipolar patients and 21 healthy subjects matched for age, gender and duration of illness. Whole brain gray matter volume of both the schizophrenia and bipolar groups was smaller than among healthy control subjects. Regional voxel-wise comparisons showed that gray matter volume was smallest within frontal and temporal regions of both patient groups. Region of interest analyses found moderately large to large differences between schizophrenia and healthy subjects in the amygdala and hippocampus. There were no group differences in the perigenual anterior cingulate. When schizophrenia and bipolar groups were directly compared, the schizophrenia group showed smaller GM volumes in right subcortical regions involving the right hippocampus, putamen, and amygdala. The hippocampal and amygdala findings confirm predictions derived from recent meta-analyses. These structural abnormalities may be important factors in the differential manifestations of these two functional psychotic disorders.
Keywords: Gray matter volume, magnetic resonance imaging, amygdala, hippocampus
1. Introduction
The issue of differences and similarities in the neuroanatomical structure between schizophrenia and bipolar disorder, and how each group differs from healthy individuals is long-standing question in psychiatry (Baumann and Gogerts, 1999). Over the past two decades, magnetic resonance imaging (MRI) has been useful in revealing neuroanatomical substrates in patients with bipolar disorder and schizophrenia. More recently, voxel-based morphometry (VBM)(Ashburner and Friston, 2000) has been increasingly applied to further elucidate structural brain changes associated with these functional mental disorders. VBM is a fully automated whole brain image analysis technique that involves the voxel-wise comparison of segmented gray and white matter between at least two groups of subjects (Ridgway et al., 2008). It allows for comprehensive exploration of whole brain structures that can be integrated with apriori hypotheses about regions of interest.
Two meta-analyses completed to date have sought to clarify the neuroanatomical differences between bipolar and schizophrenia patients and to identify differences between each patient group and healthy controls. In a meta-analysis of VBM studies that encompassed 2058 schizophrenia patients and 366 with bipolar disorder, Ellison-Wright and Bullmore (2010) found widespread gray matter reductions in the schizophrenia group compared with healthy controls that included the insula bilaterally, dorsolateral prefrontal cortex, superior temporal cortex, bilateral hippocampal-amygdala region, thalamus, anterior cingulate, medial frontal gyrus and posterior cingulate. Increases were found in the right globus pallidus extending to the head of the left caudate nucleus. In the same meta-analysis, smaller gray matter volumes were found in the right and left insula, perigenual anterior cingulate, and subgneual anterior cingulate in the bipolar studies, though not in the bilateral hippocampal-amygdala regions. The areas of reduced gray matter observed in studies of biplolar patients overlapped substantially with areas of reduced gray matter in schizophrenia patients, though the reductions seen bilaterally in the hippocampal-amygdala area among schizophrenia patients were not observed in bipolar patients. An area of the perigenual association cortex was selectively smaller among bipolar patients than healthy controls, a region where no differences between schizophrenia patients and healthy controls have been found. Arnone and colleagues (2009) meta-analysis of volumetric studies, using a variety of methods to segment magnetic resonance images, found smaller volumes in the right amygdala among schizophrenia patients when they were directly compared with bipolar patients (Arnone et al., 2009). Moreover, Arnone found that age, illness duration, and gender were significant sources of heterogeneity in study findings (Arnone et al., 2009).
Although meta-analyses generally increase both statistical power and generalizability of results compared with individual studies, the results of meta-analysis are constrained by the quality of the studies comprising the analysis. A limitation of the literature available for the Ellison-Wright and Bullmore (2010) study was that few published VBM studies have directly compared schizophrenia with bipolar I patients. In our own PubMed search of the literature (December, 2010), we found only three papers when searching with the terms “VBM and schizophrenia and bipolar” or “voxel based morphometry and schizophrenia and bipolar” that reported using voxel based morphometry to study anatomical differences between schizophrenia and bipolar patients (Cui et al., 2010; McIntosh et al., 2004; McIntosh et al., 2006). Two of the papers reported on the same samples of patients and did not directly compare the two groups (McIntosh et al., 2004; McIntosh et al., 2006). The sparsity of VBM studies where schizophrenia and bipolar patients were matched on variables, such as chronicity, age, gender, and symptom severity, which are known to influence volumetric data, means that important sources of variation might not have been optimally controlled in current meta-analyses of the two patient groups (See further. Bose et al., 2009; Nesvåg 2009; Sarnicola et al., 2009; Yoshihara et al., 2008). Volumetric studies of schizophrenia patients and controls using methods besides VBM have directly compared schizophrenia patients and controls (Arnone et al., 2009). Among these studies, however, the wide variety of tissue segmentation, coregistration, normalization, and anatomical labeling methods used are an important uncontrolled source of variation.
Some clinical trials experts recommend performing a direct comparison of study medications whenever a meta-analysis indicates improved outcome for a particular drug (Chalmers, 1988). The aim of the direct comparison is to test hypotheses derived from meta-analysis in a controlled study. This recommendation appears useful when evaluating the results from any meta-analysis, especially from imaging meta-analyses that draw conclusions about morphometric differences among patient groups by comparing each group with healthy controls. Such conclusions not only rest on the assumption that patient groups do not differ on variables that might influence brain structure other than diagnosis, they also involve the assumption that the control groups used to study one patient group are comparable to controls used to study other patient groups. The meta-analyses reported by Ellison-Wright and Bullmore (2010) and by Arnone and colleagues (2009) identified several anatomical regions where morphometric measurements might differentiate schizophrenia and bipolar patients in a study of well matched patient groups. These include the perigenual anterior cingulate and hippocampal region reported by Ellison-Wright and Bullmore (2010) and the amygdala identified by both meta-analyses. The one VBM analysis that directly compared schizophrenia and bipolar patients did not confirm any of these findings, though the authors did not use regions of interest to directly confirm the findings of the previous meta-analyses (Cui et al., 2010).
