Abstract
In functional magnetic resonance imaging (fMRI) studies, researchers often attempt to ensure that group differences in brain activity are not confounded with group differences in mean reaction time (RT). However, even when groups are matched for performance, they may differ in terms of the RT-BOLD relationship: the degree to which brain activity varies with RT on a trial-by-trial basis. Group activation differences might therefore be influenced by group differences in the relationship between brain activity and time on task. Here, we investigated whether correcting for this potential confound alters group differences in brain activity. Specifically, we reanalyzed data from a functional MRI study of response conflict in children and adults, in which conventional analyses indicated that conflict-related activity did not differ between groups. We found that the RT-BOLD relationship was weaker in children than in adults. Consequently, after removing the effect of RT on brain activity, children exhibited greater conflict-related activity than adults in both the posterior medial prefrontal cortex and the right dorsolateral prefrontal cortex. These results identify the RT-BOLD relationship as an important potential confound in fMRI studies of group differences. They also suggest that the magnitude of the RT-BOLD relationship may be a useful biomarker of brain maturity.
Keywords: fMRI, RT-BOLD relationship, development, response conflict, posterior medial prefrontal cortex
Introduction
Functional neuroimaging is becoming an increasingly popular tool for studying group differences. For example, recent studies have identified differences in brain activity between children and adults (Church et al., 2008), psychiatric patients and controls (Glahn et al., 2005), and Americans and East Asians (Han and Northoff, 2008). These studies have revealed fundamental group differences in the neural substrates of executive control, emotional processing, and social cognition.
However, group differences in brain activity are sometimes confounded with group differences in behavioral performance. For example, children sometimes respond more slowly than adults (Gaillard et al., 2001), and patients sometimes perform more poorly than controls (Kerns et al., 2005). Such confounds complicate the interpretation of group activation differences: do they reflect genuine differences in brain function or differences in behavioral performance? To avoid such ambiguity, Church and colleagues (2010) recommend several strategies to address potential group differences in behavioral performance. For example, investigators may calibrate task parameters to hold performance constant across groups, identify matched sub-groups of participants after data collection, or include performance differences as covariates during data analysis.
While each of these strategies can effectively match groups for mean behavioral performance in fMRI studies, none can ensure that groups are matched for the RT-BOLD relationship: the degree to which activity varies with RT on a trial-by-trial basis. In adults, trials with relatively slow RTs are associated with relatively high activity in a fronto-parietal network that is linked to attention and executive control (Hahn et al., 2007; Prado et al., 2011; Weissman et al., 2006). This effect has been observed across a diverse range of cognitive tasks (Yarkoni et al., 2009). Thus, fronto-parietal activity may be sensitive to demands on task-general cognitive processes (e.g., sustained attention, arousal, or effort) whose recruitment increases with time on task. In line with this possibility, conditional differences in brain activity in the fronto-parietal network are sometimes eliminated after using the RT-BOLD relationship to remove the effect of RT on brain activity (Carp et al., 2010; Grinband et al., 2011). Such effects can make differences in brain activity challenging to interpret when conditions differ in mean RT. In these situations, do activation differences reflect (1) task-specific processes that are hypothesized to vary across conditions or (2) task-general processes whose recruitment varies with time on task (Yarkoni et al., 2009)? This issue has stirred significant controversy in the past year, with different investigators drawing strikingly different conclusions from similar data (Brown, 2011; Grinband et al., 2011; Nachev, 2011; Yeung et al., 2011).
Similarly, group differences in the RT-BOLD relationship could complicate the interpretation of group activation differences. If the RT-BOLD relationship differs across groups, then removing the effect of RT on conditional differences in brain activity will alter activity more in some groups than in others. Thus, group activation differences could be magnified, reduced, or even newly revealed where none were previously observed. Such findings might dramatically alter conclusions about the nature of group differences in brain activity. To our knowledge, however, no prior study has investigated whether the RT-BOLD relationship differs across groups, or the impact of removing this potential confound on group activation differences.
The present study thus investigated whether the RT-BOLD relationship varies across groups and, if so, whether removing the effect of RT on activity alters group activation differences. To this end, we reanalyzed a prior fMRI study investigating the neural substrates of conflict processing in children and adults (Fitzgerald et al., 2010). The original study investigated developmental differences in conflict-related activity in the posterior medial prefrontal cortex (pMFC), a region of the fronto-parietal network that has been implicated in conflict detection (Botvinick et al., 1999; Carter et al., 1998). The results indicated that children and adults were well matched for both overall RT and RT differences between conditions. Further, the groups did not differ with regard to conflict-related pMFC activity. A plausible conclusion, suggested by Fitzgerald and colleagues, was that the neural substrates of conflict processing are equivalent in children and adults. However, their analysis did not assess possible group differences in the RT-BOLD relationship.
