Abstract
There are a number of symptoms, both neurological and behavioral, associated with a single episode of r mild traumatic brain injury (mTBI). Neuropsychological testing and conventional neuroimaging techniques are not sufficiently sensitive to detect these changes, which adds to the complexity and difficulty in relating symptoms from mTBI to their underlying structural or functional deficits. With the inability of traditional brain imaging techniques to properly assess the severity of brain damage induced by mTBI, there is hope that more advanced neuroimaging applications will be more sensitive, as well as specific, in accurately assessing mTBI. In this study, we used resting state functional magnetic resonance imaging to evaluate the default mode network (DMN) in the subacute phase of mTBI. Fourteen concussed student-athletes who were asymptomatic based upon clinical symptoms resolution and clearance for aerobic exercise by medical professionals were scanned using resting state functional magnetic resonance imaging. Nine additional asymptomatic yet not medically cleared athletes were recruited to investigate the effect of a single episode of mTBI versus multiple mTBIs on the resting state DMN. In concussed individuals the resting state DMN showed a reduced number of connections and strength of connections in the posterior cingulate and lateral parietal cortices. An increased number of connections and strength of connections was seen in the medial prefrontal cortex. Connections between the left dorso-lateral prefrontal cortex and left lateral parietal cortex showed a significant reduction in magnitude as the number of concussions increased. Regression analysis also indicated an overall loss of connectivity as the number of mTBI episodes increased. Our findings indicate that alterations in the brain resting state default mode network in the subacute phase of injury may be of use clinically in assessing the severity of mTBI and offering some insight into the pathophysiology of the disorder.
Keywords: Default Mode Network, Resting State fMRI, Sub-acute mTBI
Introduction
There is a range of neuropsychological and behavioral symptoms that accompany mild traumatic brain injury (mTBI) despite no clear morphological brain lesions (Milman et al., 2005). What is also puzzling is the time it takes for mTBI symptoms to resolve. Many physical symptoms clear up within three months, yet some individuals report physical, cognitive, and emotional symptoms that persist more than one year post- injury (Witt et al., 2010). Neuropsychological testing and traditional anatomical imaging are not sufficiently sensitive to detect differences within the subacute phase (less than 3 weeks) of mTBI (Mayer et al., 2011). This makes it difficult to determine relationships between the myriad of symptoms associated with mTBI and the underlying structural or functional deficits using conventional brain imaging techniques (see Schrader et al., 2009 for review). Together with its insensitivity to detect a single episode of mTBI, neuropsychological testing cannot differentiate individuals who have suffered from previous episodes of mTBI (Iverson et al., 2006). The number of mTBIs and time between these injuries are other important factors that also need to be considered in mTBI research. Recurrent brain injuries are likely to lead to cumulative neurological and cognitive deficits putting these patients at higher risk for further injuries and/or development of chronic post-concussion syndrome (Cantu, 2006). Clinicians in collegiate athletics currently use a combination of a subjective self-report symptoms survey (SRSS); a cognitive assessment measure like the SCAT-2 (McCrory et. al. 2008) and Balance Error Scoring System (BESS) as the minimum to determine an athlete’s ability to return to play. (NCAA: Sports Medicine Handbook 2010-2011). Neuropsychological testing is further recommended by the National Collegiate Athletic Association (NCAA); however these evaluation methods have been proven to lack specificity and sensitivity (Randolph et al., 2005, Mayer et al., 2011) once symptoms resolution has occurred beyond 10 days. In addition, athletes’ self-reported symptoms have proven to be unreliable and such scales are susceptible to falsification by the student athlete in an effort to return to activity sooner (Broglio et al., 2007).
Advances in brain imaging methodologies have revealed important information regarding both structural (Bazarain et al., 2007; Wozniak et al., 2007; Wilde et al., 2008) and functional (McAllister et al., 2001, 2006; Slobounov et al., 2010, 2011) alterations in subjects suffering from mTBI. There is evidence suggesting a variety of functional deficits occur in individuals with mTBI that correlate with advanced brain imaging data (Ptito et al., 2007). Many studies investigating mTBI have looked at the prefrontal cortex, an area associated with executive function, and have shown abnormal functional magnetic resonance imaging (fMRI) activation (McAllister et al. 2001, 2006; Smith et al. 2009, Slobounov et al., 2010). However, there are still some discrepancies in the fMRI literature whether or not mTBI subjects demonstrate increased activation (Jantzen et al., 2004, McAllister et al., 2006, Slobounov et al., 2010) or reduced activation (Chen et al., 2004). Together with fMRI studies, additional advanced neuroimaging studies of mTBI have utilized diffusion tensor imaging (DTI) and magnetic resonance spectroscopy (MRS) to examine alterations in white matter integrity and brain metabolites, respectively. DTI studies (Lipton et al., 2008, Mayer et al., 2010, Zhang et al., 2010) have mainly focused on the corpus callosum, which is a region known to be susceptible to axonal injury in TBI (Gasparovic et al., 2009) and have shown variations of fractional anisotropy and mean diffusivity in mTBI. MRS studies have shown widespread disruption in brain metabolite ratios within all lobes of the brain (Govind et al., 2010), including: prefrontal, primary motor cortex (Henry et al., 2010), splenium of the corpus callosum, and parietal white matter regions (Belanger et al., 2007).
These technological advances in brain imaging offer tremendous promise for improving clinical applicability of fMRI with specific focus on spontaneous modulations in the blood oxygenation level–dependent (BOLD) signal that occur during resting state conditions (see Fox and Raichle, 2007 for review). Resting state refers to the state in which an individual is awake lying quietly with eyes closed (Raichle et al., 2001) and does not require a specific experimental task or stimulus (Wolf et al., 2011). As one of the resting state networks (RSN) of the brain, the default mode network (DMN) (Li et al., 2011) includes the precuneus/posterior cingulate cortex (PCC), medial prefrontal cortex (MPFC), and medial, lateral, and inferior parietal cortex (see Broyd et al., 2009 for review). Although the DMN is active during rest it is not actively involved in attention or goal-oriented tasks (Raichle et al., 2001). Despite the fact that the DMN is deactivated during specific tasks, the presence of the DMN during rs-fMRI has been reported and validated in several studies (Beckmann et al., 2005, De Luca et al., 2006, Damoiseaux et al., 2006, Greicius et al., 2002).