The aim of the present study was to use whole brain and region of interest data to directly compare gray matter (GM) volumes in a cross-sectional design involving subjects with chronic schizophrenia, chronic bipolar I disorder, and healthy controls matched for age, gender and duration of illness. Based on previous meta-analytic studies, we tested the following hypotheses: 1) patients with schizophrenia as compared to matched patients with bipolar I disorder would show smaller GM volumes in the hippocampus and amygdala; 2) bipolar I disorder patients would show smaller GM volumes in the perigenual cortex compared with matched schizophrenia patients. To aid in the interpretation of the results of the hypothesis testing, we also examined whether broad tissue compartments (gray matter, white matter, and ventricular volume) differed among the three groups studied and examined correlations of indices of psychopathology with GM volume.
2. Methods
2.1 Subjects
Seventeen subjects with schizophrenia (SZ) (8 males and 9 females), 15 subjects with bipolar I disorder (BP) (7 males and 8 females), and 21 healthy control (HC) subjects (10 males and 11 females) without psychiatric illness were studied (Table 1). BP and SZ subjects were recruited from outpatient mental health clinics at the University of California at San Diego and Veterans Affairs San Diego Healthcare System. Healthy controls were recruited form a community sample.
Table 1.
Demographic and clinical characteristics of the study group*
| Variable | Schizophrenia Patients (n=17) | Bipolar I Patients (n=15) | Healthy Control Subjects (n=21) | Group Difference (p-value) |
|---|---|---|---|---|
| Age, years | 44.8(6.8) | 46.2(10.6) | 45.0(10.2) | 0.901 |
| Gender, Male/Female, Number | 8/9 | 7/8 | 10/11 | NA |
| Educational Level, years | 12.2(1.6) | 15.5(2.0) | 14.8(3.1) | 0.001† |
| Duration of Illness, years | 19.1(6.2) | 18.9(6.9) | NA | 0.934 |
| MMSE | 29.4(0.6) | 29.0(0.9) | NA | 0.151 |
| HDRS scores | 13.0(5.3) | 20.9(11.9) | NA | 0.019‡ |
| PANSS scores | ||||
| Positive | 12.3(4.2) | 12.1(2.7) | NA | 0.826 |
| Negative | 13.5(2.9) | 9.8(2.4) | NA | 0.001§ |
| General psychopathology | 30.2(3.6) | 33.1(8.2) | NA | 0.207 |
| Current Medication | ||||
| Antipsychotics(Atypical:Typical) | 17(16:1) | 3(2:1) | NA | NA |
| Mood stabilizers | 0 | 11 | NA | NA |
| Antidepressants | 5 | 9 | NA | NA |
| Benzodiazepines | 0 | 3 | NA | NA |
| Anti-cholinergics | 2 | 0 | NA | NA |
| Total intracranial volume | 1466.9(180.4) | 1542.8(173.4) | 1515.0(146.0) | .423 |
| Gray matter volume | 597.6(68.0) | 617.1(58.6) | 642.3(53.2) | .038# |
| White matter volume | 529.2(76.4) | 514.9(65.3) | 515.1(53.1) | .146# |
| Cerebrospinal fluid | 340.2(86.3) | 410.8(137.3) | 357.6(105.9) | .283# |
Note.
Abbreviations: NA, not applicable; MMSE, Mini-Mental State Examination; HDRS, Hamilton Depression Rating Scale; PANSS, Positive and Negative Syndrome Scale.
Data are given as mean (SD) except where noted otherwise.
Schizophrenic patients showed a significant lower educational level than bipolar I disordered patients and healthy controls (Tukey honestly significant difference tests, p<0.05)
Bipolar I disordered patients showed significantly higher scores in HDRS than schizophrenic patients (p<0.05).
Schizophrenic patients showed significantly higher negative scores of PANSS than bipolar I disordered patients (p<0.05).
Total intracranial volume covariate
Study inclusion criteria were 1) age: 25-70; 2) duration of illness: over 3 years; and 3) DSM-IV criteria for schizophrenia or bipolar I disorder, as determined by the structured clinical interview (SCID) (First et al., 1995). Exclusion criteria were 1) lifetime history of neurologic illness; 2) head trauma leading to loss of consciousness; 3) history of electroconvulsive therapy; 4) DSM-IV substance abuse or substance dependence disorder; 5) Mini-Mental State Examination (MMSE) < 25; and 6) contraindication to magnetic resonance (MR) scanning. The SCID was also administered to all HC subjects to exclude subjects with axis I or axis II psychiatric disorder.
Clinical evaluations (Table 1) included the Mini-Mental State Examination (MMSE) to exclude cognitive impairment (Folstein et al., 1975), the 28-item version of the Hamilton Depression Rating Scale (HDRS) to rate severity of depressive symptoms (Hamilton, 1960) and positive and negative syndrome scale (PANSS) to rate severity of psychotic symptoms (Kay et al., 1987). HC subjects with neurologic or psychiatric histories, or with histories or current use of psychotropic medications were excluded. All subjects signed University of California, San Diego Institutional Review Board approved informed consent prior to undergoing study procedures.
2.2 MRI image acquisition
Scans were performed with a 1.5-Tesla Siemens Magnetom Vision scanner. Earplugs and headphones were provided to block the scanner noise. Structural images were obtained using a magnetization prepared rapid gradient-echo (MPRAGE) protocol (TR = 24ms, TE = 5 ms, flip angle = 10°, 180 contiguous axial slices of 1.0 mm thickness, voxel size = 1.0 × 1.0 × 1.0 mm). Precautions were taken to minimize subjects’ motion during the MRI study by instructing subjects to remain as still as possible and tightly packing the area around their heads with foam padding. Every scan was checked for image artifacts and gross anatomical abnormalities.