In this re-analysis, we hypothesized that the RT-BOLD relationship in the pMFC would be weaker in children than in adults. This hypothesis was motivated by recent findings that impaired attention and executive control in sleep deprived individuals co-occur with an attenuated RT-BOLD relationship in the fronto-parietal network (Chee et al., 2008). Further, the RT-BOLD relationship is weakest in subjects who exhibit the largest reductions of accuracy following sleep deprivation (Chee and Tan, 2010). These results suggest that individuals with relatively weak executive control are less able to recruit task-general attentional or executive processes as RT slows, leading to an attenuated RT-BOLD relationship in fronto-parietal regions. Given these results, findings that children exhibit weaker executive control than adults (Verhaeghen and Cerella, 2002) suggest that children may also show a weaker RT-BOLD relationship than adults. Based on this hypothesis, we predicted that removing the effect of RT on pMFC activity would reduce conflict-related activity less in children than in adults. Consequently, we hypothesized that removing this confound would reveal greater conflict-related activity in children than in adults, even though no developmental difference in conflict-related activity was observed in the original study.
Methods
Participants
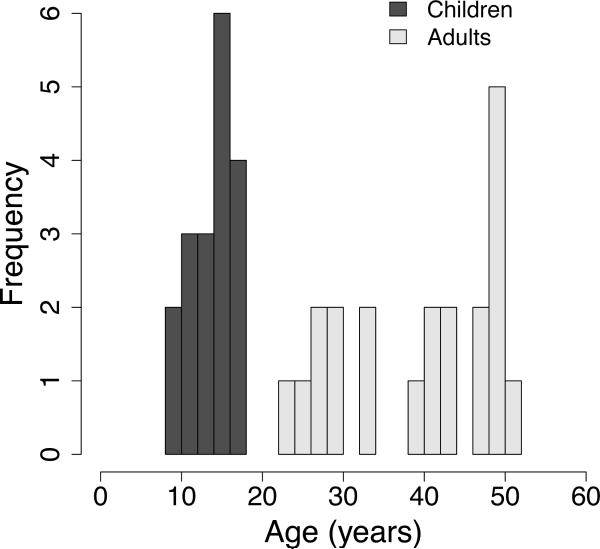
Eighteen children (mean age = 14.0 years, range 8–18 years, eight female) and twenty-one adults (mean age = 39.8 years, range 23–51 years, six female) participated in the experiment (Figure 1). Participants were screened for neurological or psychiatric illness, head trauma, and mental retardation. Analyses of these data unrelated to the present investigation were described in previous reports (Carp et al., 2010; Fitzgerald et al., 2010; Stern et al., 2009).
Figure 1.
Age range of the sample.
Task
Participants performed an event-related version of the Multi-Source Interference Task (MSIT; Bush et al., 2003), which is described only briefly here; a more detailed description can be found in the original study reporting these data (Fitzgerald et al., 2010). In each trial, participants were required to identify the unique digit (1, 2, or 3) among a set of three alphanumeric characters. The digits 1, 2, and 3 were mapped to the thumb, index finger, and middle finger of the right hand, respectively. In congruent trials, the unique digit appeared among letters (e.g., “x2x”), and its position (e.g., center) was compatible with its associated response (e.g., index finger). In incongruent trials, the unique digit appeared among digits (e.g., “311”), and its position (e.g., left) was incompatible with its associated response (e.g., middle finger).
Participants performed five runs of the MSIT. Each run included 24 incongruent trials and 24 congruent trials. Each alphanumeric stimulus was presented for 500 ms, followed by a 2500 ms fixation cross. Twelve 3000-ms fixation trials were randomly interspersed among the 48 MSIT trials in each run.
Data acquisition
Neuroimaging data were collected during task performance using a 3T GE Signa MRI scanner. Functional images were acquired using a reverse spiral sequence (repetition time [TR = 2000 ms, echo time [TE] = 30 ms, flip angle [FA] = 90°, field of view [FOV] = 20 cm). Each functional image included 40 3-mm slices with an in-plane resolution of 3.44 by 3.44 mm. Ninety-four images were collected in each functional run. The first four images were discarded to allow for the equilibration of the BOLD signal. High-resolution T1-weighted anatomical images (3D SPGR, slice thickness 1.5 mm, 0 skip) were also collected to facilitate subsequent spatial normalization of the functional images to Montreal Neurological Institute (MNI) space.
Data pre-processing
Functional images were slice-time corrected, realigned to the first volume, spatially normalized to the MNI brain atlas, and spatially smoothed using a Gaussian kernel (FWHM = 6 mm) as described by Fitzgerald and colleagues (2010).
Data analysis
Functional images were analyzed using the general linear model in SPM5 (Wellcome Department of Cognitive Neurology, London, UK, www.fil.ion.ucl.ac.uk). BOLD responses evoked by congruent and incongruent trials were modeled with separate regressors. We also included two parametric regressors, one for congruent trials and one for incongruent trials, to model the trial-by-trial RT-BOLD relationship (Weissman et al., 2006). Each parametric regressor was mean-centered, rendering it orthogonal to its corresponding activity regressor. Incorrect trials, trials with no responses, and trials in which the RT was greater than three standard deviations from the conditional mean were modeled separately and discarded from subsequent analyses. In total, 11.44% of the trials were excluded in children and 3.55% of the trials were excluded in adults.
Whole-brain analyses used a height threshold of p < 0.005 and an extent threshold of k >= 30 voxels. Monte Carlo simulations using the Resting-State fMRI Data Analysis Toolkit (REST, Song Xiao-Wei et al., http://www.restfmri.net) showed that these thresholds yielded a corrected cluster-wise false positive rate of p < 0.01. This empirical threshold derivation procedure maintains precise control of the cluster-wise false positive rate and increases sensitivity up to fivefold relative to methods that rely solely on voxel-level thresholds (Forman et al., 1995).
