Abstract
Activation of the immune system via administration of cytokines is used for the treatment of chronic viral infections such as hepatitis C and for cancers resistant to radiotherapy. Cytokine-based treatments induce a range of “sickness” behaviors (e.g. depression, anxiety, pain, anorexia, and fatigue). Activation of the hypothalamic pituitary-adrenal axis via the induction of corticotropin releasing factor (CRF) may underlie these unwanted side effects. This study used repeated systemic injections of the pro-inflammatory cytokine interleukin-1β (IL-1β) to model the sickness behaviors and biochemical effects of immune system activation. We assessed the ability of CRF type I receptor (CRF1) antagonism to reduce biochemical and behavioral signs of sickness induced by IL-1β treatment. Forty Wistar rats were assigned to one of four groups: 1) saline + vehicle; 2) saline + DMP904 (CRF1 antagonist); 3) IL-1β + vehicle; 4) IL-1β + DMP904. Rats received intraperitoneal injections of either DMP904 or vehicle and of IL-1β or saline for six days. Sickness behavior was evaluated using body weight assessments and forced swim testing (FST). Blood and brain samples were collected to measure cytokine, p38 mitogen activated protein kinase (MAPK), and phospho-p38 MAPK levels using multiplex techniques. There were significant reductions in body weights and FST immobility times associated with IL-1β administration. Rats administered IL-1β had significantly higher serum levels of IL-10, but not interferon-γ. Within the hippocampus, IL-1β reduced levels of p38 MAPK, but had no impact on levels of phospho-p38 MAPK except in the presence of DMP904. When administered alone, DMP904 had no significant effect on p38 MAPK or phospho-p38 MAPK in the hippocampus, but when given with IL-1β led to increased phosphorylation of p38 MAPK. IL-1β and DMP904 reduced levels of p38 MAPK within the hypothalamus, while co-administration of IL-1β and DMP904 abolished the effects of either drug alone. IL-1β decreased immobility time in the FST, and led to reductions in body weight, changes in serum cytokine levels and p38 MAPK regulation within the hippocampus and hypothalamus. DMP904 blocked some of the neurochemical effects of IL-1β, but did not impact the behavioral measures, or serum cytokines. Thus, additional studies will be needed to determine whether CRF1 antagonism is an effective treatment for cytokine-induced sickness.
1. Introduction
Activation of the immune system via administration of cytokines is used for the treatment of chronic viral infections such as hepatitis C and for cancers that are resistant to radiotherapy (Loftis and Hauser, 2004; Wood et al., 2006a). Unfortunately, a host of common side effects collectively termed “sickness” behavior frequently accompany treatment with pro-inflammatory cytokines. These side effects, which have overlapping features with major depressive disorder, generally fall into one of two dimensions, neurovegetative or psychological (Capuron et al., 2002). The neurovegetative dimension affects the majority of patients and is characterized by fatigue, loss of appetite and sleep disruption. The psychological dimension affects a subset of patients and is characterized by depressed mood, anxiety and cognitive dysfunction. The neurochemical mechanisms that underlie these two dimensions appear to differ and therefore respond differentially to treatment by drugs such as antidepressant medications (Capuron et al., 2002; Capuron et al., 2004). In particular, the neurovegetative symptoms are largely resistant to treatment with traditional selective serotonin re-uptake inhibitor (SSRI) antidepressants (e.g. paroxetine) (Capuron et al., 2002; Papakostas et al., 2003). The side effects of cytokine-based treatments present significant barriers to patient adherence, therefore therapeutics that reduce these unwanted symptoms are needed.
Systemic administration of the pro-inflammatory cytokine interleukin-1β (IL-1β) induces sickness behaviors such as taste aversions (Tazi et al., 1988), impairments in operant responding for food (Crestani et al., 1991), and reductions in social behavior (Kent et al., 1992) in rodents. In humans, IL-1β levels significantly correlate with fatigue in cancer patients (Greenberg et al, 1993; Bower et al., 2002) and with depressive symptoms in patients with hepatitis C (Loftis et al. 2008). IL-1β is critically involved in the innate immune response and is primarily produced by activated macrophages and blood monocytes, but is also found in dendritic cells, natural killer cells and B lymphocytes (Dinarello, 2009). Within the central nervous system, IL-1β is produced by astrocytes, oligodendrocytes, microglia and perivascular macrophages/monocytes (Sairanen et al., 1997). The physiological effects of activation of the IL-1 receptor 1 (IL-1R1) are hypotension, fever, neutrophilia, thrombocytosis and production of acute-phase proteins (Gabay et al., 2010). Typically, toll-like receptor stimulation in response to microbial infection results in binding of IL-1β to the IL-1R1 and leads to activation of: nuclear factor κ B (NFκB), p38 mitogen-activated protein kinase (p38 MAPK) as well as other kinases, and the hypothalamic pituitary-adrenal (HPA) axis. These pathways share common effector proteins with signals that are putatively involved in depression (Felger et al. in press). For example, MAPK regulation of the serotonin transporter is associated with behavioral despair [measured by the tail suspension test (TST) and forced swim test (FST)] induced by lipopolysaccharide (LPS) treatment in mice (Zhu et al., in press), and blockade of p38 MAPK activation disrupted the ability of the chemotherapy drug VP-16 to induce sickness behavior (Wood et al., 2006b). Thus, administration of inflammatory agents such as IL-1β and LPS causes activation of p38 MAPK, the HPA axis, and NFκB which appears to mediate some of the sickness and depressive behavioral effects of these compounds. Consequently, for this study, we used seven days of repeated IL-1β treatment to model the increases in inflammatory factors observed in major depressive disorder (Owen et al., 2001; Leo et al., 2006; Huang and Lee, 2007; reviewed in Howren et al., 2009) and the repeated dosages of pro-inflammatory cytokines used in cancer and viral (e.g. hepatitis C) treatments (Capuron et al., 2004; Felger et al., 2011; reviewed in Loftis and Hauser, 2004).