2.3 MRI image processing
Data analysis was performed using the SPM5 software package (Wellcome Department of Cognitive Neurology, London, UK) running under MATLAB 2006a (The MathWorks, Natick, MA, USA), as well as the VBM5 toolbox (http://dbm.neuro.uni-jena.de/vbm/vbm5-for-spm5/), which utilizes and extends the new unified segmentation approach implemented in SPM5 (Ashburner and Friston, 2005). The unified segmentation provides a generative model of VBM preprocessing that integrates tissue classification, image registration and MRI inhomogeneity bias correction. In addition, the VBM5 toolbox uses Hidden Markov Field (HMRF) model on the segmented tissue maps in order to increase the quality of segmentation (Cuadra et al., 2005). The HMRF algorithm provides spatial constraints based on neighboring voxel intensities within a 3 × 3 × 3 voxel cube. It removes isolated voxels which are unlikely to be a member of a certain tissue class and also closes holes in a cluster of connected voxels of a certain class resulting in a higher signal-to-noise ratio of the final tissue probability maps. SPM5 writes the estimated tissue maps by using Bayes rule for combining the likelihood of certain voxels belonging to a tissue class with the a priori knowledge of the respective ICBM (International Consortium for Brain Mapping) tissue prior. The final tissue maps of gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) were modulated with the Jacobian determinants of the deformation parameters obtained by normalization to the MNI (Montreal Neurological Institute) standard space. In order to analyze relative volume differences between study groups, images were modulated for non-linear warping only. Specifically, volume changes due to affine normalization were not considered which automatically corrected for different brain sizes of study participants thus it was not necessary to globally scale data in the statistical model (for details see http://dbm.neuro.uni-jena.de/vbm/segmentation/modulation/). The modulated GM partitions were smoothed with an 8 mm FWHM Gaussian kernel and entered statistical analysis. Additional calculations were performed using the “Tools” algorithms of the VBM5 toolbox: Global GM, WM and CSF volumes as well as total intracranial volumes were computed from native-space tissue maps for SZ, BP, and HC group and reported in Table 1.
Two quality assurance steps were taken to assess the adequacy of the VBM gray matter volumes that were central to the analysis. One step involved visual inspection of all native and gray matter segmented images for coarse abnormalities, such as movement abnormalities, unclear gray-white boundaries, cysts and other intracranial lesions, inadequate skull stripping and globally poor tissue segmentation. Only one participant had a positive finding. A bipolar patient had an abnormally large anterior cranial fossa, especially at the level of the superciliary ridge, that was filled with cerebrospinal fluid. The tissue segmentation step assigned these voxels to the cerebrospinal fluid class; consequently, no abnormality in the gray matter segmentation of this individual was observed, and this person was retained in subsequent analyses. As a second assessment of the quality of the VBM segmentation, tissue segmentation was performed using an alternative pathway. This second pathway began with the use of N3 to correct radiofrequency intensity biases followed by the use of AFNI’s 3dSkullStrip, a modified version of the brain extraction algorithm, to remove skull and other non-brain tissue (Sled, et al., 1998 //afni.nimh.nih.gov/pub/dist/doc/program_help/3dSkullStrip.html). FSL’s FAST routine was used to segment voxels into cerebrospinal fluid, gray matter, and white matter compartments (/www.fmrib.ox.ac.uk/fsl/fast4/index.html). The correlation of VBM and FAST gray matter volumes across all subjects is presented in section 3.1.
2.4 Statistical analysis
Smoothed images were compared between diagnostic groups using an ANCOVA model controlling for the possible effects of age and gender as implemented in SPM5. In the first step of the statistical analysis, a t-test contrasts was constructed in order to examine regional GM volume increases/decreases between the BP and SZ groups on a voxel-by-voxel basis. Then GM volume differences between the SZ and HC, as well as between BP and HC group were examined. The significance of differences was estimated using the theory of random Gaussian fields (Friston et al., 1996). Significance was set at a p value of p < 0.001, with a minimum cluster size of 200 voxels (Adler et al., 2005; Wilke et al., 2004). The significance for clusters was set a p-value of < 0.05 (corrected for multiple comparisons). To determine the magnitude of our between-group findings, we calculated the correlation ratio, η2, which assesses the amount of variance accounted for by the group effect tested. Following Cohen’s nomenclature, small, medium and large values of η2 are .01, .06, and .14, respectively (Cohen, 1988).
Two regions of interest (ROI) approaches were used to test study hypotheses. Both ROI methods were performed on gray matter density maps smoothed to 8mm FWHM. The first approach used apriori defined anatomical regions involving the perigenual, hippocampal and amygdala areas extracted from the Wake Forrest University PickAtlas (Maldjian, et al., 2003; Maldjian et al., 2004). The perigenual anterior cingulate was derived by trimming the PickAtlas anterior cingulate so that its superior edge was level with the roof of the third ventricle whereas the inferior edge was level with the anterior commissure. The second approach used empirically derived, directed ROIs identified from the VBM analysis where significant differences were found when comparing the two patient groups in the VBM analysis. The directed approach was performed on a subset of schizophrenia and bipolar patients matched on total cortical volume, and permitted the separation of a large cluster of group difference into anatomically meaningful components. The values derived from the directed ROI method were also correlated with clinical variables.
In addition, group comparisons of the demographic and clinical variables, as well as gray matter, white matter and CSF volumes were performed using one-way ANOVA and planned contrasts comparing HC with the two patient groups and the two patient groups with one another. Statistical significance was defined at the 0.05 level and was two-tailed. All statistical calculations were performed with SPSS 13.0 for Windows XP (SPSS Inc., Chicago, IL, USA).
3. Results
As shown in Table 1 groups were well matched on age (p=0.854), gender (p = .976) and duration of illness (p=0.934). For all of these measures the amount of variance accounted for by group was less than 1%. As expected, SZ group showed a significantly lower education level than BP and HC group (Tukey honestly significant difference tests, p=0.001 and p=0.004, respectively). MMSE was not significantly different among the three groups, although the observed effect size was in the moderate range, η2 = .07. Neither positive nor general scores on the PANSS differed between the BP and SZ groups, PANSS Positive η2 < .01; PANSS General η2 = .05. BP group had significantly higher HDRS scores than SZ group (p=0.019, η2 = .17), whereas SZ group had significantly higher negative scores on PANSS than did BP group (p=0.001, η2 = .33), consistent with expected clinical symptom profiles.
