Abstract
With the non-specific toxicity of anticancer drugs to healthy tissues upon systemic administration, formulations capable of enhanced selectivity in delivery to the tumor mass and cells are highly desirable. Based on the diversity of the drug payloads, we have investigated a combinatorial-designed strategy where the nano-sized formulations are tailored based on the physicochemical properties of the drug and the delivery needs. Individually functionalized C2 to C12 lipid-, thiol-, and poly(ethylene glycol) (PEG)-modified dextran derivatives were synthesized via “click” chemistry from O-pentynyl dextran and relevant azides. These functionalized dextrans in combination with anticancer drugs form nanoparticles by self-assembling in aqueous medium having PEG surface functionalization and intermolecular disulfide bonds. Using anticancer drugs with logP values ranging from −0.5 to 3.0, the optimized nanoparticles formulations were evaluated for preliminary cellular delivery and cytotoxic effects in SKOV3 human ovarian adenocarcinoma cells. The results show that with the appropriate selection of lipid-modified dextran, one can effectively tailor the self-assembled nano-formulation for intended therapeutic payload.
Keywords: Combinatorial-designed, dextran macrostructures, “click” chemistry, anticancer drugs, and systemic chemotherapy
INTRODUCTION
Since the discovery of nitrogen mustard and folate antagonists in 1940’s, chemotherapy has become one of the main arsenals against the war on cancer [1]. Although the discovery of novel chemotherapeutic agents has led to significant excitement, one of the major challenges of cancer chemotherapy is the lack of specificity of the drugs against tumor cells. Majority of chemotherapeutic agents inhibit cellular proliferation and, as such, do not discriminate between healthy and neoplastic cells. In addition to the toxicity, poor bioavailability and short residence of systemic chemotherapy is also associated with the development of multidrug resistance (MDR) in cancer. Systemic delivery of anticancer agents that can achieve tumor specificity is highly desirable.
In an attempt to circumvent these limitations and improve systemic anticancer therapy, tremendous research efforts have been concentrated on the development of drug delivery systems, such as nanoparticles [2]. Polymeric materials, in particular, play a significant role as drug carriers and therapeutic agents can either be either physically incorporated into a polymeric matrix or covalently bound to the polymer backbone [3]. The drug carrier systems, such as encapsulated polymeric nanoparticles [4], emulsions [5], micelles [3], liposomes [6], have emerged as promising approaches in anticancer treatment with major advantages. The preferential drug localization at target sites through the “enhanced permeability and retention (EPR)” effect and lower distribution in healthy tissues and capacity to deliver hydrophobic drugs, high drug loading, and control drug release rate [7, 8] are among these advantages. With the United States Food and Drug Administration approval of albumin-taxol nanoparticles (Abraxane®)[9], doxorubicin long-circulating liposomes (Doxil®) [10], and a formulation of rapamycin encapsulated in microemulsion system (Rapamune®) [11], the development of nanoscale delivery systems for other drugs with the aim of targeting drug more onto the cancer cells and less onto healthy tissues is needed.
Synthetic and natural polymeric materials used for preparing drug delivery systems should be biocompatible such as poly(epsilon-caprolactone), poly(D,L-lactide-co-glycolide), polysaccharides, and proteins. Because of their biocompatibility, biodegradability, and cell surface recognition sites, polysaccharides are a popular class of material among them [12]. Also polysaccharides, such as dextran, chitosan and cellulose, have a large number of reactive hydroxyl groups and variable molecular weight, contributing to their structural diversity and property for intended applications. Dextran is composed of α-(1→6) and partly α-(1→3) linked D-glucose units with varying branches depending on the dextran-producing bacterial strain and it has been used clinically for more than five decades as plasma volume expansion, peripheral flow promotion, and antithrombolytic agents [13]. Dextran has no surface charge, providing additional advantage for a drug delivery system as the systems without surface charge could reduce plasma protein adsorption and increase the rate of nonspecific cellular uptake [14]. Due to the presence of high amount of hydroxyl groups facilitating the introduction of drugs into the polymer backbone, Dextran has been functionalized with various pharmaceutical agents, like naproxen [15], daunorubicin [16], mitomycin C [17], and cisplatin [18] as efficient prodrugs. Dextran is fully water-soluble and hydrophobically modified dextran forms micelles which can be used to entrap drug. Recently, Susa, et al. [19] reported the use of a lipid-modified dextran-based polymeric nanosystem for doxorubicin loading and small interfering RNA delivery in tumor cells [20]. This nanosystem showed pronounced antiproliferative effects against osteosarcoma cell lines and had potential for reversing MDR in osteosarcoma.