A complex interaction exists between cytokines acting in the brain and the periphery. The HPA axis contributes to the complexities associated with this bi-directional communication and plays a central role in many of the observed sickness behaviors associated with inflammatory cytokine-based treatments (Gilbertson-White et al., in press). Activation of corticotropin releasing factor (CRF) neurons by IL-1β is a major mechanism through which the HPA axis is triggered, and in vitro studies demonstrate that CRF causes activation of the p38 MAPK pathway (Dermitzaki et al., 2002; Park et al., 2005). CRF is centrally involved in endocrine and behavioral responses to stress (Vaughan et al., 1995) and may contribute to localized inflammation (Jones et al., 1989). Postmortem brains of depressed patients exhibited a large increase in the number of CRF expressing neurons within the paraventricular nucleus of the hypothalamus (Raadsheer et al., 1994). CRF activation is also associated with increased susceptibility to viral infections, slower wound healing and a poorer response to vaccination (Sternberg, 2006). Collectively, these findings and others show that CRF activation mediates some of the sickness behaviors resulting from immune system activation (Capuron et al. 2002; Dunn and Swiergiel 2008).
Although cytokine-induced depression is well-established (Loftis and Hauser, 2004; Anisman, 2009), the specific mechanisms that underlie the activation of the innate immune system and expression of sickness symptoms remain poorly understood. Cytokines affect several biological systems involved in depression and sickness behavior, such as the HPA axis and the serotonergic and noradrenergic networks (Hsieh et al., 2010; reviewed in Dantzer et al., 2011). Current pharmacological treatments for depression primarily target serotonin and other monoamine pathways in the brain. Therapies that alter the regulation of the HPA axis have also been suggested as possible treatments (Schüle et al., 2009). Pharmacological agents that inhibit the activity of CRF; a hormone that regulates the activity of the HPA axis, like CRF receptor 1 (CRF1) antagonists, demonstrate antidepressant activity in human (Zobel et al., 2000; Valdez, 2006; Valdez, 2009; Zorrilla and Koob, 2010) and animal (Nielsen et al., 2006) studies. Subsequent studies in humans, however, indicate that CRF1 antagonism may have limited efficacy as an antidepressant (e.g. Binneman et al. 2008). Nevertheless, CRF1 antagonists have fewer known side effects than current pharmacological therapies (Nielsen et al., 2006), and may be effective in patients unresponsive to currently available treatments. In addition, their impact on depressive symptoms may provide insights regarding the specific biological systems involved in depressogenesis and sickness behavior.
The purpose of this study was to determine the impact of repeated IL-1β administration on sickness behavior, brain-region specific p38 MAPK activation, and the peripheral immune response. We used body weight changes to examine the neurovegetative symptoms, and the FST to assess psychological symptoms (i.e. behavioral despair). Interleukin 10 (IL-10) and interferon γ (IFN-γ) were used as markers of anti- and pro-inflammatory cytokines, respectively. We also examined whether co-administration of the CRF1 receptor antagonist DMP904, along with IL-1β could reduce the behavioral or biochemical effects of IL-1β. Expression and phosphorylation of p38 MAPK was measured in the hypothalamus and hippocampus. The peripheral immune response was measured using blood serum samples.
2. Materials and methods
2.1 Animals
Forty male Wistar rats (Charles River Laboratories, Wilmington, MA) between 328 and 429 g were housed individually in Lexan plastic cages. Food and water were available ad libitum in the home cage. Animals were kept on a 12h-12h light/dark cycle (lights on from 0700-1900). All experiments and injections took place during the light cycle. Wistar rats were chosen for this study because they are more sensitive to CRF1 antagonists on the FST than Sprague-Dawley rats (Li et al., 2005; Nielsen, 2006). Animals were randomly assigned to 1 of the following 4 treatment groups (n = 10/group): saline and vehicle (Sal-Veh), saline and DMP904 (Sal-DMP), IL-1β and vehicle (IL-Veh), and IL-1β and DMP904 (IL-DMP). All experiments were carried out with approval from the local institutional animal care and use committee at Reed College, Portland, Oregon, and in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
2.2 Reagents
Recombinant human IL-1β (PeproTech, Rocky Hills, NJ; catalog # 200-01B) was reconstituted using 0.9% sterile saline to a concentration of 2 μg/ml. Reconstituted IL-1β was divided into daily aliquots, and stored at −20°C. DMP904 (Bristol-Myers Squibb, New Brunswick, NJ) was dissolved in 10% dimethyl sulfoxide (DMSO), 5% Tween 80, and deionized water to obtain a concentration of 6 mg/ml (Wang et al., 2001). DMP904 solutions were prepared within 3 hours of use. A 10% DMSO, 5% Tween 80, in deionized water vehicle was prepared daily.
2.3 Drug dosage
Animals received daily intraperitoneal (i.p.) injections of either IL-1β (2 μg/rat) or an equal volume of sterile saline, and of DMP904 (4-(3-pentylamino)-2,7-dimethyl-8-(2-methyl-4-methoxyphenyl)-pyrazolo-[1,5-a]-pyrimidine) (6 mg/ml/kg) or an equal volume of vehicle (Fig. 1). Dosage for IL-1β was based on previous studies using repeated IL-1β administration schedules. Administration of 2 and 4 μg/day (but not 0.5 μg/day) for 7 days i.p. induces weight loss and sickness behavior in rats, as indicated by fever, and reductions in food intake and body weight gain (Hermus et al., 1992). A dose of 2 μg/day also decreases social behavior when administered for either 1 or 14 days (Dantzer et al., 1991). Previous research on DMP904 primarily employed oral administration (Hogan et al., 2005; Lelas et al., 2004). Dosage for repeated i.p. administration was based on the recommended therapeutic dose provided by Bristol-Myers Squibb (Nicholas Lodge, Ph.D., personal communication).
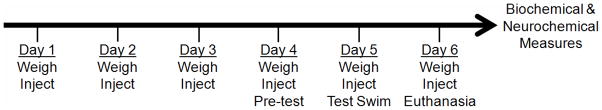
Figure 1.
Time line of experimental events. All animals were weighed and injected daily with saline, IL-1β, vehicle, or DMP904. Pre-test, test swim and animal euthanasia took place 1 h after injections.