3.1 Global anatomical variables
Global volumes for the whole brain and each of the three main tissue classes (GM, WM, and CSF) are shown in Table 1. Total intracranial volume did not significantly differ among the three groups (p = .423; η2 = .03). The three groups differed in VBM GM volume when total intracranial volume was used as a covariate, F(2, 50) = 3.56, p = .038, η2 = .12. The mean VBM GM volume of the two patient groups was significantly smaller than the HC volume, t(50) = 2.664, p = .01, η2 = .12. The two patient groups did not significantly differ from one another, t(5) = -0.021, p = .983, with the observed amount of variance in VBM gray matter volume accounted for by differences between the patient groups virtually zero. GM volume estimated by the VBM method was strongly correlated across all subjects with GM volume estimated from the FAST pipeline, r = .83, p < .001.
Although the mean CSF volumes were numerically larger in the BP group compared with the HC and SZ groups, the within group variation in CSF volumes was large compared with the means leading to large coefficients of variation and a non-significant group effect (p = .283, η2 = .05). A re-analysis of the CSF volume was performed after the BP patient with an abnormally large anterior cranial fossa filled with CSF was removed from the analysis. Omitting this patient from the analysis reduced the apparent group differences in CSF volume and cut the size of the between group effect in half (η2 = .025). Finally, no group differences were observed in total white matter volume, although the observed effect size was moderately large (p = .146, η2 = .08).
3.2 Apriori Regions of Interest
Gray matter volume in the amygdala was significantly smaller in the SZ group compared with the HC group (p = .007, η2 = .15). Mean BP amygdala volume did not differ from HC though the observed effect size fell into the moderate range (p = ,124, η2 = .08). Hippocampal gray matter volume was significantly smaller among SZ patients than healthy controls (p = .044, η2 = .11). Hippocampal volume in the BP group did not differ significantly from the HC volume (p=.244, η2 = .05). Although no significant group differences in gray matter volume were found in the perigenual anterior cingulate, the observed effect size for the contrast of the SZ volume with the CN volume was moderately large (p = .113, η2 = .07). The BP CN perigenual contrast was nonsignificant and small (p = .772, η2 < .01). No differences in ROI volumes were observed when the two patient groups were directly compared.
3.3 VBM Cluster Analyses
The SZ group displayed smaller GM volumes than the HC group mainly at three cortical and subcortical regions: (1) right middle and medial frontal cortex (2) right superior and middle temporal gyri (3) bilateral thalamic region (Fig 1 and Table 2). BP patients displayed smaller GM volume relative to the HC group at five cortical regions: (1) right superior and middle frontal region (2) right superior and middle temporal region (3) left superior temporal region (4) left pre- and post- central region (5) left occipito-parietal region (Fig 1 and Table 2). In no region were the GM volumes of the SZ and BP groups greater than the HC group.
Figure 1.
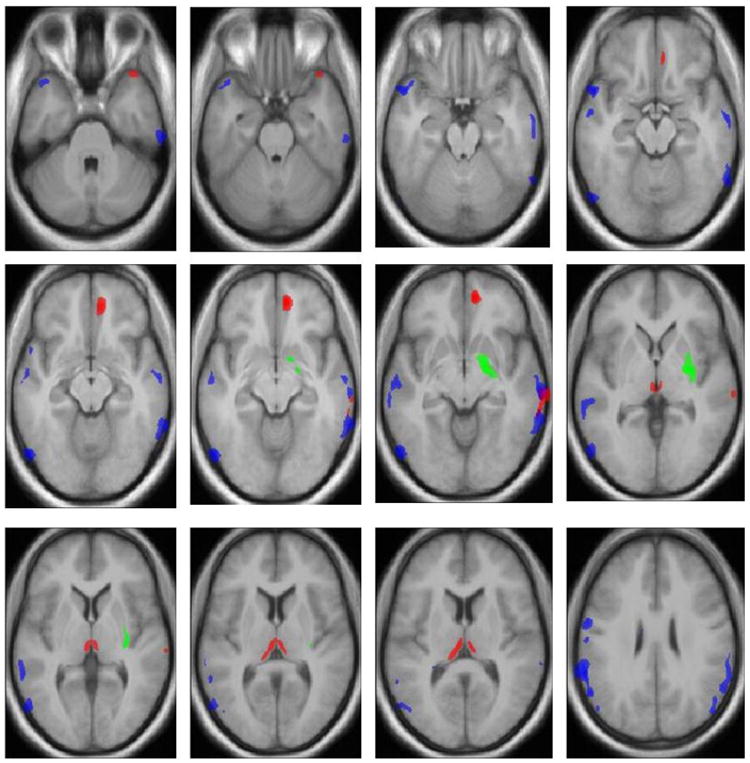
Voxel-by-voxel comparison map of bipolar I disorder (n=15), schizophrenia(n=17) and healthy control(n=21) subjects, rendered on a T1-weighted anatomic imagea
aStatistically significant differences in gray matter volume were defined p≤.001, with a cluster size of 200 and corrected cluster level significance p<0.05. The location of the significant difference results from each comparison was plotted as a dot (Montreal Neurological Institute coordinates are provided in Table 2). Green dot signifies location of gray matter reduction in schizophrenic patients compared with bipolar patients. Blue dot signifies location of gray matter reduction in bipolar I patients compared with control subjects. Red dot signifies location of gray matter reduction in schizophrenic patients compared with control subjects.
Table 2.