Few studies to date (Cao and Slobounov, 2010, Kumar et al., 2009, Marquez de la Plata et al., 2011, Mayer et al., 2011, Slobounov et al., 2011, Sponheim et al., 2011) have investigated functional connectivity in the brain of mTBI, more specifically the DMN. Previous functional connectivity research using fMRI demonstrated altered patterns of the DMN in Alzheimer’s disease (AD) (Greicius et al., 2004), schizophrenia (Pomarol-Clotet et al., 2008), depression (Greicius et al., 2007), and attention-deficit hyperactivity disorder (ADHD) (Castellanos et al., 2008), and has shown promise for possible clinical applications (Broyd et al., 2009). A recent study by Mayer et al. (2011) investigated the resting state DMN of subacute mTBI and showed that these subjects displayed decreased BOLD connectivity within the DMN and hyper-connectivity between the right prefrontal and posterior parietal cortices involved in the fronto-parietal task-related network (TRN). However, there is an issue of subject population homogeneity, which can lead to possible discrepancies. Specifically, inhomogeneities such as: (a) differential diagnosis of mTBI including the presence or absence of loss of consciousness (LOC); (b) time since injury; and (c) detailed subject’s medical history that includes information on past head injuries may be serious confounding factors influencing fMRI data. Accordingly, in this study we examined the resting state DMN following mTBI using resting state fMRI with specific focus on recruiting a homogeneous subject population and controlling for the number of concussions.
It was hypothesized that despite clinical findings, which suggest restoration of pre-morbid levels of function, there will be altered connectivity measures of the resting state DMN within the mTBI subject pool. More specifically, we hypothesized that there will be reduced connectivity, both in terms of the number and the magnitude of connections after mTBI despite the absence of neurological and/or neuropsychological deficits at the time of testing. Additionally, we suspect that the as the number of concussions increases there will be a greater departure from resting state DMN.
Methods
Participants
Fifteen neurologically normal student-athletes with no history of mTBI (7 male, 8 female, mean age 20.4 +/- 0.8 years) and fourteen student-athletes (5 male, 9 female, mean age 20.6 +/- 1.2 years) who had recently suffered a sports-related mTBI (collegiate rugby, ice hockey, lacrosse, etc.) were recruited for this study. All injured subjects suffered from grade 1 mTBI (Cantu Data Driven Revised Concussion Grading Guideline, 2006). The initial diagnosis of mTBI was made on the field by certified athletic trainers (AT) and as a part of the routine protocol of the Sport Concussion Program at the Pennsylvania State University. All athletes were evaluated by a physician with expertise in evaluating and treating athletes with concussion. Each athlete had completed the clinical cognitive assessment (SCAT-2) (McCrory et al., 2008) and Balance Error Score System (BESS) administered by the physician and had returned to their clinical baseline accordingly. In addition, each athlete had reported at least a 24-hour self-reported symptom-free period at which point they had been cleared for aerobic activity (<70% maximum heart rate) (3rd International Consensus Statement on Concussion). Scanning took place within 24 hours of clinical symptoms resolution and medical clearance for the first stage of aerobic activity by their supervising physician, which was on average 10 (+/- 2) days post-injury. All subjects were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971) with a handedness score above 90. All subjects signed an informed consent form and the Institutional Review Board of the Pennsylvania State University approved this protocol. Any mTBI subjects reporting any symptoms before or during scanning were excluded. To further investigate the effects of multiple concussions on the resting state DMN, nine more mTBI subjects (5 female, 4 male, mean age 19.9 +/- 1.5 years) were recruited. These additional subjects had no self-reported symptoms for 24 hours and had been cleared by their physician for the first stage of aerobic activity, but unfortunately they were not scanned within the initial 24 hours of this clearance. Despite not being scanned within that first 24 hours timeframe, scanning took place on average 10 (+/4) days post injury. In order to maintain the homogeneity of the time from medical clearance to scanning of the initial cohort of mTBI, these nine supplementary subjects were only used for regression analysis in exploring the effect of multiple episodes of mTBI on the resting state DMN. More detailed information about the subjects is presented in Table 1. No radiological findings such as lesions or hyperintense signals were present in either the mTBI or NV.
Table 1.
Information on subjects including age, gender, sport, and number of concussions.
| Subject | Gender | Age | # of Concussions | Sport |
|---|---|---|---|---|
| NV | M | 21 | 0 | Gymnastics |
| NV | F | 21 | 0 | Field Hockey |
| NV | F | 20 | 0 | Field Hockey |
| NV | M | 20 | 0 | Gymnastics |
| NV | M | 20 | 0 | Gymnastics |
| NV | F | 21 | 0 | Gymnastics |
| NV | M | 22 | 0 | Gymnastics |
| NV | F | 21 | 0 | Gymnastics |
| NV | F | 20 | 0 | Swimming |
| NV | M | 20 | 0 | Gymnastics |
| NV | M | 19 | 0 | Gymnastics |
| NV | M | 21 | 0 | Gymnastics |
| NV | F | 21 | 0 | Gymnastics |
| NV | F | 20 | 0 | Gymnastics |
| NV | F | 19 | 0 | Gymnastics |
| mTBI | M | 20 | 2 | Ice Hockey |
| mTBI | M | 21 | 3 | Cheerleading |
| mTBI | M | 22 | 2 | Rugby |
| mTBI | F | 19 | 2 | Rugby |
| mTBI | F | 19 | 1 | Rugby |
| mTBI | M | 22 | 3 | Lacrosse |
| mTBI | F | 19 | 3 | Ice Hockey |
| mTBI | F | 22 | 1 | Rugby |
| mTBI | F | 21 | 3 | Volleyball |
| mTBI | F | 21 | 2 | Rugby |
| mTBI | F | 21 | 1 | Cheerleading |
| mTBI | F | 21 | 1 | Rugby |
| mTBI | F | 21 | 2 | Ice Hockey |
| mTBI | M | 19 | 2 | Gymnastics |
| mTBI* | F | 21 | 1 | Tennis |
| mTBI* | F | 21 | 1 | Swimming and Diving |
| mTBI* | M | 19 | 2 | Football |
| mTBI* | M | 21 | 3 | Rugby |
| mTBI* | F | 18 | 3 | Rugby |
| mTBI* | M | 20 | 3 | Rugby |
| mTBI* | F | 18 | 1 | Fencing |
| mTBI* | M | 22 | 2 | Lacrosse |
| mTBI* | F | 19 | 2 | Rugby |
denotes additional subjects used for regression analysis
MRI Data acquisition
In this study, functional connectivity characteristics between matched normal volunteers (NV) and subjects recovering from mTBI were assessed using resting state functional magnetic resonance imaging (rs-fMRI). By eliminating the task associated with fMRI, rs-fMRI eliminates bias based upon performance which is important in certain psychiatric and neurologic conditions (Wolf et al., 2011). As in certain neurological disorders such as Alzheimer’s disease (AD), mTBI patients suffer from problems with attention and memory. Therefore the use of rs-fMRI which has already been used to assess the DMN in AD (Greicius et al., 2002 and Koch et al., 2010) was implemented in this study.