However, due to the fact that many newer generations of anticancer agents have varying degrees of physicochemical properties, such as molecular weight, charge, hydrophobicity, and intracellular target, there is a need to develop a versatile platform of nanoparticles that can encapsulate variety of different types of payloads. In this study, we report the development of a comprehensive and flexible dextran-based polymeric nanoparticle platform that can be customized to encapsulate therapeutic drugs with varying physicochemical properties. Individual functional blocks having (1) lipid chains (C2 to C12) for self-assembly in aqueous solution, (2) thiol groups for intermolecular disulfide crosslinking, and (3) poly(ethylene glycol) (PEG, Mw. 2kDa) for surface functionalization were synthesized from dextran (40 kDa) with controlled functionalization by “click” chemical conjugation method. With the use of combinatorial-design principles, representative anticancer drugs from the class of anthracyclines, topoisomerase inhibitors, and taxanes having different physicochemical properties were encapsulated using different combination of functional blocks utilizing different encapsulation techniques to develop a library of nanoparticle formulations. The optimized nanoparticle formulations were characterized and evaluated for preliminary cellular delivery and cytotoxic effects in SKOV3 human ovarian adenocarcinoma cells.
MATERIALS AND METHODS
Materials
All reagents were purchased from Sigma-Aldrich and used as received without further purification. Dextran from Leuconostoc mesenteroides strain with Mw 40 kDa was purchased from Sigma Chemicals (St. Louis, MO) and used as received. Rhodamine-conjugated PTX was purchased from Natural Pharmaceuticals (Beverley, MA). Cell Titre 96 Aqueous One Solution Proliferation Assay kit was purchased from Promega Corporation (Madison, WI). SKOV3 human ovarian adenocarcinoma cells were purchased from American Type Culture Collections (Manassas, VA).
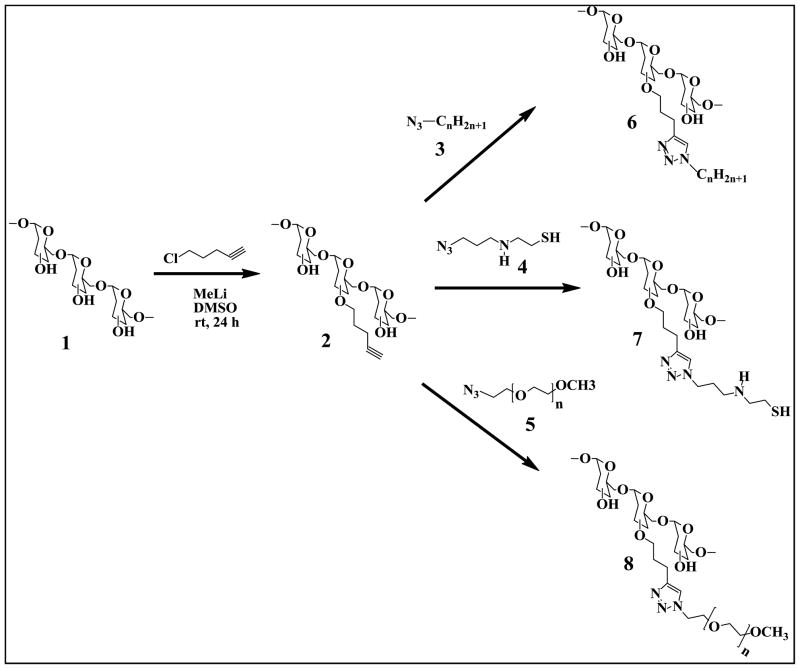
Synthesis of O-Pentynyl Dextran (2)
For conjugation of dextran, O-pentynyl moiety was attached as shown in Scheme 1. Dextran (Mw = 40kDa, 1 g, 6.17 mmol) was dissolved in dry DMSO (40 mL). After mixing to form a clear solution, 1.5 M MeLi (18.5 mL, 1.5 eq.) was added. The mixture was cooled in an ice-bath and 5-chloro-1-pentyne (0.6 mL, 0.3 eq.) was added slowly. Stirring was continued for 24 h under nitrogen atmosphere. The product was isolated by precipiting in 350 mL of ethanol, washing two times with 50 mL of ethanol, and purifying by dialysis against demineralized water and freeze drying (1.25 g, whitish solid) with degree of substitution (DS) of approximately 10%, which was determined with 1H-NMR.
Scheme 1.
The synthesis of lipid-modified (6), thiol-modified (7) and poly(ethylene glycol)-modified (8) dextrans via “click” reactions.
“Click” Synthesis of Lipid-Modified Dextran (6)
Experiments were carried out with O-pentynyl dextran and alkyl bromides (CnH2n+1, n=2, 4, 6, 8, 10, 12). In a representative experiment, O-pentynyl dextran (DS 10%, 250 mg) was dissolved in water (25 ml) and added to a round-bottom flask containing bromoethane (2g, 18.35 mmol), sodium azide (2.38 g, 36.71 mmol), and copper(II) sulfate pentahydrate (8 mg, 0.032 mmol ), sodium ascorbate (19 mg, 0.129 mmol). After stirring the mixture at room temperature for 24 h, product was purified by dialysis against demineralized water and freeze-dried (180 mg, pale green solid).