Removing the effect of RT on activity
For each participant, we used a multiple regression approach to estimate and remove the effect of conditional differences in mean RT on conditional differences in brain activity between incongruent and congruent trials (i.e., conflict-related activity) (Carp et al., 2010). First, for each voxel, we estimated the slope of the RT-BOLD relationship (βRT) in correct congruent trials. Second, we estimated the amount of activity that would have been present in correct congruent trials whose RTs equaled the mean RT in correct incongruent trials (RT-equated congruent trials, CongruentEQ). To do so, we multiplied each voxel's RT-BOLD slope by the difference in mean RT between incongruent and congruent trials and added the result to the regression-derived estimate of mean activity in correct congruent trials (Congruent). Thus, for each voxel, we calculated activity in RT-equated congruent trials using the following formula:
| (1) |
Next, we used this estimate of CongruentEQ to remove the effect of conditional differences in mean RT on conflict-related activity. Specifically, we estimated RT-equated conflict-related activity (ConflictEQ) by comparing mean activity in incongruent trials to activity in RT-equated congruent trials using the following formula:
| (2) |
This approach assumes a linear relationship between RT and brain activity. To verify this assumption, we tested for quadratic, cubic, and quartic effects of RT on activity in a separate analysis, as in our previous work (Prado et al., 2011).
Region of Interest (ROI) analysis
We also conducted region of interest (ROI) analyses in brain regions that are thought to underlie the detection and resolution of response conflict. Prior studies have linked the posterior medial frontal cortex (pMFC) to the detection of response conflict (e.g., Botvinick et al., 1999; van Veen et al., 2001). We therefore investigated conflict-related activity in the pMFC (x = 2, y = 16, z = 46). In addition, computational models of conflict processing posit that conflict detection in the pMFC recruits conflict resolution mechanisms in the dorsolateral prefrontal cortex (DLPFC; Botvinick et al., 2001; Yeung et al., 2004). Thus, we also investigated conflict-related activity in both the left DLPFC (x = −40, y = 26, z = 30) and the right DLPFC (x = 42, y = 24, z = 28). ROIs were defined as spheres of 8 mm in radius; coordinates were obtained from a recent meta-analysis of studies investigating the neural correlates of interference resolution (Nee et al., 2007).
Results
Behavioral data
Behavioral data were analyzed using mixed ANOVAs including a within-subjects factor of congruency (congruent, incongruent) and a between-subjects factor of age group (children, adults). Descriptive statistics are presented in Table 1.
Table 1.
Behavioral performance as a function of congruency and age group.
| Reaction Time (ms) | Accuracy (% correct) | |||
|---|---|---|---|---|
| Children | Adults | Children | Adults | |
| Congruent | 737.3 ± 50.1 | 804.6 ± 43.0 | 97.88 ± 0.69 | 99.31 ± 0.23 |
| Incongruent | 1020.6 ± 82.1 | 1041.9 ± 42.9 | 82.80 ± 3.57 | 95.32 ± 1.31 |
As described in the original report of these data (Fitzgerald et al., 2010), mean RTs were significantly slower in incongruent than in congruent trials (F(1, 37) = 181.27, p < 0.001). Mean RT did not vary between age groups (F < 1), nor did the congruency by age group interaction approach significance (F(1, 37) = 1.42, p = 0.24).
As reported by Fitzgerald and colleagues (2010), response accuracy was significantly lower in incongruent than in congruent trials (F(1, 37) = 32.46, p < 0.001). In addition, children responded less accurately than adults (F(1, 37) = 12.53, p = 0.0011). These main effects were qualified by an interaction between congruency and age group (F(1, 37) = 10.98, p = 0.0021): the reduction in accuracy in children, relative to adults, was more pronounced in incongruent than in congruent trials (see Table 1).
Functional MRI
Voxel-wise analyses
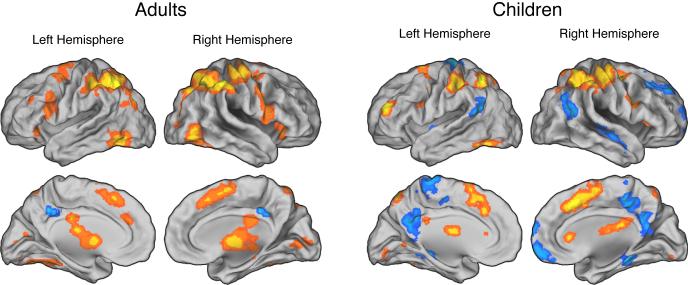
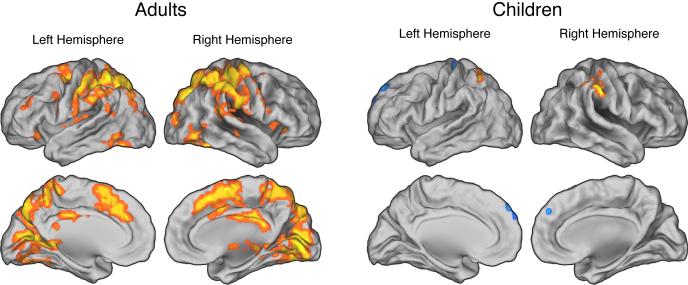
As reported by Fitzgerald et al. (2010), incongruent trials evoked significantly greater activity than congruent trials throughout the fronto-parietal network in both children and adults (Table 2; Figure 2). In addition, as described by Carp and colleagues (2010), trials with relatively slow RTs were associated with relatively high fronto-parietal activity in adults (Table 3; Figure 3). As predicted, however, the present analysis revealed that the RT-BOLD relationship was largely absent in children, among whom effects of RT on brain activity were relatively weak and restricted to bilateral regions of the posterior parietal cortex (Table 3; Figure 3). Finally, consistent with prior work (Prado et al., 2011), we observed no higher-order relationships between RT and brain activity in either children or adults (i.e., we observed no significant second-, third-, or fourth-order effects of RT on activity).