Functional and anatomical images were acquired on a 3.0 Tesla Siemens Trio whole-body scanner (Siemens, Erlangen, Germany) using a 12-channel head coil. T1 anatomical images and fMRI images were acquired in the axial plane parallel to the anterior and posterior commissure axis covering the entire brain. Anatomical images were collected using a three-dimensional isotropic T1-weighted magnetization prepared rapid gradient echo (MP-RAGE: 0.9mm × 0.9mm × 0.9mm resolution, TE= 3.46ms, TR= 2300ms, TI= 900ms, flip angle= 9°, 160 slices, NSA= 1). Two-dimensional BOLD echo planar fMRI resting-state images (3.1mm × 3.1mm × 5mm resolution, TE= 25ms, TR= 2000ms, EPI factor=64, flip angle= 79°, 30 slices, NSA= 1, acquisition time= 6:04) were obtained. During resting state fMRI acquisition, subjects were asked to lie motionless with their eyes closed.
Data Analysis
The voxel-based correlation approach was used to evaluate the temporally correlated BOLD signal (Marquez de la Plata et al., 2011) associated with the functional connectivity of the DMN. For this study, we focused on the PCC, MPFC, and lateral parietal lobes because of their strong involvement in the DMN (Broyd et al., 2009), as well as the parahippocampal gyrus since hippocampal injuries, together with memory deficits, are common after TBI (Marquez de la Plata et al., 2011). Region of interest (ROI) based correlations were also implemented with those same areas of interest as seed points.
Voxel-based and ROI-based correlational analyses were performed using the Functional Connectivity (CONN) toolbox (http://web.mit.edu/swg/software.htm) of SPM 8. After preprocessing, images were then band-pass filtered to 0.01Hz~0.09Hz and motion regressed to reduce the influence of noise. The CONN toolbox performs seed-based analysis by computing the temporal correlation between the BOLD signals from a given voxel to all other voxels in the brain. White matter, cerebrospinal fluid (CSF), and physiological noise source reduction were taken as confounds, following the implemented CompCor strategy (Behzadi et al., 2007). Whole brain BOLD signal was excluded as a regressor to eliminate erroneous anti-correlations (Murphy et al., 2009). CONN also allows for ROI-based analysis by grouping voxels into ROIs based upon Brodmann areas. Bi-variate correlations were calculated between each pair of ROIs as reflections of connections. All Brodmann areas were imported as possible connections for our selected seed ROIs. Fisher transformed Z-scores were introduced to validate multiple comparisons and SPM functions were called by the CONN toolbox for spatial statistical tests. ROI-based analyses were performed for all subjects’ data with a general linear model (GLM) test to determine significant resting state DMN connections at the individual level (1st level). Based upon 1st level results correlation coefficients were converted into standard scores, and an unpaired t-test was used with a threshold set at p<0.05 false discovery rate (FDR) corrected to determine significant connections between the NV and mTBI groups. Regression analysis was then used to determine the effect of a single concussion versus multiple concussions on the resting state DMN.
Results
As shown in Fig. 1, the PCC of the NVs showed significant connectivity with the following areas: MPFC, dorsolateral prefrontal cortex (DLPFC), inferior temporal gyrus, parahippocampal cortex, and lateral parietal cortex. However the mTBI group showed no significant connections from the PCC to either the DLPFC or parahippocampal gyrus. The between-group t-tests showed significant connections in the NV group for PCC – left DLPFC (p=0.027), PCC – right DLPFC (p=0.010), and PCC – left Parahippocampus (p=0.012). Conversely, the mTBI group (Fig. 2) showed more connections from the MPFC, including the connection between the MPFC and left lateral parietal cortex.
Fig. 1.
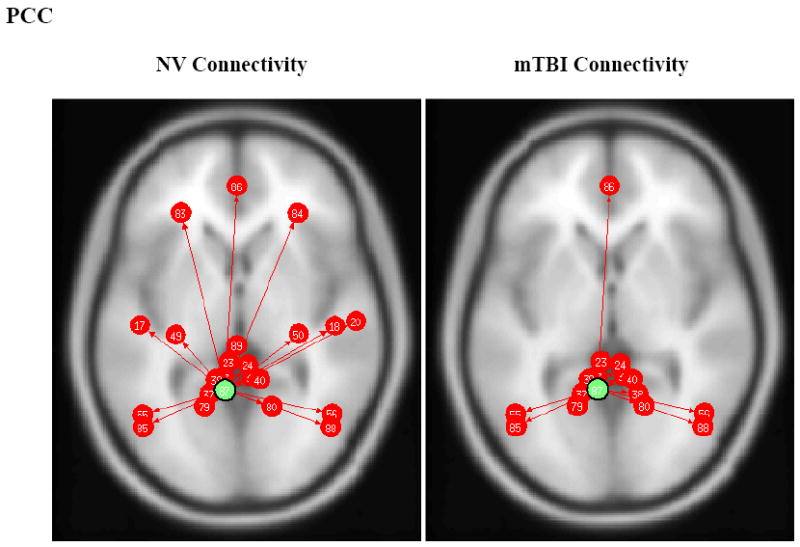
PCC ROI based connectivity maps for NV (left) and mTBI (right) subjects. Green dot designates the PCC ROI seed and red arrows and dots indicate significant positively correlated (p<0.05 FDR) brain regions (note image produced from CONN toolbox and numbers do not represent Brodmann’s Area numbers). Left and Right Inferior Temporal Gyrus (#17/18), Left and Right Parahippocampal Gyrus (#49/50), Left and Right Angular Gyrus (#55/56), Left and Right Dorsolateral Prefrontal Cortex (#83/84), and Medial Prefrontal Cortex (#86).
Fig. 2.