“Click” Synthesis of Thiol-Modified Dextran (7)
Sodium azide (NaN3, 0.5 g, 3.17 mmol) was added to a solution of 1-bromo-3-chloropropane (0.2 g, 3.17 mmol) in 15 mL of DMF at room temperature. The reaction mixture was allowed to stir for overnight. The reaction mixture was partitioned between ether and water, and the organic layer was washed with water, dried over Na2SO4 and concentrated to give 1-azido-3-chloropropane (0.3 g, 92%) as a colorless viscous liquid. Solution of cysteamine (0.19 g, 2.5 mmol) in THF (15 ml) was added to a stirred suspension of 1-azido-3-chloropropane (0.3 g, 2.5 mmol) in THF (15 ml). After stirring under nitrogen for 3 days at rt, the solvent was evaporated in vacuo and the yellow solid residue was washed with THF/hexane (1/5) to afforded product 4. This product was dissolved in 25 ml water and added to a round-bottom flask containing O-pentynyl dextran (DS 10%, 250 mg), copper(II) sulfate pentahydrate (8 mg, 0.032 mmol ), sodium ascorbate (19 mg, 0.129 mmol). After stirring the mixture at rt for 24 h, product was purified by dialysis against demineralized water and freeze-dried (110 mg, pale yellow solid).
“Click” Synthesis of PEG-Modified Dextran (8)
Methoxypolyethylene glycol azide 2000 (250 mg) and O-pentynyl dextran (DS 10%, 250 mg) were dissolved in 25ml water in a round-bottom flask. Copper(II) sulfate pentahydrate (8 mg, 0.032 mmol ) and sodium ascorbate (19 mg, 0.129 mmol) were added and stirred the mixture at rt for 24 h. The product was purified by dialysis against demineralized water and freeze-dried (320 mg, pale green solid)
Preparation of Drug-Encapsulated Self-Assembled Nano-Structures
Drug-loaded nanoparticles were prepared by three different processes: nano-precipitation, solvent evaporation and dialysis. Nano-precipitation method was applied to the hydrochlorides of three anthracylines and topotecan as follows: Dex-lipid (19.5 mg), Dex-thiol (15 mg) and Dex-PEG (15 mg) were dissolved together in 4 ml water and the anthracycline drug (0.5 mg) in water (1mL) was added and homogenized for 5 min at 6,000 rpm using a Silverson homogenizer. The mixture was then liophilized and the solid material obtained was dispersed in 5 ml water. Etoposide-loaded dextran nanoparticles were prepared by solvent evaporation method. Briefly, 0.5 mg of etoposide dissolved in 0.5mL of chloroform was added to 5 mL of water containing Dex-lipid (19.5 mg) and stirred vigorously. The organic solvent was evaporated by blowing nitrogen at room temperature. Then, Dex-thiol (15 mg) and Dex-PEG (15 mg) were added to the mixture and stirred well. The mixture was then liophilized and the solid material obtained was dispersed in 5 ml water. Preparation of campothecin, docetaxel and paclitaxel loaded-nanoparticles was carried out by a dialysis process. 0.5 mg of drug and Dex-lipid (19.5 mg) was dissolved in 3 ml DMSO was dialyzed against distilled water (Spectra/Por® membrane, MW cut off 8000) for 1 day. The deionized water was exchanged 5 times in the one day period. Then, Dex-thiol (15 mg) and Dex-PEG (15 mg) were added to the mixture and stirred well. The mixture was then liophilized and the solid material obtained was dispersed in 5 ml water. All of these dextran nanoformulations can be freeze-dried and stored in solid at −20 °C for several months.
Characterization Self-Assembled Nano-Structures
Particle Size and Zeta Potential
The nanoparticle size and size distribution were determined by dynamic light scattering using a ZETASIZER (3000HS, Malvern Co., UK). at a 90° fixed angle and at 25 °C temperature. The nanoparticle solution was diluted for particle size analysis with deionized distilled water (0.5 mL of nanoparticle solution in 1.5 mL of water) before analysis, and the average hydrodynamic diameter and the polydispersity index was determined. For the zeta potential, diluted nanoparticle solutions with deionized distilled water were placed in the electrophoretic cell of the Instrument’s ZetaPALS and the average surface charge was determined.
Transmission Electron Microscopy (TEM)
TEM analysis was used to determine the nanoparticle morphology. For this analysis, nanoparticle solutions were placed on Formvar-coated copper grids (Electron Microscopy Science, Hatfield, PA) and excess liquid was drained off with a Whatman filter paper. Sample grids were negatively stained with 10 μL of 2% (w/v) phosphotungstic acid, and the staining was allowed to proceed for 1 min at room temperature. After excess liquid was drained off with a Whatman filter paper, the grid containing the nanoparticle sample as a dry film was observed with a JEOL 100X transmission electron microscope (Peabody, MA).