Table 2.
Effects of response conflict on brain activity.
| Region | Number of voxels | MNI Coordinates | Peak t-value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Brain regions showing higher activation for incongruent than congruent trials in adults. | |||||
| Medial frontal gyrus | 349 | −3 | 18 | 51 | 5.70 |
| Medial frontal gyrus | 349 | 0 | −3 | 60 | 4.90 |
| L. inferior frontal gyrus | 157 | −33 | 27 | −6 | 4.10 |
| R. inferior frontal gyrus | 3218 | 36 | 24 | 0 | 5.52 |
| L. middle frontal gyrus | 131 | −39 | 0 | 60 | 4.85 |
| R. middle frontal gyrus | 3218 | 33 | −6 | 63 | 6.82 |
| L. inferior parietal lobule | 3218 | −42 | −51 | 51 | 6.35 |
| R. inferior parietal lobule | 3218 | 36 | −51 | 45 | 8.41 |
| L. thalamus | 2609 | −12 | −6 | 9 | 4.26 |
| R. thalamus | 2609 | 9 | −9 | 6 | 6.16 |
| L. middle occipital gyrus | 2609 | −51 | −60 | −9 | 4.97 |
| R. middle occipital gyrus | 2609 | 45 | −69 | 0 | 5.75 |
| Brain regions showing higher activation for incongruent than congruent trials in children. | |||||
| Cingulate gyrus | 2633 | 3 | 15 | 45 | 4.72 |
| L. middle frontal gyrus | 2633 | −27 | 0 | 66 | 3.87 |
| R. middle frontal gyrus | 2633 | 33 | −3 | 63 | 3.90 |
| L. middle frontal gyrus | 52 | −54 | 6 | 42 | 3.92 |
| R. inferior frontal gyrus | 100 | 48 | 3 | 30 | 4.48 |
| L. middle frontal gyrus | 131 | −36 | 42 | 24 | 5.44 |
| L. thalamus | 128 | −3 | −12 | 15 | 5.58 |
| L. inferior parietal lobule | 2633 | −48 | −36 | 45 | 5.10 |
| R. inferior parietal lobule | 2633 | 42 | −45 | 51 | 5.97 |
| L. superior parietal lobule | 2633 | −6 | −72 | 57 | 4.61 |
| L. fusiform gyrus | 98 | −48 | −60 | −18 | 4.59 |
Figure 2.
Effects of response conflict on brain activity. In both adults (left panels) and children (right panels), incongruent trials evoked greater activity than congruent trials in a widespread network of frontal and parietal regions. All maps are thresholded at p < 0.005 and 30 contiguous voxels.
Table 3.
Effects of trial-by-trial variations in RT on brain activity.
| Region | Number of voxels | MNI Coordinates | Peak t-value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Brain regions showing higher activity for slow versus fast trials in adults. | |||||
| Medial frontal gyrus | 349 | −3 | 18 | 51 | 5.70 |
| Medial frontal gyrus | 349 | 0 | −3 | 60 | 4.90 |
| L. inferior frontal gyrus | 157 | −33 | 27 | −6 | 4.10 |
| R. inferior frontal gyrus | 3218 | 36 | 24 | 0 | 5.52 |
| L. middle frontal gyrus | 131 | −39 | 0 | 60 | 4.85 |
| R. middle frontal gyrus | 3218 | 33 | −6 | 63 | 6.82 |
| L. inferior parietal lobule | 3218 | −42 | −51 | 51 | 6.35 |
| R. inferior parietal lobule | 3218 | 36 | −51 | 45 | 8.41 |
| L. thalamus | 2609 | −12 | −6 | 9 | 4.26 |
| R. thalamus | 2609 | 9 | −9 | 6 | 6.16 |
| L. middle occipital gyrus | 2609 | −51 | −60 | −9 | 4.97 |
| R. middle occipital gyrus | 2609 | 45 | −69 | 0 | 5.75 |
| Brain regions showing higher activity for slow versus fast trials in children. | |||||
| L. superior parietal lobule | 36 | −33 | −57 | 54 | 4.12 |
| R. inferior parietal lobule | 157 | 48 | −36 | 57 | 4.83 |
| R. superior parietal lobule | 32 | 24 | −63 | 39 | 3.73 |
Figure 3.