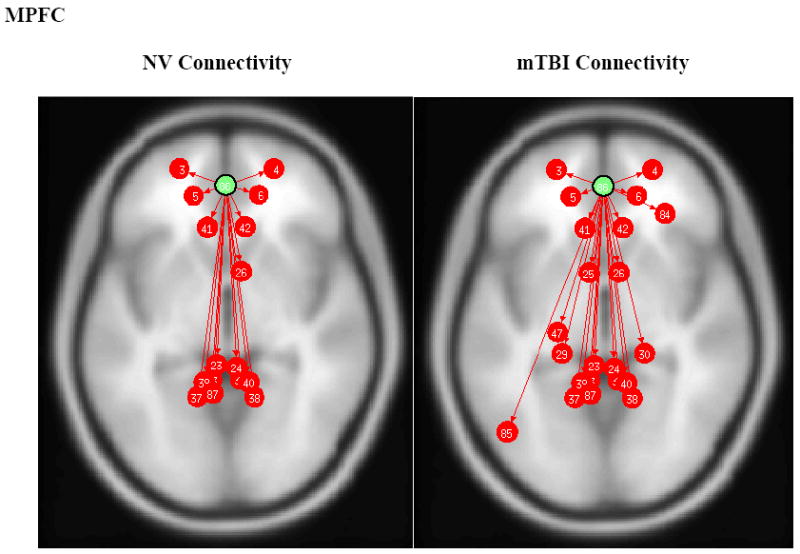
MPFC ROI based connectivity maps for NV (left) and mTBI (right) subjects. Green dot designates the MPFC ROI seed and red arrows and dots indicate significant positively correlated (p<0.05 FDR) brain regions. Left and Right Anterior Prefrontal Cortex (#3/4), Left and Right Orbitofrontal Cortex (#5/6), Anterior Cingulate Cortex (#41/42), Precuneus/Posterior Cingulate Cortex (#87), and Left Lateral Parietal (#85).
Fig. 3 illustrates that the lateral parietal lobe was connected to the PCC/precuneus area in both groups. However, the NV group showed connections between the lateral parietal and inferior temporal gyrus.
Fig. 3.
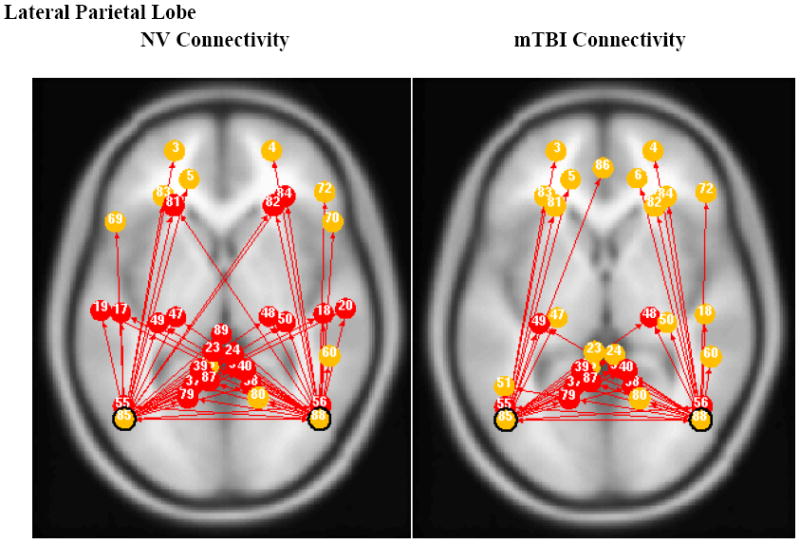
Left and Right Lateral Parietal ROI based connectivity maps for NV (left) and mTBI (right) subjects. Red dots indicate shared connections from both left and right lateral parietal ROI, while yellow dots indicate non-shared connections.
Meanwhile, both left and right lateral parietal cortices were connected bilaterally to the DLPFC in the NV group, while the mTBI group showed only ipsilateral connections between lateral parietal cortex and DLPFC. The t-tests for group comparison showed significant differences (p<0.05) for the following connections: left DLPFC – left lateral parietal (p=0.022), right DLPFC – left lateral parietal (p=0.014), left lateral parietal – right lateral parietal (p=0.020), left DLPFC – right lateral parietal (p=0.009), right DLPFC – right lateral parietal (p=0.014). Overall, the NV group had a higher average connection strength than the mTBI group.
As depicted in Fig. 4, there were no connections between the parahippocampal gyrus and PCC in the mTBI group. Connections between the parahippocampal gyrus and the left and right parietal lobes were also absent.
Fig. 4.
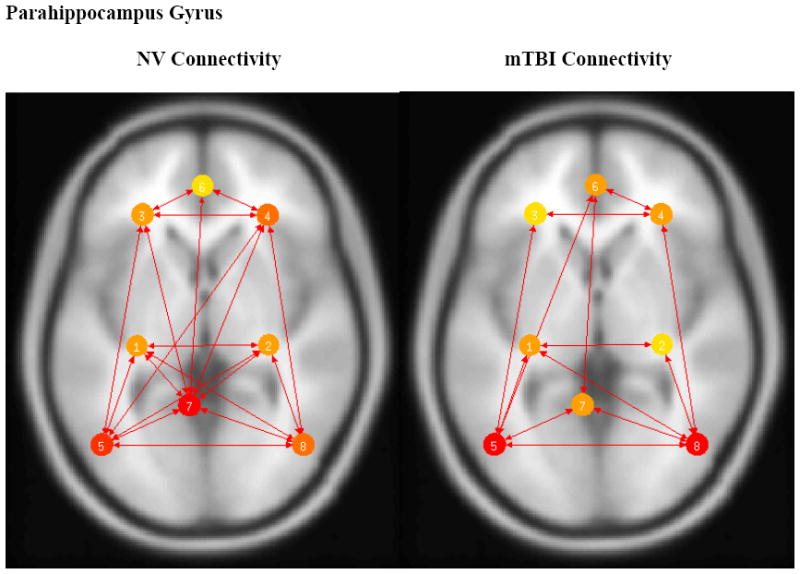
Left and Right Parahippocampal Gyrus based connectivity maps for NV (left) and mTBI (right) subjects. Connectivity significance level set at p<0.05 FDR. Left and Right Parahippocampus Cortex (#1/2), Left and Right Dorsolateral Prefrontal Cortex (#3/4), Left and Right Lateral Parietal (#5/8), Medial Prefrontal Cortex (#6), and Posterior Cingulate Cortex (#7). Color corresponds to number of connections with warmer (red) colors having more connections.
The connection between the MPFC and left lateral parietal was only observed in the mTBI group. The average z-scores used to determine strength of connections are depicted in Fig. 5. The details of these analyses are shown in Table 2.
Fig. 5.