2.4 Forced swim test (FST)
Rats were placed in a clear acrylic cylinder (40 cm height; 18 cm diameter) filled to 30 cm with water. To avoid temperature-related stress responses, the water temperature was maintained at 25°C (±2°C). The water was sufficiently deep that the rats would swim or float in the water without limbs or tail touching the bottom of the container. Rats were exposed to a 15 minute practice swim (pre-test), followed 24 hours later by a 5 minute test swim. The test swim was recorded by a video camera positioned above the container. Due to experimenter error, data from three animals was not included in the analysis (n = 1 animal from each of the following groups: Sal-Veh, Sal-DMP, IL-DMP). Two blinded raters independently scored video recordings of test swims. Raters were trained to distinguish 3 behaviors: swimming, climbing and immobility. These behaviors were defined by Cryan et al. (2002):
Immobility – floating in the water without struggling and using only small movements to keep the head above water
Swimming – moving limbs in an active manner (more than required to keep head above water) causing movement around the cylinder
Climbing – making active movements with the forepaws in and out of the water, usually directed against the wall.
Raters scored the predominant behavior during 5 second intervals of the 5 minute test swim. The main dependent variable for this task was time spent immobile, and the inter-rater reliability for this measure was good (Pearson’s r = 0.742, p < 0.001).
2.5 Experimental schedule
Animals were weighed and injected at the same time (during the light cycle) daily for six consecutive days (Fig. 1). The pre-test for the FST was given on Day 4, the test swim on Day 5, and animals were euthanized on Day 6. Previous findings suggest that both IL-1β (Kent et al., 1992; Song et al., 2006) and DMP904 (Li et al., 2005; Lelas et al., 2004) are maximally effective 1–2 hours following administration. For this reason, the pre-test, test swim, and blood and brain collection procedures were all performed approximately 1 hour after the daily injection.
2.6 Blood and brain collection
Animals were deeply anesthetized with a ketamine/xylazine cocktail for cardiac blood collection (Vacutainer SST; BD Diagnostics, Franklin Lakes, NJ) and brain dissection. Following cardiac blood draws, rats were decapitated by guillotine and brains were extracted and microdissected on ice to obtain the hypothalamus and hippocampus, brain regions known to be involved in the stress response (Conrad and Bimonte-Nelson, 2010). Brain tissue was snap frozen on dry ice and stored at −80°C until analyzed. Blood samples were spun at 1660 × g in vacuum collection tubes and the resultant serum isolated and stored at −80°C until analyzed.
2.7 Cytokine multiplex assays
Serum samples were analyzed using a Rat Cytokine/Chemokine LINCOplex kit (Millipore, Billerica, MA formerly Linco Research, St. Charles, MO) and Luminex100 (Luminex Corporation, Austin, TX) system. We measured IFN-γ [to assess type 1 helper T cell activation (Th1); minimum detectable concentration = 0.7 pg/mL], and IL-10 [to assess type 2 helper T cell activation (Th2); minimum detectable concentration = 1.47 pg/mL]. Serum from each animal was run in duplicate, and the resultant determinations were averaged. In some cases, the concentration of cytokine in a specific well was below the detection threshold. If the duplicate well had a measurable concentration, this value was used for the animal. In cases where both wells of the same sample were below the detection threshold (IFN-γ, n = 2 (1 animal that received IL-1β, and 1 animal that received saline); IL-10, n = 14 (all were from the groups that received saline)), the lowest detected concentration was substituted. This substitution shifted values closer to the overall mean, decreasing the likelihood of a type I error.
2.8 Determination of p38 and phospho-p38 MAPK brain expression
Levels of p38 and phospho-p38 (p-p38) in rat hippocampus and hypothalamus were measured in duplicate by commercial assay services (AssayGate, Inc., Ijamsville, MD) using bead-based suspension protein arrays and the Bio-Plex 200 Bead Reader System (described in Petty et al., 2009). Antibodies were labeled with different concentrations of fluorophores to generate distinct bead sets. Bead sets were coated with capture antibody specific for each protein or phospho-protein. Bound analyte was detected using a biotinylated detection antibody and streptavidin-phycoerythrin. Analyses were done using a dual laser, flow-based, sorting and detection platform. One laser was bead-specific and determined a given antibody bound to protein. The other laser determined the magnitude of phycoerythrin-derived signal, which is in direct proportion to the amount of protein bound. The protein concentrations for the tissue homogenates were similarly determined by AssayGate, Inc. Data are reported as pmol per mg total protein, and as ratios of p-p38 to total p38.
2.9 Statistical analysis
Data were analyzed by analysis of variance (ANOVA) followed, when appropriate, by ANOVAs or t-tests to detect differences between experimental groups. Between subject factors were IL-1β administration and DMP904 treatment. Days was included as a within subject factor in the analysis of weight changes. Huynh-Feldt corrections were applied to repeated measures ANOVAs when sphericity assumptions were violated. The cytokine data (IL-10 and IFN-γ) was transformed using a natural logarithm to reduce skewness and/or kertosis. Transformations were not necessary for any of the other dependent measures. Spearman’s correlations were used to examine relationships between the concentration of individual cytokines, sickness and depressive behaviors and brain region-specific p38 and p-p38. Statistical analyses were performed using SPSS 12.0.1 for Windows (SPSS Inc., Chicago, IL). Graphs were created using GraphPad Prism software, version 4.03 (GraphPad Software, Inc., La Jolla, CA). Statistical significance was maintained at p < 0.05, with trends reported at p < 0.10.