Cellular Uptake and Intracellular Drug Distribution
SKOV3 human ovarian adenocarcinoma cell lines were grown in RPMI medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cell cultures were maintained in a humidified 95% O2/5% CO2 atmosphere at 37 °C. For the subculture, cells growing as monolayer were detached from the tissue culture flasks by treatment with 0.05% trypsin/EDTA. The viability and cell count were monitored routinely using trypan blue dye exclusion method.
Drug-loaded dextran nanoparticles were prepared as previously described for the cellular uptake test. The cells were seeded at 3 × 103 cells/well in a 96-well plate and incubated for 24 h. Then the cells were exposed to a medium containing drug alone or nanoparticles loaded with drug for further 4 hour incubation. To counter-stain nuclei, the cells were incubated with 1 μg/ml Hoechst 33342 (Invitrogen, Carlsbad, CA) for 1 minute. After washing the cells with RPMI medium three times, brightfield and fluorescence microscopy images were obtained at 60X original magnification on an Olympus fluorescence microscope.
Drug Cytotoxicity Determination
The cytotoxicity studies were performed with both free drug and the nanoparticle formulations containing graded concentrations of drug. Approximately 3,000 cells per well were seeded into 96-well plates and allowed to adhere overnight. The media was replaced with above graded concentration of drug solutions alone and in formulations. Controls included all of the blank nanoparticle formulations and media that did not have any drug. RPMI growth media was used as a negative control and treatment with 0.25 mg/mL poly(ethyleneimine) (molecular weight 10 kDa), a cationic cytotoxic polymer, was used as positive control. Four replicates were made for each test condition, and plates were incubated for 3 days. At the end, the media was replaced with 100 μL of RPMI media and MTS reagent 20 μL per well and the plates were incubated for 2 h. Cell viability was determined spectrophotometrically by measuring the conversion of the MTS substrate to the formazan product which is directly proportional to the number of viable cells. The plates were read at 590 nm using Bio-Tek Synergy HT plate reader (Winooski, VT), and the percent cell viability values were determined relative to the negative control. The concentrations of drug in solution and drug-loaded nanoparticles producing 50% inhibition of cell viability (IC50) were calculated using GraphPad Prism 5.
RESULTS AND DISCUSSION
“Click” Synthesis of Dextran Derivaties (6–8)
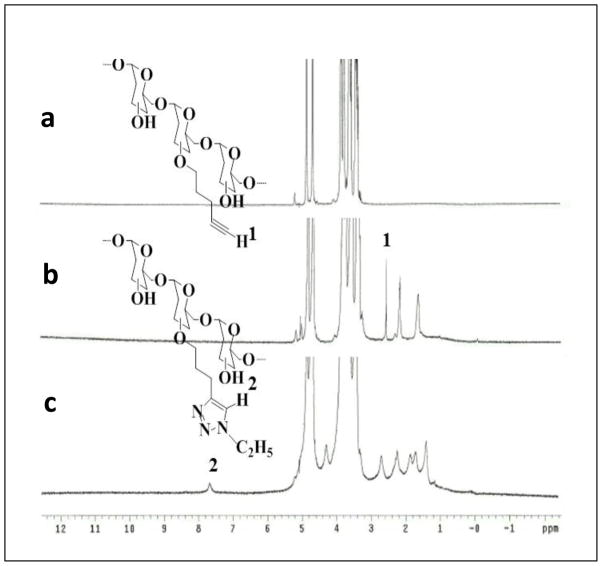
In order for “click chemistry” to be applied to the synthesis of lipid, thiol and PEG-modified dextrans, dextran needs to contain an alkyne or azide moiety. O-pentynyl dextran (2) was synthesized by etherification of dextran (1) in DMSO with 5-chloro-1-pentyne using MeLi as base. The transformation of O-pentynyl dextran dissolved in H2O with relevant azides forming a 1,4-disubstituted 1,2,3-triazole linker for the covalent attachment of fatty acid chains, PEG chains, and thiol groups to the polymer backbone in the presence of copper( II) sulfate pentahydrate and sodium ascorbate was successful as presented in Scheme 1. To obtain lipid-modified dextran library (6), in situ prepared alkyl azides by a heterogeneous nucleophilic displacement reaction in H2O with sodium azide were used. Azide 4 was obtained from cysteamine with the reaction of 1-azido-3-chloropropane prepared from 1-bromo-3-chloropropane following published procedures [21, 22]. PEG modified dextran was made with the direct use of commercially available methoxy-polyethylene glycol azide 2000MW. 1H NMR spectrum was used to confirm the modification of dextran. 1H NMR spectra of dextran, O-pentynyl dextran and Dex-lipid (C2H5) are shown in Figure 2 for a representative example. The alkyne proton peak at 2.5 ppm is observed in 1H NMR spectrum of O-pentynyl dextran (Figure 2b). In the 1H NMR spectrum of Dex-lipid (C2H5) (Figure 2c), the alkyne signal disappears and the appearance of a signal at 7.6 ppm confirm the formation of 1,4-disubstituted 1,2,3-triazole. The degree of substitution (DS) of O-pentynyl dextran was calculated by the proton peak areas of CH2 of pendant pentynyl (at 1.6 ppm) and the proton peak areas of dextran (at 4.9 ppm) in the 1H NMR spectrum.