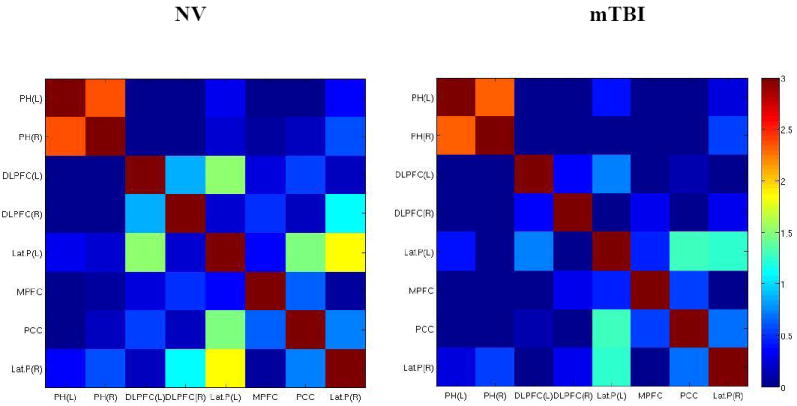
Correlation matrices showing resting state DMN connectivity for NV (left) and mTBI (right) groups. The color map was defined with Z-transformed Z-scores from correlation coefficients. The colder (blue-end) boxes represent low correlations between regions, whereas warmer (red-end) boxes represent stronger correlations.
Table 2.
Seed-based connection table. Brodmann’s Area (BA); Z-score (Z); FDR corrected p value (p (FDR))
| NV | mTBI | |||
|---|---|---|---|---|
| ROI | BA | Z | Z | p (FDR) |
| PCC/Precuneus | ||||
| Left DLPFC | 9 | 0.96(0.62) | 0.17(0.56) | 0.027* |
| Right DLPFC | 9 | 0.44(0.20) | -0.26(0.77) | 0.010* |
| Right MPFC | 0.64(0.96) | 0.55(0.87) | 0.816 | |
| Left Parahippocampus | 36 | 0.08(0.24) | -0.31(0.39) | 0.012* |
| Right Parahippocampus | 36 | 0.20(0.20) | -0.21(0.78) | 0.098 |
| Left Lateral Parietal | 1.45(1.04) | 1.30(0.67) | 0.690 | |
| Right Lateral Parietal | 0.73(0.90) | 0.69(0.90) | 0.903 | |
| Right DLPFC | ||||
| Left Parahippocampus | 36 | -0.24(0.77) | -0.96(0.72) | 0.033* |
| Right Parahippocampus | 36 | -0.07(0.88) | -1.02(0.65) | 0.010* |
| Left Lateral Parietal | 0.22(0.47) | -0.58(0.96) | 0.014* | |
| Right Lateral Parietal | 1.08(0.68) | 0.28(0.76) | 0.014* | |
| Left DLPFC | 9 | 1.07(0.69) | 0.34(0.69) | 0.036* |
| Left Parahippocampus | ||||
| Right Parahippocampus | 36 | 2.35(0.63) | 2.33(0.69) | 0.929 |
| Left Lateral Parietal | 0.31(0.34) | 0.41(0.67) | 0.627 | |
| Right Lateral Parietal | 0.37(0.60) | 0.28(0.75) | 0.744 | |
| Left DLPFC | ||||
| Left Lateral Parietal | 1.54(0.81) | 0.74(0.70) | 0.022* | |
| Right Lateral Parietal | 0.33(0.68) | -0.49(0.71) | 0.009* | |
| Left Lateral Parietal | ||||
| Right Lateral Parietal | 1.99(0.64) | 1.24(0.75) | 0.020* | |
| MPFC | 0.33(1.06) | 0.69(0.44) | 0.320 |
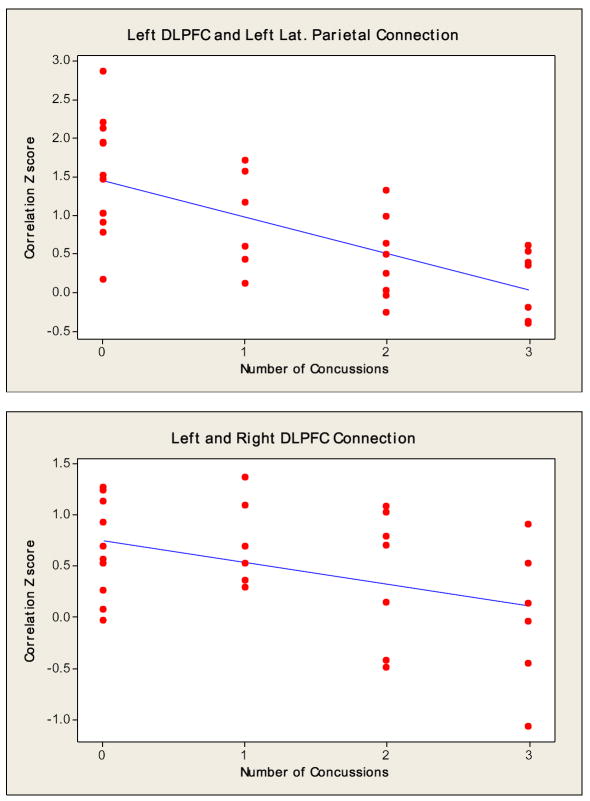
Regression analysis revealed a significant reduction (p=0.014 and R2= 24.1%) in the magnitude of connections between the left DLPFC and left lateral parietal cortex as a function of number of concussive episodes. Despite not showing any statistically significant connections between the left and right DLPFC (p=0.412 and R2= 18.3%) and/or the left DLPFC and PCC (p=0.387 and R2= 16.9%), the regression analysis seen in Fig. 6 showed a downward trend in the number of connections as the number of concussions increased.
Fig 6.
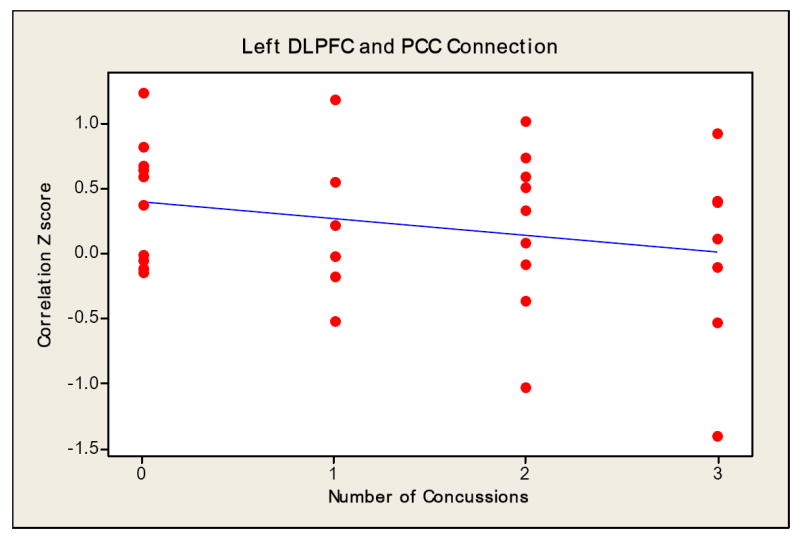
Regression slope indicating the relationship between the number of concussive episodes and magnitude of connectivity.