3. Results
3.1 CRF antagonism does not affect IL-1β induced reductions in body weight
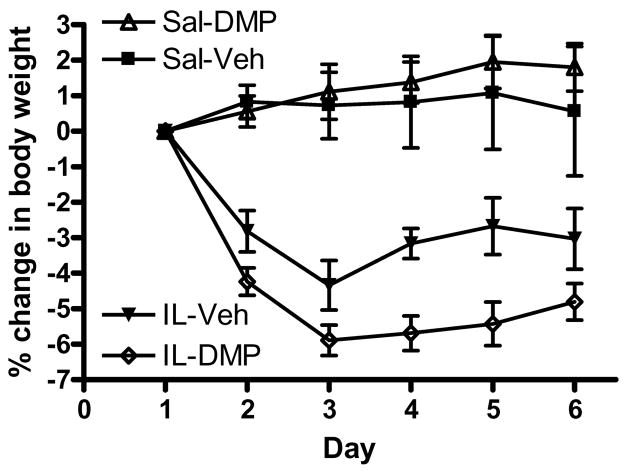
An ANOVA of animal weights from day 1 indicated no between group differences in body weights prior to IL-1β administration or DMP904 treatment. An ANOVA (within subject factor: Day, between subject factors: IL-1β, DMP904) indicated significant Day [F(1.9,68.3) = 7.78, p < 0.01], Day × IL-1β [F(1.9,68.3) = 20.23, p < 0.001], and IL-1β [F(1,36) = 51.61, p < 0.001] effects on % change in body weight during treatment. In addition, there was a trend for an IL-1β × DMP904 interaction [F(1,36) = 3.17, p = 0.08], while treatment with DMP904 alone had no effect. As depicted in Fig. 2, rats that received IL-1β exhibited an initial decrease in body weight, which tended to be exacerbated by co-administration of DMP904. Two-way ANOVAs (between subject factors: IL-1β, DMP904) of % change in body weight data for days 2 through 6 indicate that rats administered IL-1β had significant reductions in body weights for all of the days examined [all F’s (1,36) =≥ 7.89, all p’s < 0.001]. Animals treated with DMP904 exhibited significant reductions in body weights on day 2 [F(1,36) = 4.63, p < 0.05], but not on days 3 through 6. There was a trend toward an IL-1β × DMP904 interaction on days 4 [F(1,36) = 3.75, p = 0.06] and 5 [F(1,36) = 3.09, p = 0.09], however this effect failed to reach significance. No other significant main effects or interactions were present.
Figure 2.
IL-1β administration led to significant body weight reductions. DMP904 did not ameliorate this effect. Animals were weighed and injected at the same time daily for six consecutive days.
3.2 Repeated IL-1β administration reduces immobility time in the forced swim test
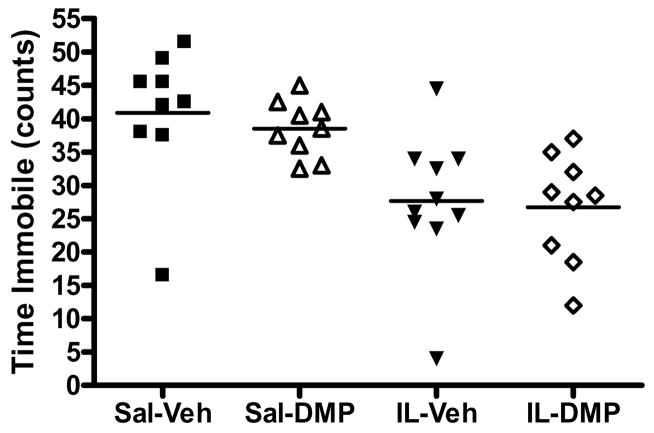
Time spent immobile (total bins) during the FST was analyzed using ANOVA (Fig 3). There was a significant main effect of IL-1β [F(1,33) = 19.13, p < 0.001], but no other significant effects or interactions. Both the IL-Veh and IL-DMP groups exhibited decreased immobility compared with Sal-Veh and Sal-DMP groups (all p’s < 0.05). Thus, administration of IL-1β caused a significant reduction in immobility time, while treatment with DMP904 had no effect.
Figure 3.
IL-1β caused a significant reduction in immobility time on the FST. Dot plots of immobility time (number of 5 s bins rated as immobile) for each subject are reported by group. Group means are denoted by the horizontal bar.
To examine potential confounding effects of weight loss on swim behavior (e.g., weight loss may cause changes in buoyancy, necessitating animals to swim harder to stay afloat), a Spearman’s correlation was run between percentage change in total body weight on day 5 (the day the final FST test occurred), and immobility time on the FST. Immobility time was positively correlated with percentage change in body weight (Spearman’s ρ = 0.468, p < 0.01), thus the decrease in body weight induced by IL-1β administration may have impacted immobility time.
3.3 CRF1 antagonism does not affect IL-1β induced increases in IL-10
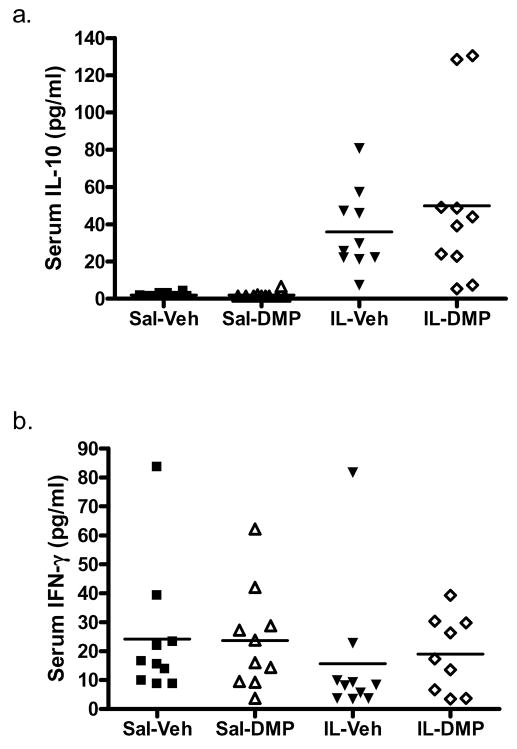
The effect of treatment group on serum cytokine levels was examined for IL-10 and IFN-γ independently using ANOVA. As shown in Fig. 4, IL-1β administration caused a robust increase in IL-10 levels [F(1,36) = 166.21, p < 0.001]. There was no effect of DMP904, or IL-1β × DMP904 interaction. Serum concentrations of IFN-γ were unaffected by IL-1β administration, DMP904 treatment, or their interaction.
Figure 4.
IL-1β, but not DMP904 administration was associated with higher serum concentrations of IL-10 (a), but not IFN-γ (b). Dot plots of cytokine concentrations for each animal are reported by group, with group means denoted as a horizontal bar. (data are presented as the non-transformed values for ease of interpretation)
3.4 Combined DMP904 and IL-1β alter the phosphorylation of p38 MAPK in the hippocampus
To determine the effects of IL-1β administration and CRF1 antagonism on the expression and phosphorylation of p38 MAPK in the hippocampus, ANOVAs were performed examining levels of p38 MAPK, p-p38 MAPK and the p-p38:p38 MAPK ratio. Administration of IL-1β significantly reduced levels of p38 MAPK [F(1,36) = 5.94, p < 0.05] (Table 1). There was no DMP904 effect, or IL-1β × DMP904 interaction on the expression of p38 MAPK. Subsequent analysis using t-tests indicated that levels of p38 MAPK were elevated in the SAL-Veh group compared to both the IL-Veh group (p < 0.05), and IL-DMP group (p < 0.05).