Effects of trial-by-trial variations in reaction time (RT) on brain activity. In adults (left panels), relatively slow RTs were associated with increased activity throughout the fronto-parietal network. In contrast, the RT-BOLD relationship was reduced in children (right panels) who exhibited only weak effects of RT on brain activity, which were confined to lateral parietal cortex. All maps are thresholded at p < 0.005 and 30 contiguous voxels.
ROI analyses in the pMFC
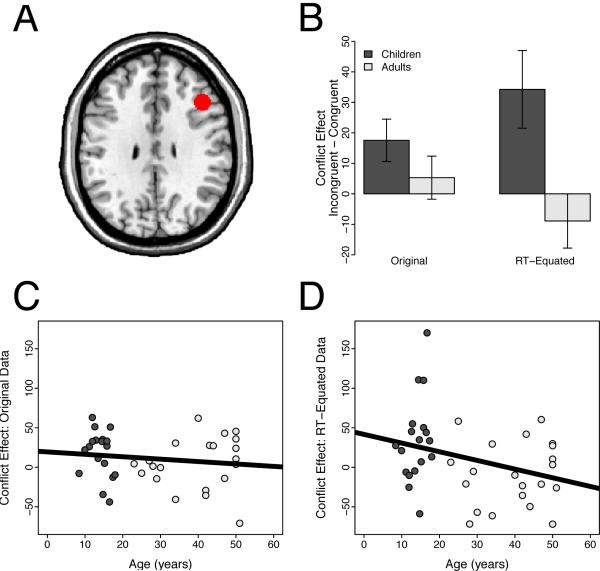
As described by Fitzgerald et al. (2010), incongruent trials evoked greater pMFC activity than congruent trials in both children (t(17) = 5.16, p < 0.001) and adults (t(20) = 4.99, p < 0.001). Moreover, as reported by Fitzgerald et al. (2010), conflict-related pMFC activity did not differ between children and adults (t(37) = 1.65, n.s.). As hypothesized, however, the present analysis revealed that the RT-BOLD relationship was significantly weaker in children than in adults (t(37) = 2.65, p = 0.012). Specifically, RT did not modulate brain activity in children (t(17) = −0.33, n.s.). In contrast, as we reported in a prior reanalysis of these data (Carp et al., 2010), adults showed a positive relationship between RT and brain activity (t(20) = 4.06, p < 0.001). Thus, consistent with our first prediction, the RT-BOLD relationship was significantly attenuated in children, relative to adults.
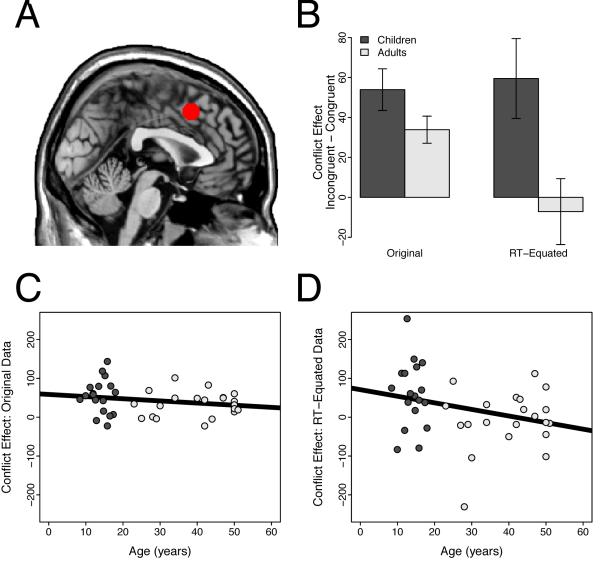
Given this developmental difference in the RT-BOLD relationship, we next explored whether correcting for this confound would reveal a group difference in conflict-related pMFC activity. As predicted, after removing the effect of RT on activity, we observed significantly greater conflict-related pMFC activity in children than in adults (t(37) = 2.59, p = 0.014; Figure 4). This group difference reflected the fact that conflict-related activity remained robust after removing the effect of RT on activity in children (t(17) = 2.98, p = 0.0085), but, as reported by Carp et al. (2010), was eliminated in adults (t(20) = −0.43, n.s.).
Figure 4.
Region of interest analyses in the posterior medial frontal cortex (pMFC). Panel A: The pMFC ROI, overlaid on the ch2bet template in MNI space. Panel B: Conflict-related activity in pMFC did not differ for children and adults before removing the effect of RT on brain activity (t(37) = 1.65, n.s.). However, after removing for this effect, children showed significantly greater conflict-related activity than adults (t(37) = 2.59, p = 0.014). Error bars show the standard error of the mean. Panel C: Developmental trajectory of the conflict effect in the pMFC using the original data. Panel D: Developmental trajectory of the conflict effect in the pMFC using the RT-equated data.
ROI analyses in the DLPFC
We next performed analogous analyses in bilateral regions of the DLPFC, which are thought to participate in resolving response conflict (Botvinick et al., 2001; Yeung et al., 2004). Analyses of these ROIs were unique to the present report.