Discussion
In this study, we investigated the connectivity within the resting state DMN of mTBI subjects who were clinically asymptomatic based on physician’s examination, 24 hours symptom free on SRSS, and who had returned to baseline on SCAT-2 and BESS clinical exam scores. Even though the subjects in this study were clinically asymptomatic and scanned within 24 hours of being cleared by a medical professional, this cohort of subjects was restricted to collegiate athletes. Although strict guidelines based upon time from medical clearance to scanning (<24 hours) were adhered to, the mTBI group was compromised of subjects with no previous history of mTBI to subjects with upwards to three episodes of mTBI. Many pathological changes can occur after mTBI, including altered perfusion and metabolism that may influence the BOLD signal and therefore influence connectivity measured when assessed through fMRI (Mayer et al., 2011). Biological processes, technical limitations, and differing neuroimaging modalities can all be sources of error. Nevertheless, there are three major findings. First, voxel-based correlation analysis suggests that there are disruptions in the connections that make up the DMN in mTBI subjects. Specifically, the mTBI resting state DMN showed no connections in the PCC with both left and right DLPFC and parahippocampal gyri, as well a reduced number of overall connections and strength of connections. Left and right lateral parietal cortex connectivity in mTBI subjects illustrated only ipsilateral connections with DLPFC as compared to bilateral DLPFC connectivity in NV groups. Second, an overall reduction in the number and strength of connections was observed in the mTBI group within the left and right parietal cortex ROI seed. However, an increased number of connections and strength of connections were seen in the MPFC in mTBI subjects. Third, connections between the left DLPFC and left lateral parietal cortex were significantly reduced in magnitude as the number of concussions increased. In addition, there was an overall downward trend in these connections, suggesting a larger departure from the typical DMN as the number of concussions increased.
These major findings are similar to those of Mayer et al. (2011) who found that the resting state DMN in mTBI subjects demonstrated an overall reduced connectivity, more specifically connections from the PCC with the ACC and right supramarginal gyrus. Moreover, that group showed an increased connectivity between the rostral anterior cingulate gyrus (rACC) with the left DLPFC and bilateral ventral lateral prefrontal cortex (VLPFC) and insula, whereas we observed hyper-connectivity in the MPFC. One reason for this discrepancy may be the method used to define the ROI seed point. Alterations in interhemispheric connectivity observed in mTBI subjects are consistent with previous studies which reported reduced connectivity after TBI. Specifically, interhemispheric connectivity in the ACC, DLPFC, primary visual cortex and hippocampal networks are reduced in mTBI subjects (Slobounov et al., 2011 and Marquez de la Plata et al., 2011). In a recent study, Marquez de la Plata et al. (2011) reported significantly less interhemispheric connectivity within the hippocampus (which is important in learning and memory) and within the ACC in addition to a more widespread DMN disruption in patients who had suffered from traumatic axonal injury. Similar to AD, autism, and low-grade glioma, patients suffering from mTBI have decreased long-distance functional connectivity and significantly increased short-distance connectivity (Cao and Slobounov, 2010). Both AD and Parkinson’s disease (PD) have also shown disruptions in DMN studies. AD patients show a marked reduction in hippocampal connectivity with the rest of the DMN in addition to reduced connectivity between MPFC and PCC that occurs due to aging (Broyd et al., 2009). DMN research in PD patients has shown that the MPFC and rostral ventromedial caudate nucleus are not connected (van Eimeren et al., 2009).
Numerous symptoms, not only physical, are associated with a single episode of mTBI including cognitive and emotional symptoms such as: trouble with memory, attention, concentration, problem solving, executive function, depression, and anxiety (Bergman and Bay 2010). Whether or not these cognitive and emotional symptoms can be solely attributed to disruptions in connectivity patterns is yet to be determined. The PCC/precuneus plays a vital role in the DMN (Fransson and Marrelec, 2008) and is involved in visuospatial imagery, episodic memory, and self-processing (Cavanna and Trimble 2006). MPFC may play a role in controlling emotional processes and there is evidence that alterations in the prefrontal cortex can lead to depression and post-traumatic stress disorder (PTSD) (Weinberg et al., 2010). Traditionally, the lateral posterior parietal cortex has been considered essential in spatial information, movement planning and control, and multisensory integration (Davidson et al., 2008). Additionally recent fMRI studies have indicated that the lateral posterior parietal cortex may be important in episodic memory retrieval. With all the similarities in symptoms and disruptions of the DMN that mTBI shares with AD and PD it is not surprising that both animal and human experiments suggest a possible link between TBI and early onset of these neurological disorders (Kiraly and Kiraly 2007).
There is little research (Guskiewicz et al., 2003, De Beaumont et al., 2007, Slobounov et al., 2009, Talavage et al., 2010, Thériault et al., 2011) on the effects of multiple episodes of mTBI and whether or not subsequent mTBI may have a cumulative effect. It has been shown that individuals who have suffered from previous mTBI are more likely to suffer from future mTBI (Guskiewicz et al., 2003). De Beaumont et al. (2007) used transcranial magnetic stimulation (TMS) and showed that long-term motor system dysfunction is exacerbated by multiple episodes of mTBI. An electroencephalography (EEG) study of athletes suffering from three or more episodes of mTBI demonstrated significantly attenuated sustained posterior contralateral negativity amplitude compared to subjects with one or two mTBIs, as well as adding evidence that more mTBI results in more disproportionately unfavorable outcomes (Thériault et al., 2011). These EEG and TMS findings are complementary to our previous observation of subjects with recurrent concussions. Specifically, the rates of recovery of “visual-kinesthetic integration” during dynamic postural tasks were significantly slower after the second concussive episode. More importantly, unlike the first concussion, the presence of “visual-kinesthetic disintegration” was evident far later than 10 days post-second concussion (Slobounov et al., 2007). Furthermore, the time between two recurrent concussive episodes appeared to be an important factor influencing the rate of recovery after the second concussion. Collectively, our data support the hypothesis that a history of previous concussions may be associated with slower recovery of neurological functions (Guskiewicz et al., 2003; Slobounov et al., 2006) which may not be obvious when conventional clinical tools are utilized. This should be considered by medical practitioners while clearing athletes for sports participation. Current guidelines given by the NCAA for return to play in athletics given to physicians managing patients with mTBI include: the presence of a 24-hour symptom free waiting period on SRSS, restoration of baseline measures on clinical cognitive (SCAT-2) and BESS assessment measures. These clinical measures precede a gradual return to pre-injury levels of activity starting with an aerobic challenge followed by increases in anaerobic and functional sport-specific challenges. As demonstrated in this study, clinicians using these basic measures should exercise caution when returning an athlete to athletic participation based on these measures alone.