Table 1.
Hippocampal and hypothalamic expression and phosphorylation of p38 MAPK
| SAL-VEH | SAL-DMP | IL-VEH | IL-DMP | |
|---|---|---|---|---|
| Hippocampus p38 | 1046 ± 113 | 759 ± 88 | 647 ± 98* | 683 ± 87* |
| Hippocampus p-p38 | 6.4 ± 0.8 | 7.3 ± 0.7 | 7.1 ± 0.5 | 10.0 ± 1.1*,#,$ |
| Hippocampus ratio (p-p38:p38) | 0.007 ± 0.001 | 0.011 ± 0.001 | 0.014 ± 0.002* | 0.017 ± 0.002** |
| Hypothalamus p38 | 434 ± 36 | 317 ± 42* | 260 ± 47** | 602 ± 91#,$$ |
| Hypothalamus p-p38 | 9.5 ± 0.6 | 13.0 ± 1.3* | 12.7 ± 1.7 | 10.2 ± 1.5 |
| Hypothalamus ratio (p-p38:p38) | 0.023 ± 0.003 | 0.047 ± 0.006** | 0.075 ± 0.023* | 0.024 ± 0.008# |
denotes p < 0.05,
denotes p < 0.01 versus SAL-VEH
denotes p < 0.05 versus SAL-DMP
denotes p < 0.05,
denotes p < 0.01 versus IL-VEH
Values listed are mean pg/ml for each treatment group ± SEM.
Administration of IL-1β [F(1,36) = 5.00, p < 0.05] or DMP904 [F(1,36) = 5.77, p < 0.05] increased levels of p-p38 MAPK, with no significant IL-1β × DMP904 interaction. Subsequent analysis using t-tests indicated that animals receiving combined treatment with IL-1β and DMP904 had increased p38 MAPK phosphorylation relative to all other groups (p’s < 0.05). There were no other significant between group differences.
Overall, treatment with IL-1β increased the p-p38:p38 MAPK ratio [F(1,36) = 9.27, p < 0.01], while DMP904 tended to increase the p-p38:p38 MAPK ratio [F(1,36) = 3.09, p = 0.09], however this effect did not reach significance. No significant IL-1β × DMP904 interactions were present for the p-p38:p38 MAPK ratio. Follow-up analyses using t-tests indicated that both the IL-Veh (p < 0.05) and IL-DMP (p < 0.01) groups had increased p-p38:p38 MAPK ratios compared to the Sal-Veh group. To summarize, within the hippocampus neither IL-1β administration nor DMP904 treatment alone led to changes in the phosphorylation of p38 MAPK; however, when co-administered, DMP904 and IL-1β significantly increased p38 MAPK phosphorylation. Treatment with IL-1β reduced levels of the inactive form of p38 MAPK independent of DMP904. This decrease was responsible for the observed changes in the p-p38:p38 MAPK ratio in the IL-Veh group, but only partly responsible for the p-p38:p38 MAPK ratio changes observed in the IL-DMP group. Treatment with DMP904 had no effect on p38 MAPK expression, but increased levels of p-p38 MAPK, however, this effect was driven by the between group difference observed for animals treated with both IL-1β and DMP904 relative to the Sal-Veh group (p < 0.05).
DMP904 attenuates the IL-β-induced increase in p38 MAPK phosphorylation in the hypothalamus
To determine the effects of repeated IL-1β administration and CRF1 antagonism on the expression and phosphorylation of p38 MAPK in the hypothalamus, ANOVAs were performed on p38 MAPK, p-p38 MAPK and the p-p38 to p38 MAPK ratio. There were no main effects of IL-1β on levels of p38 MAPK, p-p38 MAPK, or the p-p38:p38 MAPK ratio (Table 1). Similarly, there were no main effects of DMP904 on p38 MAPK expression [F(1,36) = 3.78, p = 0.06], p-p38 MAPK, or the p-p38:p38 MAPK ratio. There were, however, significant IL-1β × DMP904 interactions observed for p38 MAPK expression [F(1,36) = 15.62, p < 0.001], p-p38 MAPK [F(1,36) = 5.05, p < 0.05], and the p-p38:p38 MAPK ratio [F(1,36) = 8.32, p < 0.01]. Follow-up t-tests were used to examine between group differences. In the presence of DMP904, IL-1β increased levels of p38 MAPK relative to IL-Veh (p < 0.01) and Sal-DMP (p < 0.05) groups. Only animals given DMP904 without IL-1β had increased p-p38 MAPK relative to animals in the Sal-Veh group (p < 0.05), though animals in the IL-Veh group trended toward an increase in p-p38 MAPK relative to the Sal-Veh group (p = 0.09). Animals in the Sal-DMP group exhibited a heightened p-p38:p38 MAPK ratio relative to both the Sal-Veh group (p < 0.01) and the IL-DMP group (p < 0.05). Animals in the IL-Veh group exhibited an increased p-p38:p38 MAPK ratio relative to the Sal-Veh group (p < 0.05), and a marginal elevation relative to the IL-DMP group (p = 0.05). To summarize, when given alone, both IL-1β and DMP904 altered p38 MAPK regulation within the hypothalamus. The combined effects of IL-1β and DMP904 were not additive, and instead seemed to counteract the effects of either drug on its own.