Areas of reduced gray matter in patients with bipolar I disorder and schizophrenia compared to healthy controls and between patients group*
| Significance | MNI Coordinates | Anatomical Region | ||||||
|---|---|---|---|---|---|---|---|---|
| KE | T | Z | η2 | X | y | z | ||
| 15 bipolar I disordered patients minus 17 schizophrenic patients | 2353 | 5.17 | 4.30 | 0.16 | 20 | -6 | -7 | Right hippocampus |
| 5.32 | 4.39 | 0.17 | 30 | -8 | -3 | Right putamen | ||
| 4.82 | 4.08 | 0.14 | 15 | 1 | -9 | Right amygdala | ||
|
| ||||||||
| 21 Healthy controls minus 17 schizophrenic patients | 992 | 6.02 | 4.93 | 0.17 | 10 | 49 | -9 | Right middle frontal gyrus |
| 4.48 | 3.94 | 0.10 | 7 | 37 | -15 | Right medial frontal gyrus | ||
|
| ||||||||
| 1122 | 4.19 | 3.74 | 0.09 | 66 | -41 | -7 | Right middle temporal gyrus | |
| 5.07 | 4.34 | 0.12 | 69 | -28 | -6 | |||
| 3.82 | 3.45 | 0.08 | 69 | -24 | 3 | Right superior temporal gyrus | ||
|
| ||||||||
| 1355 | 5.32 | 4.51 | 0.13 | 36 | 23 | -40 | Right superior temporal gyrus | |
| 3.89 | 3.51 | 0.08 | 39 | 24 | -28 | |||
|
| ||||||||
| 1631 | 4.43 | 3.91 | 0.10 | 6 | -21 | 9 | Right thalamus | |
| 4.52 | 3.97 | 0.10 | -5 | -21 | 10 | Left thalamus | ||
|
| ||||||||
| 21 Healthy controls minus 15 bipolar I disordered patients | 731 | 4.98 | 4.25 | 0.11 | 39 | 6 | 58 | Right middle frontal gyrus |
| 3.65 | 3.32 | 0.06 | 28 | 2 | 59 | |||
|
| ||||||||
| 604 | 5.41 | 4.53 | 0.13 | 20 | 9 | 66 | Right superior frontal gyrus | |
| 4.47 | 3.91 | 0.09 | 21 | -1 | 66 | |||
|
| ||||||||
| 2381 | 5.98 | 4.86 | 0.15 | 67 | -25 | -7 | Right middle temporal gyrus | |
| 4.65 | 4.04 | 0.10 | 63 | -10 | -16 | |||
| 4.57 | 3.98 | 0.10 | 69 | -26 | -30 | |||
|
| ||||||||
| 5231 | 5.41 | 4.52 | 0.13 | 58 | -57 | 20 | Right superior temporal gyrus | |
| 5.08 | 4.32 | 0.12 | 63 | -49 | 20 | |||
| 4.74 | 4.09 | 0.10 | 64 | -36 | 19 | |||
|
| ||||||||
| 1543 | 6.59 | 5.20 | 0.18 | -53 | 11 | -18 | Left superior temporal gyrus | |
| 4.67 | 4.05 | 0.10 | -38 | 17 | -35 | |||
| 4.32 | 3.81 | 0.09 | -45 | 17 | -23 | |||
|
| ||||||||
| 9237 | 5.30 | 4.46 | 0.13 | -58 | -59 | 24 | Left superior temporal gyrus | |
| 5.30 | 4.46 | 0.13 | -57 | -71 | -11 | Left middle occipital gyrus | ||
| 6.44 | 5.12 | 0.17 | -62 | -42 | 23 | Left inferior parietal lobule | ||
|
| ||||||||
| 8881 | 5.26 | 4.43 | 0.12 | -46 | -46 | 47 | Left inferior parietal lobule | |
| 5.28 | 4.44 | 0.12 | -45 | -17 | 63 | Left precentral gyrus | ||
| 5.72 | 4.71 | 0.14 | -61 | -16 | 29 | Left postcentral gyrus | ||
Regional differences in gray matter were reported as significant if they contained 200 voxels at p≤.001 and corrected cluster level significance p<0.05 within SPM5.
d, Cohen’s d
VBM analysis revealed a cluster where the SZ group had smaller GM volume compared to the BP group. The cluster was in a right subcortical region that involved the right hippocampus, putamen, and amygdala (Fig 1, Tables 2 and 3). This large subcortical area was separated into right hippocampal, putamenal, and amygdalar regions. When SZ and BP patients matched pair-wise on total gray matter volume were contrasted, group means in all three areas remained significantly different (amygdala p < .001; hippocampus p < .001; putamen p = .008). There were no regions where the BP group had smaller mean GM volume compared with the SZ group. To determine the diagnostic utility of these volumetric changes, we predicted the diagnostic classification of the two patient groups from the cluster volumes in the right hippocampus, right putamen, and right amygdala. The cross-validated classification rate was 78.1% with two SZ patients and five BP patients misclassified.
Table 3.
Gray matter volumes in empirically derived regions of interest (mm3)
| Variable | Schizophrenia Patients (n=17) | Bipolar I Patients (n=15) | Healthy Control Subjects (n=21) |
|---|---|---|---|
| Hippocampus | 200 (30) | 250 (30) | 230 (70) |
| Amygdala | 250 (40) | 330 (40) | 320 (110) |
| Putamen | 330 (40) | 380 (40) | 360 (80) |
Note. Data are given as mean (SD) mm3.
Compatible with the above findings, the magnitude of the PANSS negative score was inversely correlated with GM volume in the right hippocampus, putamen, and amygdala when the two patient groups were combined into one sample. A comparison of the within group correlations of PANSS negative score with GM volumes indicated that most of the covariance underlying the PANSS negative/GM relationship was due to between-group variation in the PANSS negative and GM values and not to within group covariation. No other symptom variables significantly correlated with GM volumes in the hippocampus, amygdala, or putamen.
4. Discussion
The study findings indicate wide spread reduction of gray matter volume in both schizophrenia and bipolar I disorder compared with healthy control subjects, especially in frontotemporal regions. The reduction appears greater in schizophrenia than in bipolar I disorder in the amygdala and hippocampus of the right hemisphere. The smaller gray matter volumes in schizophrenia and bipolar patients in wide-spread cortical areas as well as the smaller amygdala and hippocampal volumes derived from apriori ROIs in schizophrenia patients compared with controls are direct replications of key findings from the Ellison-Wight and Bullmore’s (2010) meta-analysis of VBM studies. The wide-spread gray matter loss in bipolar I and schizophrenia patients has been reported in both early and recent volumetric studies of the two groups (Lim et al., 1999; Rimol et al., 2010).