Figure 2.
1H-NMR spectra of dextran (Mw = 40 kDa) (a); O-pentynyl dextran (40k) (b); and (c) C2 lipid-modified dextran (40k).
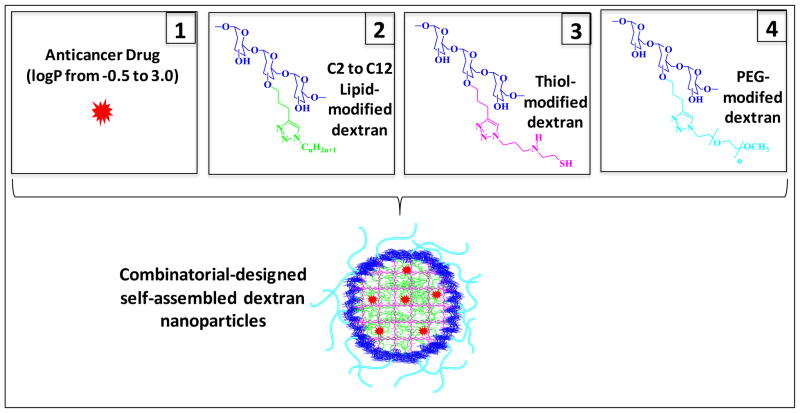
Combinatorial-Designed Nanoparticle Preparation and Characterization
The lipid-modified dextran could easily self-assemble in aqueous milieu as a result of hydrophobic interactions to form spherical nanoparticles called micelles. Thus, hydrophobic drugs can be entrapped into the micelle core non-specifically through hydrophobic interactions. Dextran micelles have been reported as a potential candidate for doxorubicin delivery due to their increased loading capacities and adjustable drug release [23]. Also different therapeutic drugs with different hydrophobicities could be loaded into the dextran micelles efficiently by adjusting the hydrophobic core environment with the variation of lipid chain length. Stability of micelles is a critical factor for effective drug delivery in vivo. The micelles must not dissociate to release the entrapped drug until they reach the target site. By adding thiol-modified dextran to the system, the micelle cores could be cross-linked reversibly by oxidation of thiol groups. This will allow specific intracellular release of drug because the disulfide cross-links could be cleaved only in the presence of strong reducing environment. The long-circulating property and preferential tumor targeting by means of the EPR effect for the nanoparticles can be obtained by having a poly(ethylene glycol) modified surface. PEG-modified dextran could be used in combination with lipid- and thiol-dextrans for this purpose. In order to develop a safe and effective systemically-administered nano delivery system for cancer, these three modified-dextrans (lipid, thiol and PEG) were used in this study. The disulfide bond cross-linked dextran nanoparticles remain more stable without drug leaching (especially with highly hydrophobic drugs such as docetaxel and paclitaxel) in reconstituting the freeze-dried samples. We have compared this stability with nanoparticles without thiol-modified dextran. This property may provide an evidence for the existence of cross-linked network in the nanostructures.
Representative anticancer drugs from three different drug classes (anthracyclines, topoisomerase inhibitors, and taxanes) selected for this study have different physicochemical properties. Their hydrophobicities, as measured by using partition coefficient (i.e., logP values), vary from −0.5 to 3 as water soluble salts to water insolubles compounds. The encapsulation efficiency of the drugs in the hydrophobic dextran core and the stability of the drug-loaded nanoparticles could be mainly based on two factors: the log P of the drug and the length of lipid chain of the modified dextran. To provide the dextran platform which can be used to load a drug efficiently, “mix and match” type screening was done with systematic variations of the lipid chain length of the modified dextran. All the lipid and drug combinations tested are summarized in Figure 1. The effect of the lipid chain on the hydrophobicity of the drug was studied and drug-loaded dextran polymer nanoparticle libraries were prepared in a combinatorial format, allowing for the preparation of up to 8 different nano-formulations with the same drug. Since anthracyclines and topotican are very hydrophilic as hydrochlorides, nano-precipitation approach was used as to incorporate the drug into the dextran core. It was found that the hydrochloride salt of the drugs is converted to the base form as a result of the slight basic condition of the solution due to the presence of triazole moieties. Additionally, anthracylines’ interactions could be strong with the core of the nanoparticle with π-π stacking interactions [24] between the heteroaromatic 1,4-triazole and the aromatic moiety of anthracycline. The highest drug loading capacity for these drugs (~ 85%) was obtained with ethyl (C2H5)-bearing lipid dextrans. The loading capacity was determined by ultracentrifugation of the nanoemulsion and drug quantification of the supernatant solution by UV-spectroscopy. Etoposide-loaded dextran nanoparticles were prepared by solvent evaporation method and butyl (C4H9) lipid dextran was found to be the most suitable one with the formation of stable nanoformulation. For the preparation of campothecin and taxanes (docetaxel and paclitaxel) loaded-nanoparticles, a dialysis process was carried out and hexyl (C6H13) and octyl (C8H17) dextran derivatives, respectively yielded stable nanoparticles (Table 1).