Exploratory biochemical and behavioral correlations
Previously we found that depressive symptoms are associated with alterations in peripheral levels of cytokines (Loftis et al., 2008). Given the significant overlap between depression and sickness behavior (Capuron et al., 2002), we used Spearman’s correlations to determine whether there were significant relationships among peripheral immune system activation (measured by serum cytokine levels), activation of brain stress pathways (measured by p38 phosphorylation and expression), time immobile in the FST, and percentage change in body weight. As reported in Table 2, serum IL-10 was negatively correlated with percentage change in body weight, and immobility time in the FST, while IFN-γ was unrelated to either factor. Measures of p38 or p-p38 MAPK expression were unrelated to the percentage change in body weight and time spent immobile in the FST. Serum IL-10 was negatively correlated with the level of p38 MAPK within the hippocampus, and positively correlated with the p-p38:p38 MAPK ratio within the hippocampus. Serum IL-10 also trended toward a positive correlation with p-p38 MAPK within the hippocampus. Thus, an increase in serum IL-10 levels was associated with weight loss, immobility time in the FST and reductions in p38 MAPK expression in the hippocampus. By contrast, IFN-γ was not correlated with any of the p38 MAPK or p-p38 MAPK measures. Hypothalamic measures of p38 MAPK expression, p-p38 MAPK, or the p-p38:p38 MAPK ratio were not related to any of the other experimental measures.
Table 2.
IL-10 correlates with p38 MAPK, weight and immobility time*.
| IFN-γ | IL-10 | Hip p38 | Hip p-p38 | Hip ratio | Hyp p38 | Hyp p-p38 | Hyp ratio | Weight | |
|---|---|---|---|---|---|---|---|---|---|
| IL-10 | ρ = −0.16 p = 0.33 |
||||||||
| Hip p38 | ρ = −0.07 p = 0.67 |
ρ = −0.32 p < 0.05 |
|||||||
| Hip p-p38 | ρ = 0.09 p = 0.60 |
ρ = 0.30 p = 0.07 |
ρ = −0.30 p = 0.06 |
||||||
| Hip ratio | ρ = 0.08 p = 0.63 |
ρ = 0.41 p < 0.01 |
ρ = −0.87 p < 0.001 |
ρ = 0.71 p < 0.001 |
|||||
| Hyp p38 | ρ = 0.20 p = 0.22 |
ρ = −0.03 p = 0.85 |
ρ = 0.27 p = 0.09 |
ρ = 0.09 p = 0.60 |
ρ = −0.14 p = 0.40 |
||||
| Hyp p-p38 | ρ = −0.24 p = 0.13 |
ρ = −0.01 p = 0.97 |
ρ = −0.02 p = 0.90 |
ρ = 0.08 p = 0.61 |
ρ = 0.05 p = 0.77 |
ρ = −0.45 p < 0.01 |
|||
| Hyp ratio | ρ = −0.19 p = 0.24 |
ρ = 0.01 p = 0.99 |
ρ = −0.23 p = 0.15 |
ρ = −0.04 p = 0.79 |
ρ = 0.13 p = 0.45 |
ρ = −0.93 p < 0.001 |
ρ = 0.72 p < 0.001 |
||
| Weight | ρ = 0.05 p = 0.75 |
ρ = −0.69 p < 0.001 |
ρ = 0.25 p = 0.11 |
ρ = −0.16 p = 0.32 |
ρ = −0.30 p = 0.06 |
ρ = −0.19 p = 0.23 |
ρ = 0.10 p = 0.55 |
ρ = 0.17 p = 0.28 |
|
| Immobility time | ρ = 0.13 p = 0.46 |
ρ = −0.64 p < 0.001 |
ρ = 0.14 p = 0.41 |
ρ = −0.19 p = 0.27 |
ρ = −0.23 p = 0.17 |
ρ = −0.09 p = 0.58 |
ρ = 0.13 p = 0.46 |
ρ = 0.10 p = 0.55 |
ρ = 0.39 p < 0.05 |
Spearman’s rho (ρ) values for correlations between biochemical, physiological and behavioral markers indicate the association of IL-10, but not IFN-γ with
several of the study variables. Significant correlations are in bold.
Discussion
The present study is the first known to examine the effects of repeated systemic IL-1β administration and CRF1 antagonism on weight regulation, behavioral despair, peripheral cytokine levels, and central p38 MAPK expression and phosphorylation. As predicted from the literature, IL-1β caused significant weight loss (Fig. 2). We did not directly measure food intake so it is unclear whether the weight loss was due to anorexia, or to other sickness-related symptoms (e.g. diarrhea). Previous studies have demonstrated that IL-1β administered either centrally, or peripherally causes reductions in food-motivated behaviors (e.g. Crestani et al., 1991; Kent et al., 1992), suggesting that the observed reductions in body weight were likely the result of anorexia. Given that DMP904 failed to prevent the IL-1β-induced body weight loss, it can be speculated that at least some IL-1β-induced symptoms may be secondary to other, CRF1-independent, mechanisms. Thus, provided that DMP904/CRF1 antagonists prove otherwise useful in the treatment of depression, the present study raises concern that they may not be as effective in the treatment of IL-1β-induced somatic/neurovegetative symptoms.
In addition to changes in body weight, we also evaluated the effects of CRF1 antagonism on behavioral despair, another sign of sickness or depressive-like behavior. Our findings support those of Li et al. (2005), who found that DMP904 does not influence immobility on the FST. However, the inability of IL-1β to increase immobility time in the FST means that future studies will need to determine the impact of DMP904 in cytokine-induced “depression” using other behavioral measures [e.g. sucrose preference testing (Sammut et al., 2001), or social interaction assessments (Konsman et al., 2008)].
To evaluate the effects of DMP904 treatment on the peripheral immune system following IL-1β administration, the expression of pro- and anti-inflammatory cytokines was investigated. The Th1 cytokine, IFN-γ, was not significantly changed in any of the treatment groups. Further, we found that IFN-γ did not correlate with any of the behavioral or biochemical measures studied. This supports the observations that activation of the immune system can induce sickness behavior in the absence of increased IFN-γ levels (Fu et al., 2010). Recently, Fu et al. (2010) reported that intracerebroventricular LPS administration (100 ng/animal) to C57/BL6J mice also did not increase IFN-γ expression.