Advanced neuroimaging techniques have discovered altered brain metabolites using MRS in mTBI (Govind et al., 2010 and Henry et al., 2010) as well as altered microstructural function of white matter tracks using DTI (Lipton et al., 2008, Mayer et al., 2010, Zhang et al., 2010) and rs-fMRI functional connectivity (Slobounov et al., 2011). These findings demonstrate a distinct dissociation between clinical findings of restoration of function and advanced imaging abnormalities. The dissociation between a ‘return to baseline’ clinical evaluation and structural / functional imaging findings suggests a possible explanation for the susceptibility of these patients to recurrent injuries. This growing body of neuroimaging findings suggests that clinicians need to up-date current clinical practice to seek out clinical measures which may expose these abnormalities. The neuroimaging community needs to continue to develop a body of data and normative values which may be used by clinicians in an effort to revise and up-date current ‘Return to Play’ guidelines. Future investigations should utilize diagnostic techniques (fMRI analysis of DMN, resting state functional connectivity, MRS, DTI, etc.) in conjunction with clinical test measures (SCAT, balance, etc.) to verify clinical tests’ specificity and validity in the mTBI population. This combination of diagnostic and objective measures may be the future of mTBI clinical management. Moreover, in order to assess the effects of multiple concussions versus a single concussion, it is important to separate subjects based upon their history of prior head trauma. By compensating for the number of concussive episodes, insight into the severity of symptoms and length of recovery might be gained. As demonstrated in this investigation, there are clear differences in resting state DMN network connectivity between the NV and mTBI groups. It is unknown if this altered resting state DMN network seen in mTBI is a short- or long-term effect. Therefore longitudinal studies with stricter emphasis on scheduling of initial and follow-up scans need to be implemented to better track changes over time.
We investigated the default mode network in subacute mTBI.
-
>
Resting-state fMRI showed that there are disruptions in the DMN in subacute mTBI.
-
>
Overall mTBI caused reduced strength and number of connections in the DMN.
-
>
The number of mTBI appears to have an impact on the connectivity of the DMN.
Acknowledgments
The authors would like to thank Devee Schoenberg for her editing assistance.
Funding: This work was supported by National Institutes of Health Grant RO1 NS056227-01A2 “Identification of Athletes at Risk for Traumatic Brain Injury” awarded to Dr. Slobounov, PI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion Tensor Imaging Detects Clinically Important Axonal Damage after Mild Traumatic Brain Injury: A Pilot Study. Journal of Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger HG, Vanderploeg RD, Curtiss G, Warden DL. Recent Neuroimaging Techniques in Mild Traumatic Brain Injury. J Neuropsychiatry Clin Neurosci. 2007;19:5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Macciocchi SN, Ferrara MS. Neurocognitive performance of concussed athletes when symptom free. J Athl Train. 2007;42:504–508. [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: A systematic review. Neuroscience & Biobehavioral Reviews. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Cantu R. Concussion classification: Ongoing Controversy. In: Slobounov Semyon, Sebastianelli Wayne., editors. Foundations of sport-related brain injuries. Springer; NY: 2006. pp. 87–111. [Google Scholar]
- Cao C, Slobounov S. Alteration of cortical functional connectivity as a result of traumatic brain injury revealed by graph theory, ICA, and sLORETA analyses of EEG signals. IEEE Trans Neural Syst Rehabil. 2010;18:11–19. doi: 10.1109/TNSRE.2009.2027704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJS, Rotrosen J, Adler LA, Milham MP. Cingulate-Precuneus Interactions: A New Locus of Dysfunction in Adult Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: an fMRI study. NeuroImage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PSR, Anaki D, Ciaramelli E, Cohn M, Kim ASN, Murphy KJ, Troyer AK, Moscovitch M, Levine B. Does lateral parietal cortex support episodic memory?Evidence from focal lesion patients. Neuropsychologia. 2008;46:1743–1755. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaumont L, Lassonde M, Leclerc S, Théoret H. Long-Term and Cumulative Effects of Sports Concussion on Motor Cortex Inhibition. Neurosurgery. 2007;61:329–337. doi: 10.1227/01.NEU.0000280000.03578.B6. 310.1227/1201.NEU.0000280000.0000203578.B0000280006. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Gasparovic C, Yeo R, Mannell M, Ling J, Elgie R, Phillips J, Doezema D, Mayer A. Neurometabolite Concentrations in Gray and White Matter in Mild Traumatic Brain Injury: A 1H–Magnetic Resonance Spectroscopy Study. Journal of Neurotrauma. 2009 doi: 10.1089/neu.2009.0896. 110306202455053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind V, Gold S, Kaliannan K, Saigal G, Falcone S, Arheart KL, et al. Whole-brain proton MR spectroscopic imaging of mild-to-moderate traumatic brain injury and correlation with neuropsychological deficits. J Neurotraum. 2010;27:483–496. doi: 10.1089/neu.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M, Flores B, Menon V, Glover G, Solvason H, Kenna H, Reiss A, Schatzberg A. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2002;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2549–55. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- Henry LC, Tremblay S, Boulanger Y, Ellemberg D, Lassonde M. Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. J Neurotraum. 2010;27:65–76. doi: 10.1089/neu.2009.0962. [DOI] [PubMed] [Google Scholar]
- Iverson GL. No cumulative effects for one or two previous concussions. British Journal of Sports Medicine. 2006;40:72–75. doi: 10.1136/bjsm.2005.020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen KL, Anderson B, Steinberg FL, et al. A prospective functional MR imaging study of mild traumatic brain injury in collegiate football players. Am J Neuroradiol. 2004;25:738–745. [PMC free article] [PubMed] [Google Scholar]
- Kiraly M, Kiraly SJ. Traumatic brain injury and delayed sequelae: a review—traumatic brain injury and mild traumatic brain injury (concussion) are precursors to later-onset brain disorders, including early-onset dementia. Scientific World Journal. 2007;7:1768–1776. doi: 10.1100/tsw.2007.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W, Teipel S, Mueller S, Benninghoff J, Wagner M, Bokde ALW, Hampel H, Coates U, Reiser M, Meindl T. Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer’s disease. Neurobiology of Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Kumar S, Rao SL, Chandramouli BA, Pillai SV. Reduction of functional brain connectivity in mild traumatic brain injury during working memory. J Neurotraum. 2009;26:665–675. doi: 10.1089/neu.2008.0644. [DOI] [PubMed] [Google Scholar]