Figure 1.
Schematic illustration for combinatorial approach in designing nanoparticle assemblies using C2 to C12 lipid-modified, thiol-modified, and poly(ethylene glycol) (PEG)-modified dextrans.
Table 1.
Particle size and surface charge of drug-encapsulated combinatorial-designed dextran nanoparticles
| Drug | Lipid-Modifed Dextran | Drug LogP | Hydrodynamic Diameter | Zeta Potential (mV) | |
|---|---|---|---|---|---|
| Mean Diameter (nm) | Polydispersity Index | ||||
| Doxorubicin | C2H5 | −0.50 | 295 ± 1.7* | 0.2 | −1.21 ± 3.31 |
| Daunorubicin | C2H5 | 0.10 | 245 ± 1.5 | 0.2 | −1.33 ± 2.55 |
| Idarubicin | C2H5 | 0.20 | 233 ± 1.5 | 0.2 | −1.45 ± 3.22 |
| Topotecan | C2H5 | 0.80 | 193 ± 1.3 | 0.3 | −1.35 ± 3.61 |
| Etoposide | C4H9 | 1.00 | 137 ± 1.4 | 0.3 | −3.38 ± 3.77 |
| Camptothecin | C6H13 | 1.74 | 187 ± 1.6 | 0.4 | −3.68 ± 2.01 |
| Docetaxel | C8H17 | 2.40 | 212 ± 1.4 | 0.2 | −3.58 ± 2.23 |
| Paclitaxel | C8H17 | 3.00 | 225 ± 1.5 | 0.2 | −4.11 ± 1.89 |
Mean ± SD, n = 3.
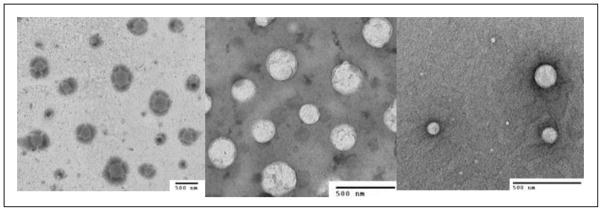
Particle size and zeta potential (surface charge) values of nanoformulations are summarized in Table 1. The average particle sizes of the nanoformulations vary from 130 nm to 300 nm. This may be caused by the physicochemical properties of the drug, length of the lipid chain and the nanoparticle fabrication method. The average surface charges of the nanoparticles in the formulation were in the range of −1.21 to − 4.11 mV. TEM micrographs of the nanoemulsions of representative drugs, doxorubicin, topotecan and paclitaxel, showed that the nanoparticles were spherical (Figure 3). In this study, we used Mw 40 kDa Dextran polymers for the preparation of nanoparticles. With the use of Mw 10 kDa Dextran, smaller size particles (~ 90 nm) could be obtained. Hence, the particle size can be controlled with the use of dextran polymer having the suitable molecular weight.
Figure 3.
Transmission electron microscopy images of drug-loaded combinatorial-designed dextran nanoparticles containing doxorubicin (a); topotecan (b); and paclitaxel (c).
Cellular Uptake and In Vitro Drug Cytotoxicity Studies
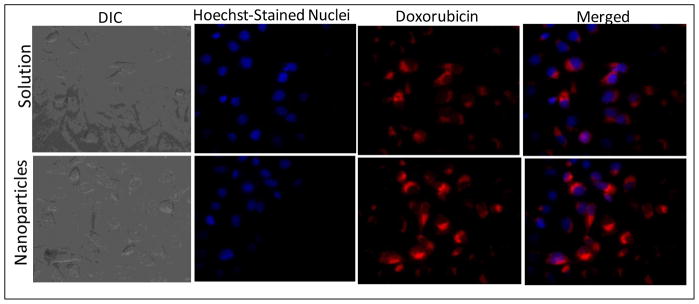
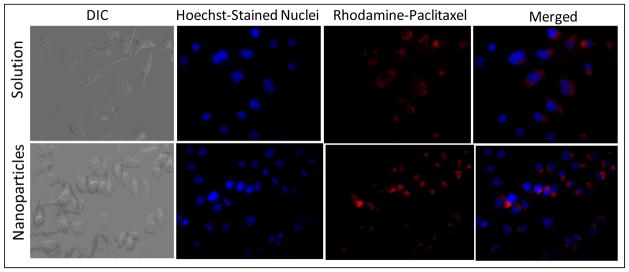
Figure 4 presents the cellular images of SKOV3 human ovarian adenocarcinoma cells after incubation with doxorubicin in solution and with C2 lipid-modified dextran nanoparticles. The red fluorescence in the cells indicates intracellular doxorubicin delivery. The results show that nanoparticle-encapsulated doxorubicin was efficiently internalized in SKOV3 cells relative to the drug in solution. There was also greater nuclear localization of doxorubicin administered in nanoparticle formulations. Figure 5 shows the images of rhodamine-paclitaxel administered either in solution or in C8 lipid-modified dextran nanoparticles. Similar to the doxorubicin data in Figure 4, the results convincingly show that there was greater rhodamine-paclitaxel accumulation in cells with the nanoparticle formulation relative to the administration of the agent in solution. The free drug molecules could internalize into the cancer cells with molecular diffusion mechanism. However, when the drug was loaded into dextran nanoparticles, the cellular uptake mechanism might be more efficient endocytosis pathway through cell membrane.