In contrast to IFN-γ, the Th2 anti-inflammatory cytokine, IL-10, was significantly higher in serum following IL-1β administration. Treatment with DMP904 did not prevent IL-1β induced increases in peripheral IL-10 levels. It has been suggested that increases in IL-10 may be a component of the intense regulation of the immune system [also described as the compensatory anti-inflammatory response (CARS)], acting as negative feedback following elevations of pro-inflammatory or Th1 cytokines (e.g., IL-1β, TNF-α) (Pestka et al., 2004; Simon et al., 2008; Adib-Conquy and Cavaillon 2009, Woiciechowsky et al. 1999). Although, a recent meta-analysis of studies examining the role of cytokines in depression found no differences in IL-10 levels between individuals with major depression and controls (Dowlati et al., 2010), it may be that cytokine-induced depression is one of several different subtypes of depression (Prins et al., 2011; Lloyd and Nemeroff, 2011). For example, mice lacking IL-10 expression exhibit increases in immobility time in the FST relative to wild-type mice, while mice over-expressing IL-10 exhibit reduced immobility in this task (Mesquita et al., 2008). Systemic administration of IL-10 to Balb/c mice caused an increase in locomotion (Harvey et al., 2006). Thus, the increase in IL-10 observed in our study may have counteracted the induction of depressive-like signs (i.e. increases in immobility) induced by IL-1β in the FST, as IL-10 levels were negatively correlated with immobility time on the FST (Table 2).
Glucocorticoids are part of the negative feedback loop associated with the HPA axis (Seckl and Olsson 1995) and may exert differential effects on p38 MAPK regulation within the hippocampus and hypothalamus. Our study demonstrated brain region specific effects of IL-1β administration on the expression and phosphorylation of p38 MAPK and on the ability of DMP904 to antagonize these effects. Combined DMP904 and IL-1β altered the phosphorylation of p38 MAPK in the hippocampus. However, we found that DMP904 antagonized the ability of IL-1β to regulate p38 MAPK within the hypothalamus, indicating that this may be a primary mechanism by which IL-1β activates the HPA axis. This hypothesis is supported by previous work showing that CRF is critically involved in IL-1β induced increases in adrenocorticotropic hormone and corticosterone (Sapolsky et al., 1987) and that p38 MAPK activation contributes to increased CRF gene expression in hypothalamic cells (see Kageyama and Suda, 2009 for review). On the other hand, we found that DMP904 alone was able to increase p38 MAPK phosphorylation and the ratio of p-p38:p38 MAPK expression within the hypothalamus (but not in the hippocampus). This effect may be due to differential expression of CRF2α and CRF2β receptors between the hippocampus and hypothalamus (Lovenberg et al., 1995).
Another explanation for the observed brain region differences in p38 MAPK regulation induced by IL-1β and DMP904 could be that the hippocampus and hypothalamus exhibit different adaptations to repeated drug administration, e.g. differences in tolerance or sensitization to IL-1β or DMP904. Noguchi et al. (2010) found a significant reduction in baseline glucocorticoid receptor mRNA in the hippocampus following repeated immobilization stress relative to animals given a single immobilization. By contrast, levels of glucocorticoid receptor mRNA within the hypothalamus were similar for animals given repeated immobilizations, or a single immobilization. Thus, differential adaptations to stress axis activation within the hippocampus and hypothalamus have been reported and could explain our observations. It should be noted that within the hippocampus, hypothalamus, spleen, adrenal glands, and plasma, IL-1β levels were unchanged immediately after forced swim testing of 15 – 30 minutes, while levels of corticosterone were significantly elevated (Deak et al. 2003). Nevertheless, the interpretation of the biochemical and neurochemical results in this study should consider that the stress of forced swim testing may influence levels of cytokines and stress-related molecules.
A potential limitation of this study was the failure of repeated IL-1β to increase immobility in the FST. This outcome may be attributed to: 1) species differences in FST behavior following immune activation, 2) dose effects of IL-1β or DMP904, 3) a fear response overcoming the sickness response, or 4) tolerance to the sickness inducing effects of repeated IL-1β. Previous work using male CD-1 mice, found that an acute i.p. injection of IL-1β led to a dose-dependent increase in time spent immobile in the FST (Dunn and Swiergiel, 2005). Similar FST results were found following treatment with the toll-like receptor 4 (TLR-4) agonist LPS (Dunn and Swiergiel, 2005). However, and in agreement with our finding, LPS treatment in rats has had limited effects on immobility (Deak et al., 2005; Pitychoutis et al., 2009) in the FST (but see also Tonelli et al., 2008). Deak et al. (2005) using either 10 μg/kg or 100 μg/kg and multiple time points prior to FST testing found no effects of LPS on immobility time in Sprague-Dawley rats. Likewise, Pitychoutis et al. (2009) found no effect of LPS on either male or female Sprague Dawley rats. Further, del Cerro and Borrell (1990) found that intracerebroventricular administration of IL-1β decreased immobility time in the FST in Wistar rats, while Minor et al. (2003) found the opposite effect. It remains unclear as to what mechanisms underlie the apparent behavioral differences in response to pro-inflammatory stimuli such as IL-1β and LPS between rats and mice. Future studies could examine the effect of chronic administration of a range of IL-1β concentrations on the FST in rats, as has previously been done in mice (Dunn and Swiergiel, 2005) to better assess IL-1β dose-response relationships. It has also been speculated that sickness behavior is a motivational state that can be overcome in certain contexts such as fear (Deak et al., 2005). Consequently, the swim stress may have temporarily masked signs of sickness or depression. Finally, it is important to consider that the FST was not originally designed as a measure of depression, but is instead applied as a screen for potential antidepressants (see Castagné et al. 2011 for review).
Taken together, the results of the present study indicate that IL-1β administration was associated with significant reductions in body weight and increases in the ratio of p-p38:p38 MAPK within both the hippocampus and hypothalamus, indicating activation of the stress axis. Similarly, IL-1β caused a large increase in serum concentrations of IL-10, which has been associated with behavioral activation (Harvey et al., 2006; Mesquita et al., 2008) and may play a role in the CARS response (Lehto et al. 2010). The CRF1 antagonist DMP904 caused brain-region dependent changes in p38 MAPK regulation and also influenced p38 MAPK activation induced by IL-1β. The lack of pre-clinical studies examining the impact of repeated treatment with pro-inflammatory cytokines such as IL-1β makes our study novel and more representative of the persistent HPA axis activation observed among some depressed patients (see Leonard and Myint 2009 for review), and long-term treatment with pro-inflammatory cytokines in cancer (e.g. Wood et al. 2006a) and chronic hepatitis C viral infection (e.g. Loftis and Hauser 2004). It remains unclear whether DMP904 will impact sickness behavior induced by persistent activation of the immune system. Future studies could focus on the effect of CRF1 antagonism on other classical symptoms of sickness behavior such as social interactions and anhedonia. The identification of adjunct compounds that reduce the behavioral and biochemical features of sickness or depressive behaviors will be an important step toward alleviating the side effect burden and improving treatment outcomes for individuals with treatment-resistant depression and for patients requiring pro-inflammatory cytokine-based therapies.