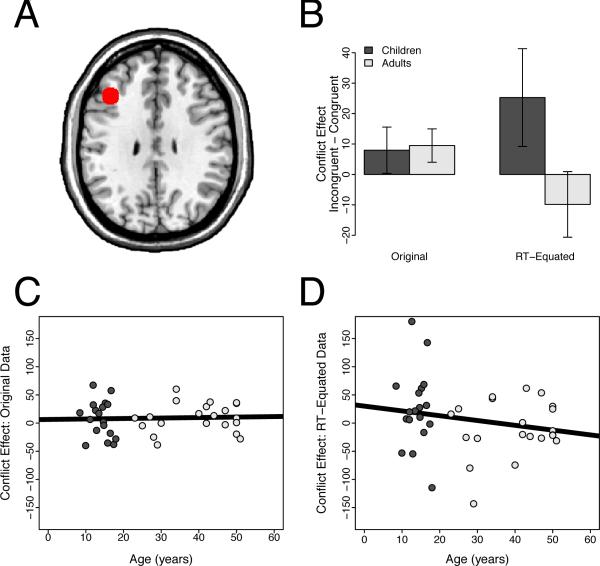
In the right DLPFC, we observed significant conflict-related activity in children (t(17) = 2.53, p = 0.022) but not in adults (t(20) = 0.75, n.s.). However, conflict-related activity did not differ between children and adults (t(37) = 1.23, n.s.). Nonetheless, as hypothesized, the RT-BOLD relationship was significantly weaker in children than in adults (t(37) = 2.23, p = 0.032). Indeed, as in the pMFC, we observed no relationship between RT and brain activity in children (t(17) = −1.11, n.s.). In contrast, we observed a significant positive RT-BOLD relationship in adults (t(20) = 2.31, p = 0.032). Consequently, removing the effect of RT on activity revealed greater conflict-related activity in children than in adults (t(37) = 2.84, p = 0.0072; Figure 5).
Figure 5.
Region of interest analyses in the right dorsolateral prefrontal cortex (DLPFC). Panel A: The right DLPFC ROI, overlaid on the ch2bet template in MNI space. Panel B: Conflict-related activity in the right DLPFC did not differ for children and adults before removing the effect of RT on brain activity (t(37) = 1.23, n.s). However, after removing this effect, children showed significantly greater conflict-related activity than adults (t(37) = 2.84, p = 0.0072). Error bars show the standard error of the mean. Panel C: Developmental trajectory of the conflict effect in the right DLPFC using the original data. Panel D: Developmental trajectory of the conflict effect in the right DLPFC using the RT-equated data.
In the left DLPFC, we found that neither children (t(17) = 1.05, n.s.) nor adults (t(20) = 1.73, n.s.) exhibited significant conflict-related activity. Further, consistent with Fitzgerald et al. (2010), conflict-related activity did not vary with age group (t(37) = −0.17, n.s.). As predicted, however, the RT-BOLD relationship was significantly weaker in children than in adults (t(37) = 3.01, p = 0.0047). In particular, we observed no RT-BOLD relationship in children (t(17) = −1.57, n.s.) while adults showed a robust positive RT-BOLD relationship (t(20) = 3.28, p = 0.0037). Finally, as expected, removing the effect of RT on left DLPFC activity revealed a marginally significant trend for greater conflict-related activity in children than in adults (t(37) = 1.86, p = 0.071; Figure 6).
Figure 6.
Region of interest analyses in the left dorsolateral prefrontal cortex (DLPFC). Panel A: The left DLPFC ROI, overlaid on the ch2bet template in MNI space. Panel B: Conflict-related activity in the left DLPFC did not differ for children and adults before removing the effect of RT on brain activity (t(37) = −0.17, n.s.). After removing this effect, children showed marginally significantly greater conflict-related activity than adults (t(37) = 1.86, p = 0.071). Error bars show the standard error of the mean. Panel C: Developmental trajectory of the conflict effect in the left DLPFC using the original data. Panel D: Developmental trajectory of the conflict effect in the left DLPFC using the RT-equated data.
Discussion
When assessing group differences in brain activity, researchers often take great pains to hold potentially confounding variables constant. For example, researchers may equate groups for average behavioral performance (e.g., Church et al., 2008; Schlaggar et al., 2002), intelligence (Eden et al., 1996), or age (Manoach et al., 2000). Nonetheless, previous studies have not explored whether the RT-BOLD relationship varies across groups and, if so, whether correcting for this potential confound alters group activation differences.
To investigate these issues, we reanalyzed a functional MRI study of developmental differences in conflict processing (Fitzgerald et al., 2010). As predicted, the RT-BOLD relationship was weaker in children than in adults in brain regions that have been implicated in conflict processing. Removing the effect of RT on activity in these regions therefore did not reduce conflict-related activity in children, but wholly eliminated such activity in adults. Consequently, after removing the effect of RT on activity, we observed greater conflict-related activity in children than in adults in both the pMFC and the right DLPFC. Given that these groups showed equivalent conflict-related pMFC activity in conventional group contrasts (Fitzgerald et al., 2010), our findings identify the RT-BOLD relationship as a potentially important confound in functional MRI studies of group activation differences. These results also suggest that the magnitude of the RT-BOLD relationship may be a useful biomarker of brain maturity.