Empirically derived, directed ROIs found that schizophrenia patients displayed smaller gray matter volumes than bipolar I patients in the amygdala, hippocampus, and putamen of the right hemisphere. Together gray matter volumes from the right amygdala, hippocampus, and putamen accurately classified about 78% of schizophrenia and bipolar patients. These right hemisphere findings persisted when schizophrenia and bipolar patients matched on gray matter volume were directly compared. The finding of smaller gray matter volumes in the hippocampus and amygdala confirmed a prediction from Ellison-Wright and Bullmore’s (2010) meta-analysis that these two medial temporal lobe structures would be smaller in schizophrenia patients than in bipolar patients whenever the two patient groups are directly compared. The finding of smaller amygdala gray matter volumes in the right hemisphere of schizophrenia patients compared with bipolar I confirms the volumetric finding from the meta-analysis of Arnone et al. (2009). Although the group difference was observed in the empirically derived ROIs, the differences were not observed for the larger anatomical ROIs derived from the Pick Atlas. The difference in effects suggests that subregions of the amydala and hippocampus might be responsible for the group differences. Future studies comparing schizophrenia and bipolar I patients should focus on investigating regional differences in the volumes of these two limbic structures.
The hippocampus and amygdala have been strongly implicated in schizophrenia. Previous structural MRI studies that specifically examined hippocampus as their region of interest have frequently found volumetric reductions within the region (Nelson et al., 1998). Furthermore, meta-analyses of clinical neuroimaging studies in schizophrenia found 5-10% mean volume reductions within amygdala when compared to healthy controls (Honea and Crow, 2005; Lawrie et al., 1998; Nelson et al., 1998; Wright et al., 2000).
We also observed that the right putamen was reduced in schizophrenia patients relative to bipolar I disorder patients. Structural imaging studies of the putamen in schizophrenia have yielded inconsistent results. Although studies of chronic schizophrenic patients with poor executive functions have reported a reduction in putamen volume relative to healthy controls (Stratta et al., 1997), several investigators reported volumetric enlargement within this structure (Brandt et al., 2008). Possible reasons for the discrepancy between our finding and those of other studies can be related to differences in study population and/or atypical antipsychotics exposure. Previous studies that compared schizophrenic patients and healthy controls showed that medication affects GM volume within the putamen where normal GM volumes are seen in patients receiving atypical antipsychotic drugs (Gur et al., 1999), antipsychotic-naïve patients (Keshavan et al., 1998), or in first-episode patients (Gunduz et al., 2002), while increased volumes are seen in patients receiving typical antipsychotic drugs, which decrease when patients switch to a second-generation antipsychotic drugs (Lang et al., 2004). All but one schizophrenic patient in our study received atypical antipsychotic medication.
Bilateral thalamic GM volumes were significantly smaller in patients with schizophrenia compared with healthy controls; the finding was not observed in bipolar disorder I patients. It has been suggested that dysfunctional cortico–subcortical connections are involved in the pathophysiology and psychopathology of schizophrenia (Carlsson and Carlsson, 1990). In this regard, the thalamus is an important structure in the relay of sensory and motor information, maintaining consciousness and attention through reciprocal connections with the cerebral cortex, cerebellum and brain stem (Spinks et al., 2002). The role of the thalamus for the pathophysiology of schizophrenia is supported by imaging and neuropathologic reports. Postmortem studies have reported reductions in neuronal and oligodendrocyte numbers in the thalamus of patients with schizophrenia (Byne et al., 2006; Pakkenberg, 1990). Also, functional neuroimaging investigations found thalamic hypoactivation during cognitive tasks involving working memory, attention and visual processing in patients with schizophrenia (Andreasen et al., 1996; Andrews et al., 2006; Buchsbaum et al., 1996). Likewise, structural MRI studies found reduced thalamic volumes in schizophrenia (Ettinger et al., 2007; Gur et al., 1999). Although there are some controversial findings for volume abnormalities, taken together, these studies support the role of the thalamus in the pathophysiology of schizophrenia (Andreasen et al., 1994).
The cluster of GM reduction in patients with schizophrenia as compared to healthy controls within the right middle frontal region extended to the medial frontal region of the orbitofrontal cortex (OFC). By contrast, it extended to superior frontal region or dorsolateral prefrontal cortex (DLPFC) in bipolar I patients as compared to healthy controls. Structural neuroimaging shows that OFC volume changes are observed in schizophrenia with more severe negative symptoms (Baare et al., 1999) and social dysfunction (Gur et al., 2000). However, previous findings in OFC volumetry have been inconsistent, where some studies showed smaller OFC volumes in schizophrenia (Gur et al., 2000), while others reported increased OFC volumes in first-episode schizophrenia (Szeszko et al., 1999) or no difference when compared to healthy controls (Baare et al., 1999). Recently, Nakamura et al (2008) reported that smaller right OFC volumes within the schizophrenia group were associated with a longer duration of illness. Interestingly schizophrenic patients in Nakumura et al (2008) study were demographically very similar to the sample described in our study in terms of age and illness duration. Therefore, it is plausible that right OFC gray matter volume reduction might be associated with illness duration in chronic schizophrenia. In contrast to OFC, DLPFC has been shown to play an important role in the pathophysiology of the bipolar disorder (Chang et al., 2004). Specifically, DLPFC lesions have been shown to be associated with inattention and mood instability (Cummings, 1993) as well as symptom factors (Bench et al., 1993) associated with bipolar disorder. Furthermore, these findings are consistent with prior PET and magnetic resonance spectroscopy (MRS) studies that showed abnormal DLPFC metabolism in bipolar disorder (Baxter et al., 1985; Osuch et al., 2000; Winsberg et al., 2000). The loss of gray matter in this region may thus confer a neuroanatomical diathesis underlying bipolar disorder.
The results failed to support the hypothesis of reduced gray matter volume in the perigenual region of the anterior cingulate. Contrary to the hypothesis, schizophrenia patients had smaller mean perigenual anterior cingulate volumes compared with bipolar I patients. Similarly no differences in ventricular volume were observed, even though enlarged ventricles distinguished bipolar I and schizophrenia groups in one of the earliest studies comparing the two groups (Lim et al., 1999). It is possible that the present study’s small sample sizes reduced statistical power below the level needed to detect the perigenual and ventricular effects.