- Li R, Chen K, Fleisher AS, Reiman EM, Yao L, Wu X. Large-scale directional connections among multi resting-state neural networks in human brain: A functional MRI and Bayesian network modeling study. NeuroImage. 2011;56:1035–1042. doi: 10.1016/j.neuroimage.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton ML, Gellella E, Lo C, Gold T, Ardekani BA, Shifteh K, Bello JA, Branch CA. Multifocal White Matter Ultrastructural Abnormalities in Mild Traumatic Brain Injury with Cognitive Disability: A Voxel-Wise Analysis of Diffusion Tensor Imaging. Journal of Neurotrauma. 2008;25:1335–1342. doi: 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- Marquez de la Plata C, Garces J, Shokri Kojori E, Diaz-Arrastia R, et al. Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic diffuse axonal injury. Arch Neurol. doi: 10.1001/archneurol.2010.342. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74:643–650. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Human Brain Mapping n/a-n/a. 2011 doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential Working Memory Load Effects after Mild Traumatic Brain Injury. NeuroImage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Flashman L, McDonal BC, Saykin AJ. Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J Neurotrauma. 2006;23:1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, Cantu R. Consensus statement on concussion in sport – The 3rd International Conference on concussion in sport, held in Zurich, November 2008; Journal of Clinical Neuroscience; 2009. pp. 755–763. [DOI] [PubMed] [Google Scholar]
- Milman A, Rosenberg A, Weizman R, Pick CG. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J Neurotraum. 2005;22:1003–1010. doi: 10.1089/neu.2005.22.1003. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Collegiate Athletic Association. Sports Medicine Handbook 2010-2011. Indianapolis, IN: 2010. pp. 52–56. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Salvador R, Sarró S, Gomar J, Vila F, Martínez Á, Guerrero A, Ortiz-Gil J, Sans-Sansa B, Capdevila A, Cebamanos JM, McKenna PJ. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychological Medicine. 2008;38 doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- Ptito A, Chen JK, Johnston K. Contribution of functional magnetic resonance imaging (fMRI) to sport concussion evaluation. NeuroRehabilitation. 2007;22:217–227. [PubMed] [Google Scholar]
- Randolph C, McCrea M, Barr W. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139–154. [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader H, Mickevičiene D, Gleizniene R, Jakstiene S, Surkiene D, Stovner L, Obelieniene D. Magnetic resonance imaging after most common form of concussion. BMC Medical Imaging. 2009;9:11. doi: 10.1186/1471-2342-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov S, Sebastianelli W, Tutwiler R, Slobounov E. Alteration of postural responses to visual field motion in mild traumatic brain injury. Neurosurgery. 2006;59:134–139. doi: 10.1227/01.NEU.0000219197.33182.3F. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Sebastianelli W, Cao C, Slobounov E, Newell K. Differential rate of recovery in athletes after first versus and second concussion episodes. Neurosurgery. 2007;61:238–244. doi: 10.1227/01.NEU.0000280001.03578.FF. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Cao C, Sebastianelli W. Differential effect of first versus second concussive episodes on wavelet information quality of EEG. Clinical Neurophysiology. 2009;120:862–867. doi: 10.1016/j.clinph.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov, Zhang K, Pennell D, Ray W, Johnson B, Sebastianelli W. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: a functional MRI study. Exp Brain Res. 2010;202:341–354. doi: 10.1007/s00221-009-2141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov SM, Gay M, Zhang K, Johnson B, Pennell D, Sebastianelli W, Horovitz S, Hallett M. Alteration of brain functional network at rest and in response to YMCA physical stress test in concussed athletes: RsFMRI study. NeuroImage. 2011;55:1716–1727. doi: 10.1016/j.neuroimage.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim SR, McGuire KA, Kang SS, Davenport ND, Aviyente S, Bernat EM, Lim KO. Evidence of disrupted functional connectivity in the brain after combat-related blast injury. NeuroImage. 2011;54:S21–S29. doi: 10.1016/j.neuroimage.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Talavage T, Nauman E, Breedlove E, Yoruk U, Dye A, Morigaki K, Feuer H, Leverenz J. Functionally-Detected Cognitive Impairment in High School Football Players Without Clinically-Diagnosed Concussion. Journal of Neurotrauma. 2010 doi: 10.1089/neu.2010.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriault M, De Beaumont L, Tremblay S, Lassonde M, Jolicoeur P. Cumulative effects of concussions in athletes revealed by electrophysiological abnormalities on visual working memory. Journal of Clinical and Experimental Neuropsychology. 2011;33:30–41. doi: 10.1080/13803391003772873. [DOI] [PubMed] [Google Scholar]
- van Eimeren T, Monchi O, Ballanger B, Strafella AP. Dysfunction of the default mode network in Parkinson disease: A functional magnetic resonance imaging study. Arch Neurol. 2009;66:877–883. doi: 10.1001/archneurol.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Johnson DC, Bhatt AP, Spencer RL. Medial prefrontal cortex activity can disrupt the expression of stress response habituation. Neuroscience. 2010;168:744–756. doi: 10.1016/j.neuroscience.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ST, Lovejoy DW, Pearlson GD, Stevens MC. Decreased prefrontal cortex activity in mild traumatic brain injury during performance of an auditory oddball task. Brain Imaging and Behavior. 2010;4:232–247. doi: 10.1007/s11682-010-9102-3. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Sambataro F, Vasic N, Schmid M, Thomann PA, Bienentreu SD, Wolf ND. Aberrant connectivity of resting-state networks in borderline personality disorder. J Psychiatry Neurosci. 2010 doi: 10.1503/jpn.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J, Krach L, Ward E, Mueller B, Muetzel R, Schnoebelen S, Kiragu A, Lim K. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: A diffusion tensor imaging (DTI) study. Archives of Clinical Neuropsychology. 2007;22:555–568. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S. Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Experimental Brain Research. 2010;204:57–70. doi: 10.1007/s00221-010-2294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]