Developmental differences in the RT-BOLD relationship
While adults showed a robust positive relationship between RT and brain activity throughout a network of frontal and parietal brain regions, this pattern was largely absent in children. Indeed, children exhibited only weak effects of RT that were restricted to lateral parietal regions. Furthermore, relative to adults, the RT-BOLD relationship in children was significantly attenuated in both the pMFC and bilateral DLPFC. The weaker RT-BOLD relationship in children dovetails with prior work indicating that (1) executive control is less efficient in children than in adults (Verhaeghen and Cerella, 2002) and (2) less efficient executive control is linked to a weaker RT-BOLD relationship in frontal and parietal regions (Chee et al., 2008).
What developmental differences in executive control could account for the attenuated RT-BOLD relationship in children? Yarkoni et al. (2009) suggested that the RT-BOLD relationship in frontal and parietal regions reflects demands on sustained attention. More specifically, they posited that activity in fronto-parietal regions related to sustaining attention is maintained in each trial until a response is made, thereby leading to a positive relationship between brain activity and time on task. From this perspective, the weaker RT-BOLD relationship in children may indicate that children have reduced attentional resources, relative to adults. Consistent with this view, children show reduced activity in brain regions implicated in attentional alerting, orienting, and control (Konrad et al., 2005). Alternatively, activity in fronto-parietal regions may simply be less sensitive to demands on sustained attention in children than in adults. Future studies exploring the link between sustained attention and the RT-BOLD relationship may therefore help to clarify the developmental differences we have observed.
Developmental differences in the RT-BOLD relationship might also reflect age differences in conflict processing. According to the conflict monitoring model of cognitive control, trials with relatively long RTs are associated with greater response conflict than trials with relatively short RTs. Thus, they are thought to make greater demands on conflict detection processes implemented by the pMFC (Yeung et al., 2011). Our finding that pMFC activity increases with RT more in adults than in children therefore suggests a group difference in conflict processing. This effect may indicate that variations of RT stem from variations of response conflict less in children than in adults. Alternatively, pMFC activity may be less sensitive to parametric variations of response conflict (as indexed by trial-by-trial variations of RT) in children than in adults. Consistent with this possibility, prefrontal activity increases with demands on response inhibition in adults, but not in children (Durston and Casey, 2006).
Quantitative versus qualitative activation differences
Yarkoni et al. (2009) argued that removing the effect of RT on activity distinguishes between quantitative and qualitative differences in brain activity. In particular, they suggested that activation differences that can be explained by conditional differences in mean RT are likely quantitative, meaning that different conditions recruit the same processes to varying degrees. In contrast, activation differences that cannot be explained by RT differences are likely qualitative, meaning that different conditions recruit distinct processes.
Following this logic, the present findings indicate that conflict-related pMFC activity is quantitative in adults but qualitative in children. This suggests that the two age groups performed the experimental task using different strategies. Consistent with this possibility, although mean RT did not vary with age group, error rates were significantly higher in children than in adults, especially in incongruent trials (see Table 1). These results imply that children showed a stronger preference for speed over accuracy than adults, possibly reflecting a greater reliance on pre-potent stimulus-response mappings.
To our knowledge, this is the first study to demonstrate that an activation difference can be quantitative in one group but qualitative in another. This result is consistent with prior research indicating developmental differences in neural responses to increased processing demands. For example, Crone and colleagues reported that increased demands on relational reasoning processes modulated prefrontal activity differently in children and adults (2009). The authors interpreted this effect as a group difference in the recruitment of common or distinct processes to meet increased processing demands. The present findings are therefore consistent with prior results suggesting that children and adults sometimes use different processing strategies.
Relevance of the present findings to developmental cognitive neuroscience
A number of previous studies have documented developmental differences in frontoparietal activity. For example, Rubia and colleagues (2006) reported increased frontoparietal activity in adults, relative to children, during task switching, response inhibition, and response conflict. Adults also exhibit greater DLPFC activity than children when maintaining (Klingberg et al., 2002) or manipulating (Crone et al., 2009) information in working memory. These results may be interesting to revisit in light of our finding that correcting for developmental differences in the RT-BOLD relationship can alter group activation differences. In particular, our results suggest that correcting for group differences in the RT-BOLD relationship might change the nature of previously reported developmental differences. Such changes might bring about important revisions to current theories in developmental cognitive neuroscience.
The present findings are also relevant to prior work showing that machine learning algorithms can predict individual differences in brain maturity using patterns of functional connectivity between regions (Dosenbach et al., 2010). These algorithms explain only about 55% of the total variance in age. Thus, augmenting them with information about the RT-BOLD relationship may enhance their predictive power.
Conclusion
The present findings identify the RT-BOLD relationship as a potentially important confound in functional MRI studies of group activation differences. Moreover, they indicate that when the RT-BOLD relationship varies across groups, removing the effect of RT on activity can alter the nature of group activation differences that are observed. This suggests that the conclusions of previous studies of group activation differences might change if the confounding effects of RT on activity were removed. We therefore encourage researchers to determine whether the RT-BOLD relationship varies across groups and, if so, to correct for the effects of this variability on group activation differences. Future research should also continue to explore the RT-BOLD relationship, with an eye to understanding its psychological underpinnings and potential use as a biomarker of brain maturation.