We acknowledge that our findings are based on a small, medicated sample and should be interpreted carefully. It is important to note that medication used in the current sample was appropriate to each diagnostic group (i.e., antipsychotics in schizophrenic and mood stabilizers in bipolar I patients), and polypharmacy, as well as anti-cholinergic use was low. Even though several prior reports suggested significant relationship between use of psychotropic and anti-depressant medication and reduced GM volumes within prefrontal regions in patients with bipolar disorder or schizophrenia compared to healthy controls (Almeida et al., 2009; Navari and Dazzan, 2009; Nugent et al., 2006), others did not find such relationship (Blumberg et al. 2006). Furthermore, a recent report showed no significant contribution of typical and/or atypical antipsychotics to GM reductions in schizophrenic group compared to healthy controls (Koutsouleris et al., 2008). Consistent with this, similar GM reductions in frontal and temporal regions as the ones observed here were recently reported in unmedicated subjects at high-risk for psychosis (Meisenzahl et al., 2008). Another factor that might potentially limit interpretation of the current results is the significantly lower education level in patients with schizophrenia compared to patients with bipolar disorder and healthy controls in our sample. Again, it is imperative to note that low education in schizophrenic group in our study is related to patients’ diagnosis. Although some evidence suggests a positive relationship between years of education and preserved white matter within inferior frontal areas (90), the effects of education on subcoritcal volumes, which showed significant differences between schizophrenic and bipolar patients in our study, have not been examined. Finally, we need to be cautious when explaining observations of GM volume difference between bipolar and schizophrenic groups in a cross-sectional study. Although some affected regions are compatible with results from previous structural and functional studies, the cause-effect relationship remains uncertain. Future studies should examine the effects of symptom profiles, disease progression, and medication on the pattern of regional gray matter volumes in these disorders.
In summary, schizophrenic compared to bipolar I patients matched for age, gender and duration of illness showed reduced GM volumes within right hippocampus, amygdala and putamen. In spite of several limitations, our results suggest differences in the affected gray matter volumes in patients with schizophrenia and bipolar disorder, even when the two groups are matched for age, gender, and illness duration. These structural abnormalities may be important factors in determining the differential diagnoses of these two functional psychotic disorders.
Acknowledgments
This research was supported by a VA Mental Illness Research, Education, and Clinical Center grant to the VA Desert Pacific Healthcare System and by an NIMH grant R01-MH57140.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler CM, Levine AD, Delbello MP, Strakowski SM. Changes in gray matter volume in patients with bipolar disorder. Biological Psychiatry. 2005;58:151–157. doi: 10.1016/j.biopsych.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Adler CM, DelBello MP, Jarvis K, Levine A, Adams J, Strakoski SM. Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biological Psychiatry. 2007;61:776–781. doi: 10.1016/j.biopsych.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Almeida JRC, Akkal D, Hassel S, Travis MJ, Banihashemi L, Kerr N, Kupfer DJ, Phillips ML. Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: Significant effects of gender and trait anxiety. Psychiatry Research. 2009;171:54–68. doi: 10.1016/j.pscychresns.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Swayze V, II, Cizadlo T, Flaum M, O’Leary D, Ehrhardt JC, Yuh WT. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J, Wang L, Csernansky JG, Gado MH, Barch DM. Abnormalities of thalamic activation and cognition in schizophrenia. American Journal of Psychiatry. 2006;163:463–469. doi: 10.1176/appi.ajp.163.3.463. [DOI] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. The British Journal of Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry: The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baare WF, Hulshoff Pol HE, Hijman R, Mali WP, Viergever MA, Kahn RS. Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive function and symptomatology. Biological Psychiatry. 1999;45:1597–1605. doi: 10.1016/s0006-3223(98)00266-2. [DOI] [PubMed] [Google Scholar]
- Baumann B, Bogerts B. The pathomorphology of schizophrenia and mood disorders: similarities and differences. Schizophrenia Research. 1999;39:141–148. doi: 10.1016/s0920-9964(99)00113-9. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Phelps ME, Mazziotta JC, Schwartz JM, Gerner RH, Selin CE. Cerebral metabolic rates for glucose in mood disorders: Studies with positron emission tomography and fluorodeoxyglucose F18. Archives of General Psychiatry. 1985;42:441–447. doi: 10.1001/archpsyc.1985.01790280019002. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Frackowiak RS, Dolan RJ. Regional cerebral blood flow in depression measured by positron emission tomography: The relationship with clinical dimensions. Psychological Medicine. 1993;23:579–590. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- Bose SK, Mackinnon T, Mehta MA, Turkheimer FE, Howes OD, Selvaraj S, Kempton MJ, Grasby PM. The effect of ageing on grey and white matter reductions in schizophrenia. Schizophrenia Research. 2009;112:7–13. doi: 10.1016/j.schres.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Brandt GN, Bonelli RM. Structural neuroimaging of the basal ganglia in schizophrenic patients: a review. Wiener Medizinische Wochenschrift. 2008;158:84–90. doi: 10.1007/s10354-007-0478-7. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Krystal JH, Bansal R, Martin A, Dziura J, Durkin K, et al. Age, Rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: A cross-sectional study. Biological Psychiatry. 2006;59:611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Someya T, Teng CY, Abel L, Chin S, Najafi A, et al. PET and MRI of the thalamus in never-medicated patients with schizophrenia. American Journal of Psychiatry. 1996;153:191–199. doi: 10.1176/ajp.153.2.191. [DOI] [PubMed] [Google Scholar]
- Byne W, Kidkardnee S, Tatusov A, Yiannoulos G, Buchbaum MS, Haroutunian V. Schizophrenia-associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nucleus. Schizophrenia Research. 2006;85:245–253. doi: 10.1016/j.schres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Schizophrenia: A subcortical neurotransmitter imbalance syndrome? Schizophrenia Bulletin. 1990;16:425–432. doi: 10.1093/schbul/16.3.425. [DOI] [PubMed] [Google Scholar]