Figure 4.
Differential interference contrast (DIC) and fluorescence microscopy images of intracellular accumulation of doxorubicin administered in aqueous solution or in C2 lipid-modified dextran nanoparticles after 4 hours of incubation with SKOV3 human ovarian adenocarcinoma cells. The cell nuclei were stained with Hoechst dye, while the doxorubicin accumulation was observed with intrinsic fluorescence. Original magnification was 60×.
Figure 5.
Differential interference contrast (DIC) and fluorescence microscopy images of intracellular accumulation of rhodamine-labeled paclitaxel administered in aqueous solution or in C8-lipid modified dextran nanoparticles after 4 hours of incubation with SKOV3 human ovarian adenocarcinoma cells. The cell nuclei were stained with Hoechst dye, while the doxorubicin accumulation was observed with intrinsic fluorescence. Original magnification was 60×.
Cytotoxicities of drug, blank, and drug-loaded dextran nanoparticles against SKOV3 human ovarian adenocarcinoma cells were investigated. Using the MTS method, the 50% cellular growth inhibitions (IC50) within 72 h (anthracyclines and taxanes) and 96 h (topoisomerase inhibitors) were determined. The blank dextran nanoparticles showed 100% cell viability relative to the negative control, indicating that the dextran materials used in this study is non-toxic. The in vitro cytotoxicity studies against drug alone and drug-loaded nano-formulation are summarized in Table 2. All the therapeutic drug formulations showed higher cytotoxicities. These results show that dextran nano-formulations have higher efficiency in delivering drugs to SKOV3 cells. These nanoparticles are pegylated with a terminal methoxy group. By introducing terminus functionalities such as hydrazide and NHS, PEG-bearing dextran can be modified to incorporate targeting ligands at tunable densities. These targeted nanoparticles may provide greater intracellular delivery of therapeutic agents to the cancer cells within solid tumors than their nontargeted analogs.
Table 2.
The 50% inhibitory concentration values of anticancer drugs with different logP in SKOV3 human ovarian adenocarcinoma cells administered either in aqueous solution or in dextran nanoparticles
| Drug | Lipid- Modified Dextran | Drug LogP | IC50 Values | |
|---|---|---|---|---|
| Drug in Solution | Drug - Loaded Dextran Nanoparticles | |||
| Doxorubicin | C2H5 | − 0.50 | 0.56 ± 0.11* μM | 0.22 ± 0.08 μM |
| Daunorubicin | C2H5 | 0.10 | 0.50 ± 0.09 μM | 0.26 ± 0.06 μM |
| Idarubicin | C2H5 | 0.20 | 0.70 ± 0.13 μM | 0.40 ± 0.11 μM |
| Topotecan | C2H5 | 0.80 | 0.36 ± 0.06 μM | 0.21 ± 0.04 μM |
| Etoposide | C4H9 | 1.00 | 1.57 ± 0.23 μM | 1.36 ± 0.14 μM |
| Camptothecin | C6H13 | 1.74 | 0.44 ± 0.05 μM | 0.26 ± 0.07 μM |
| Docetaxel | C8H17 | 2.40 | 13.67 ± 1.41 nM | 7.51 ± 1.52 nM |
| Paclitaxel | C8H17 | 3.00 | 17.33 ± 2.08 nM | 10.45 ± 1.31 nM |
Mean ± SD, n = 4.
CONCLUSIONS
Dextran was individually functionalized with lipid, thiol and PEG via a click chemistry methodology. The combined molecules self-assemble into nanoparticles in water, with PEG surface modification and intramolecular disulfide bond formation, making them an interesting novel potential drug delivery system. It has been shown that different therapeutic drugs with different physicochemical properties can be loaded into the nanosystem from a combinatorial approach where encapsulation efficiency and stability of the formulation depends on the lipid chain length and the preparation technique. Cell uptake and in vitro cytotoxicity studies indicated that the dextran therapeutic drug nano-formulations were more effective for suppressing SKOV3 cells than the drug solution. Overall, the results suggest that designing dextran nano-formulations based on combinatorial principles is a promising approach for the delivery of wide array of anticancer drugs.