Acknowledgements
This research was supported by a National Defense Science & Engineering Graduate Fellowship to Joshua Carp, a National Institute of Mental Health (NIMH) grant to Kate Fitzgerald (1K23-MH082176), two NIMH grants to Stephan Taylor (R01-MH064148 and R01-MH071821), and by startup funds from the University of Michigan awarded to Daniel Weissman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brown J. Medial prefrontal cortex activity correlates with time-on-task: What does this tell us about theories of cognitive control? NeuroImage. 2011;57:314–315. doi: 10.1016/j.neuroimage.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The Multi-Source Interference Task: Validation study with fMRI in individual subjects. Molecular Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Carp J, Kim K, Taylor S, Fitzgerald K, Weissman D. Conditional differences in mean reaction time explain effects of response congruency, but not accuracy, on posterior medial frontal cortex activity. Frontiers in Human Neuroscience. 2010;4 doi: 10.3389/fnhum.2010.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chee M, Tan J. Lapsing when sleep deprived: Neural activation characteristics of resistant and vulnerable individuals. NeuroImage. 2010;51:835–843. doi: 10.1016/j.neuroimage.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Chee MW, Tan JC, Zheng H, Parimal S, Weissman DH, Zagorodnov V, Dinges DF. Lapsing during sleep deprivation is associated with distributed changes in brain activation. The Journal of Neuroscience. 2008;28:5519–5528. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J, Coalson R, Lugar H, Petersen S, Schlaggar B. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cerebral Cortex. 2008;18:2054–2065. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J, Petersen S, Schlaggar B. The “Task B problem” and other considerations in developmental functional neuroimaging. Human Brain Mapping. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E, Wendelken C, van Leijenhorst L, Honomichl R, Christoff K, Bunge S. Neurocognitive development of relational reasoning. Developmental Science. 2009;12:55–66. doi: 10.1111/j.1467-7687.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N, Nardos B, Cohen A, Fair D, Power J, Church J, Nelson S, Wig G, Vogel A, Lessov-Schlaggar C, Kelly A, Dubis J, Feczko E, Coalson R, Pruett J, Barch D, Petersen S, Schlaggar B. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Advances in Developmental Cognitive Neuroscience. 2006;44:2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Eden GF, VanMeter JW, Rumsey JM, Maisog JM, Woods RP, Zeffiro TA. Abnormal processing of visual motion in dyslexia revealed by functional brain imaging. Nature. 1996;382:66–69. doi: 10.1038/382066a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K, Perkins S, Angstadt M, Johnson T, Stern E, Welsh R, Taylor S. The development of performance-monitoring function in the posterior medial frontal cortex. NeuroImage. 2010;49:3463–3473. doi: 10.1016/j.neuroimage.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gaillard W, Grandin C, Xu B. Developmental aspects of pediatric fMRI: Considerations for image acquisition, analysis, and interpretation. NeuroImage. 2001;13:239–249. doi: 10.1006/nimg.2000.0681. [DOI] [PubMed] [Google Scholar]
- Glahn D, Ragland D, Abramoff A, Barrett J, Laird A, Bearden C, Velligan D. Beyond hypofrontality: A quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Human Brain Mapping. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinband J, Savitskaya J, Wager T, Teichert T, Ferrera V, Hirsch J. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. NeuroImage. 2011;57:303–311. doi: 10.1016/j.neuroimage.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross T, Stein E. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cerebral Cortex. 2007;17:1664–1671. doi: 10.1093/cercor/bhl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Northoff G. Culture-sensitive neural substrates of human cognition: A transcultural neuroimaging approach. Nature Reviews Neuroscience. 2008;9:646–654. doi: 10.1038/nrn2456. [DOI] [PubMed] [Google Scholar]
- Kerns J, Cohen J, MacDonald A, Johnson M, Stenger A, Aizenstein H, Carter C. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. American Journal of Psychiatry. 2005;162:1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel C, Specht K, Hanisch C, Fan J, Herpertz-Dahlmann B, Fink G. Development of attentional networks: An fMRI study with children and adults. NeuroImage. 2005;28:429–439. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biological Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- Nachev P. The blind executive. NeuroImage. 2011;57:312–313. doi: 10.1016/j.neuroimage.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Nee D, Wager T, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective & Behavioral Neuroscience. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Prado J, Carp J, Weissman DH. Variations of response time in a selective attention task are linked to variations of functional connectivity in the attentional network. NeuroImage. 2011;54:541–549. doi: 10.1016/j.neuroimage.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith A, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B, Brown T, Lugar H, Visscher K, Miezin F, Petersen S. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Stern E, Welsh R, Fitzgerald K, Taylor S. Topographic analysis of individual activation patterns in medial frontal cortex in schizophrenia. Human Brain Mapping. 2009;30:2146–2156. doi: 10.1002/hbm.20657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Aging, executive control, and attention: A review of meta-analyses. Neuroscience and Biobehavioral Reviews. 2002;26:849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Barch D, Gray J, Conturo T, Braver T. BOLD correlates of trial-by-trial reaction time variability in gray and white matter: A multi-study fMRI analysis. PLoS ONE. 2009;4 doi: 10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Botvinick M, Cohen J. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Yeung N, Cohen J, Botvinick M. Errors of interpretation and modeling: A reply to Grinband et al. NeuroImage. 2011;57:316–319. doi: 10.1016/j.neuroimage.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]