- Chalmers TC. Meta-analysis in clinical medicine. Transactions of the American Clinical and Climatological Association. 1988;99:144–150. [PMC free article] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder. Archives of General Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1988. [Google Scholar]
- Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Transactions on Medical Imaging. 2005;24:1548–1565. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar and schizophrenia: A meta-analysis. Schizophrenia Research. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Picchioni M, Landau S, Matsumoto K, van Haren NE, Marshall N, Hall MH, Schulze K, Toulopoulou T, Davies N, Ribchester T, McGuire PK, Murray RM. Magnetic resonance imaging of the thalamus and adhesio interthalamica in twins with schizophrenia. Archives of General Psychiatry. 2007;64:401–409. doi: 10.1001/archpsyc.64.4.401. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Gunduz H, Wu H, Ashtari M, Bogerts B, Crandall D, Robinson DG, Alvir J, Lieberman J, Kane J, Bilder R. Basal ganglia volumes in first-episode schizophrenia and healthy comparison subjects. Biological Psychiatry. 2002;51:801–808. doi: 10.1016/s0006-3223(01)01345-2. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Archives of General Psychiatry. 2000;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. American Journal of Psychiatry. 1999;155:1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. American Journal of Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Gaser C, Jager M, Bottlender R, Frodl T, Holzinger S, Schmitt GJ, Zetzsche T, Burgermeister B, Scheuerecker J, Born C, Reiser M, Möller HJ, Meisenzahl EM. Structural correlates of psychopathological symptom dimensions in schizophrenia: a voxel-based morphometric study. Neuroimage. 2008;39:1600–1612. doi: 10.1016/j.neuroimage.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Lang DJ, Kopala LC, Vandorpe RA, Rui Q, Smith GN, Goghari VM, Lapointe JS, Honer WG. Reduced basal ganglia volumes after switching to olanzapine in chronically treated patients with schizophrenia. American Journal of Psychiatry. 2004;161:1829–1836. doi: 10.1176/ajp.161.10.1829. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. British Journal of Psychiatry. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data Sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach Atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead WJ, Harrison LK, Forrester K, Lawrie SM, Johnstone EC. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biological Psychiatry. 2004;56:544–552. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead WJ, Harrison LK, Whalley HC, Johnston EC, Lawrie SM. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2006;141B:76–83. doi: 10.1002/ajmg.b.30254. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Koutsouleris N, Gaser C, Bottlender R, Schmitt GJE, McGuire P, Decker P, Burgermeister B, Born C, Reiser M, Möller HJ. Structural brain alterations in subjects at high-risk of psychosis: a voxel-based morphometric study. Schizophrenia Research. 2008;102:150–162. doi: 10.1016/j.schres.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, Levitt JJ, Cohen AS, Kawashima T, Shenton ME, McCarley RW. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain. 2008;131:180–195. doi: 10.1093/brain/awm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychological Medicine. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Rirdan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Archives of General Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nesvåg R, Saetre P, Lawyer G, Jönsson EG, Agartz I. The relationship between symptom severity and regional cortical and grey matter volumes in schizophrenia. Progress in Neuropsychopharmacology & Biological Psychiatry. 2009;33:482–490. doi: 10.1016/j.pnpbp.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, Zarate CA, Pine DS, Price JL, Drevets WC, et al. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. NeuroImage. 2006;30:485–97. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Archives of General Psychiatry. 1990;47:1023–1028. doi: 10.1001/archpsyc.1990.01810230039007. [DOI] [PubMed] [Google Scholar]
- Osuch EA, Ketter TA, Kimbrell TA, George MS, Benson BE, Willis MW. Regional cerebral metabolism associated with anxiety symptoms in affective disorder patients. Biological Psychiatry. 2000;48:1020–1023. doi: 10.1016/s0006-3223(00)00920-3. [DOI] [PubMed] [Google Scholar]
- Ridgway GR, Henley SMD, Rohrer JD, Scahill RI, Warren JD, Fox NC. Ten simple rules for reporting voxel-based morphometry studies. Neuroimage. 2008;40:1429–1435. doi: 10.1016/j.neuroimage.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvǻ R, Fennema-Notestine C, Hagler DJ, Jr, Pung CJ, Jennings RG, Haukvik UK, La Nakstad PH, Melle I, Andreassen OA, Dale AM, Agartz I. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biological Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Sarnicola A, Kempton M, Germanà C, Haldane M, Hadjulis M, Christodoulou T, Koukopoulos A, Girardi P, Tatarelli R, Frangou S. No differential effect of age on brain matter volume and cognition in bipolar patients and healthy individuals. Bipolar Disorder. 2009;11:316–322. doi: 10.1111/j.1399-5618.2009.00670.x. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Spinks R, Magnotta VA, Andreasen NC, Albright KC, Ziebell S, Nopoulos P, Cassell M. Manual and automated measurement of the whole thalamus and mediodorsal nucleus using magnetic resonance imaging. NeuroImage. 2002;17:631–42. [PubMed] [Google Scholar]
- Stratta P, Mancini F, Mattei P, Daneluzzo E, Casacchia M, Rossi A. Association between striatal reduction and poor Wisconsin card sorting test performance in patients with schizophrenia. Biological Psychiatry. 1997;42:816–820. doi: 10.1016/s0006-3223(97)00017-6. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Bilder RM, Lencz T, Pollack S, Alvir JM, Ashtari M, Ashtari M, Wu H, Lieberman JA. Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Research. 1999;90:1–15. doi: 10.1016/s0925-4927(99)00002-5. [DOI] [PubMed] [Google Scholar]
- Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: First results. Psychiatry Research. 2004;131:57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Winsberg ME, Sachs N, Tate DL, Adalsteinsson E, Spielman D, Ketter TA. Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder. Biological Psychiatry. 2000;47:475–481. doi: 10.1016/s0006-3223(99)00183-3. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Sugihara G, Matsumoto H, Suckling J, Nishimura K, Toyoda T, Isoda H, Tsuchiya KJ, Takebayashi K, Suzuki K, Sakahara H, Nakamura K, Mori N, Takei N. Voxel-based structural magnetic resonance imaging (MRI) study of patients with early onset schizophrenia. Annals of General Psychiatry. 2008;7:25. doi: 10.1186/1744-859X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]