Acknowledgments
This study was supported by the National Cancer Institute’s Alliance in Nanotechnology for Cancer Platform Partnership grant U01-CA151452. Transmission electron microscopy analysis was performed by Ms. Jing Xu at the Electron Microscopy Center of Northeastern University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Papac RJ. Origins of cancer therapy. Yale J Biol Med. 2001;74(6):391–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Haag R. Supramolecular drug-delivery systems based on polymeric core-shell architectures. Angew Chem Int Ed Engl. 2004;43(3):278–82. doi: 10.1002/anie.200301694. [DOI] [PubMed] [Google Scholar]
- 3.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2(5):347–60. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 4.Hartman KB, Wilson LJ, Rosenblum MG. Detecting and treating cancer with nanotechnology. Mol Diagn Ther. 2008;12(1):1–14. doi: 10.1007/BF03256264. [DOI] [PubMed] [Google Scholar]
- 5.Terwogt JM, et al. Alternative formulations of paclitaxel. Cancer Treat Rev. 1997;23(2):87–95. doi: 10.1016/s0305-7372(97)90022-0. [DOI] [PubMed] [Google Scholar]
- 6.Yowell SL, Blackwell S. Novel effects with polyethylene glycol modified pharmaceuticals. Cancer Treat Rev. 2002;28(Suppl A):3–6. doi: 10.1016/s0305-7372(02)80002-0. [DOI] [PubMed] [Google Scholar]
- 7.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54(5):631–51. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 8.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 9.Blum JL, et al. Phase II study of weekly albumin-bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanes. Clin Breast Cancer. 2007;7(11):850–6. doi: 10.3816/CBC.2007.n.049. [DOI] [PubMed] [Google Scholar]
- 10.Muggia FM. Doxil in breast cancer. J Clin Oncol. 1998;16(2):811–2. doi: 10.1200/JCO.1998.16.2.811. [DOI] [PubMed] [Google Scholar]
- 11.Sehgal SN. Rapamune (Sirolimus, rapamycin): an overview and mechanism of action. Ther Drug Monit. 1995;17(6):660–5. doi: 10.1097/00007691-199512000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, et al. Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev. 2008;60(15):1650–62. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Thoren L. The dextrans--clinical data. Dev Biol Stand. 1980;48:157–67. [PubMed] [Google Scholar]
- 14.Alexis F, et al. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–15. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harboe E, et al. Macromolecular prodrugs. XV. Colon-targeted delivery--bioavailability of naproxen from orally administered dextran-naproxen ester prodrugs varying in molecular size in the pig. Pharm Res. 1989;6(11):919–23. doi: 10.1023/a:1015981126732. [DOI] [PubMed] [Google Scholar]
- 16.Levi-Schaffer F, et al. Reduced toxicity of daunorubicin by conjugation to dextran. Cancer Treat Rep. 1982;66(1):107–14. [PubMed] [Google Scholar]
- 17.Kojima T, et al. Mitomycin C-dextran conjugate: a novel high molecular weight pro-drug of mitomycin C. J Pharm Pharmacol. 1980;32(1):30–4. doi: 10.1111/j.2042-7158.1980.tb12840.x. [DOI] [PubMed] [Google Scholar]
- 18.Ohya Y, et al. Design of macromolecular prodrug of cisplatin using dextran with branched galactose units as targeting moieties to hepatoma cells. Biomacromolecules. 2001;2(3):927–33. doi: 10.1021/bm010053o. [DOI] [PubMed] [Google Scholar]
- 19.Susa M, et al. Doxorubicin loaded Polymeric Nanoparticulate Delivery System to overcome drug resistance in osteosarcoma. BMC Cancer. 2009;9:399. doi: 10.1186/1471-2407-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Susa M, et al. Inhibition of ABCB1 (MDR1) expression by an siRNA nanoparticulate delivery system to overcome drug resistance in osteosarcoma. PLoS One. 2010;5(5):e10764. doi: 10.1371/journal.pone.0010764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao L, Smith BT, Aube J. Base-promoted reactions of bridged ketones and 1,3- and 1,4-haloalkyl azides: competitive alkylation vs azidation reactions of ketone enolates. J Org Chem. 2004;69(5):1720–2. doi: 10.1021/jo0356098. [DOI] [PubMed] [Google Scholar]
- 22.Cressier D, et al. Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorg Med Chem. 2009;17(14):5275–84. doi: 10.1016/j.bmc.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 23.Du YZ, et al. Synthesis and antitumor activity of stearate-g-dextran micelles for intracellular doxorubicin delivery. ACS Nano. 2010;4(11):6894–902. doi: 10.1021/nn100927t. [DOI] [PubMed] [Google Scholar]
- 24.Crescenzi V, et al. Novel hydrogels via click chemistry: synthesis and potential biomedical applications. Biomacromolecules. 2007;8(6):1844–50. doi: 10.1021/bm0700800. [DOI] [PubMed] [Google Scholar]