Behavioral and biochemical effects of repeated IL-1β injection were examined.
IL-1β reduced body weight and immobility time in an antidepressant screen.
IL-1β altered p38 MAP kinase regulation.
CRF1 antagonism altered the biochemical but not behavioral effects of IL-1β.
Acknowledgments
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Clinical Sciences Research and Development. JML and MSH are supported by career development awards from the Department of Veterans Affairs, Veterans Health Administration. This work was also supported by Reed College (Portland, Oregon, USA) and the National Institute of Mental Health (# 1-F32-MH071137-01). The authors would also like to thank Bristol Myers Squibb for generously providing the DMP904.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CJW performed the majority of statistical analysis, data analysis and interpretation, and manuscript preparation. AMC performed the majority of the experiments, preliminary statistical analysis, was involved in conceiving the study, and wrote an initial version of the manuscript, which was adapted from his Bachelor of Arts thesis. DJM and MSH were involved in data analysis and interpretation. JML was involved in conceiving the study, performing the experiments, statistical analysis, data analysis and interpretation, and manuscript preparation. All authors have read and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Clare J. Wilhelm, Email: wilhelmc@ohsu.edu.
Aaron Murphy-Crews, Email: aaron.murphycrews@gmail.com.
Daniel J. Menasco, Email: dmenasco@mac.com.
Marilyn S. Huckans, Email: Marilyn.huckans@va.gov.
References
- Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101:36–47. [PubMed] [Google Scholar]
- Anisman H. Cascading effects of stressors and inflammatory immune activation: implications for major depressive disorder. J Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-week randomized, placebo-controlled trial of CP-316-311 (a selective CRH1 antagonist) in the treatment of major depression. Am J Psychiatry. 2008;165:617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interfon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmaoclogy. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18:205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Castagné V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2011;Chapter 8(Unit8. 10A) doi: 10.1002/0471142301.ns0810as55. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Bimonte-Nelson HA. Impact of the hypothalamic-pituitary-adrenal/gonadal axes on trajectory of age-related cognitive decline. Prog Brain Res. 2010;128:31–76. doi: 10.1016/S0079-6123(10)82002-3. [DOI] [PubMed] [Google Scholar]
- Crestani F, Seguy F, Dantzer R. Behavioural effects of peripherally injected interleukin-1: role of prostaglandins. Brain Res. 1991;542:330–335. doi: 10.1016/0006-8993(91)91587-q. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Kelley KW. Androgen-dependent vasopressinergic neurotransmission attenuates interleukin-1-induced sickness behavior. Brain Res. 1991;557:115–20. doi: 10.1016/0006-8993(91)90123-d. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D’Agostino LG. Exposure to forced swim stress does not alter central production of IL-1. Brain Res. 2003;972:53–63. doi: 10.1016/s0006-8993(03)02485-5. [DOI] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D’Agostino LG, Rosanoff M, McElderry NK, Bordner KA. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav Brain Res. 2005;160:125–134. doi: 10.1016/j.bbr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- del Cerro S, Borrell J. Interleukin-1 affects the behavioral despair response in rats by an indirect mechanism which requires endogenous CRF. Brain Res. 1990;24:162–164. doi: 10.1016/0006-8993(90)90212-t. [DOI] [PubMed] [Google Scholar]
- Dermitzaki E, Tsatsanis C, Gravanis A, Margioris AN. Corticotropin-releasing hormone induces Fas ligand production and apoptosis in PC12 cells via activation of p38 mitogen-activated protein kinase. J Biol Chem. 2002;277:12280–12287. doi: 10.1074/jbc.M111236200. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Hermann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav. 2005;81:688–93. doi: 10.1016/j.pbb.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Effects of acute and chronic stressors and CRF in rat and mouse tests for depression. Ann N Y Acad Sci. 2008;1148:118–126. doi: 10.1196/annals.1410.022. [DOI] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Pace TW, Woolwine BJ, Hu F, Raison CL, Miller AH. Early activation of p38 mitogen activated protein kinase is associated with interferon-alpha-induced depression and fatigue. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.02.015. ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Zunich SM, O’Connor JC, Kavelaars A, Dantzer R, Kelley KW. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures. J Neuroinflammation. 2010;7:43. doi: 10.1186/1742-2094-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- Gilbertson-White S, Aouizerat BE, Miaskowski C. Methodologic issues in the measurement of cytokines to elucidate the biological basis for cancer symptoms. Biol Res Nurs. doi: 10.1177/1099800410379497. In press. [DOI] [PubMed] [Google Scholar]
- Greenberg DB, Gray JL, Mannix CM, Eisenthal S, Carey M. Treatment-related fatigue and serum interleukin-1 levels in patients during external beam irradiation for prostate cancer. J Pain and Symptom Manage. 1993;8:196–200. doi: 10.1016/0885-3924(93)90127-h. [DOI] [PubMed] [Google Scholar]
- Harvey D, Smith R, English K, Mahon B, Commins S. Interleukin-10 (IL-10) but not Lipopolysaccharide (LPS) produces increased motor activity and abnormal exploratory patterns while impairing spatial learning in Balb/c mice. Physiol Behav. 2006;87:842–847. doi: 10.1016/j.physbeh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Hermus RM, Sweep CG, van der Meer MJ, Ross HA, Smals AG, Benraad TJ, Kloppenborg PW. Continuous infusion of interleukin-1 beta induces a nonthyroidal illness syndrome in the rat. Endocrinology. 1992;131:2139–2146. doi: 10.1210/endo.131.5.1425414. [DOI] [PubMed] [Google Scholar]
- Hogan JB, Hodges DB, Jr, Lelas S, Gilligan PJ, McElroy JF, Lindner MD. Effects of CRF1 receptor antagonists and benzodiazepines in the Morris water maze and delayed non-matching to position tests. Psychopharmacology. 2005;178:410–419. doi: 10.1007/s00213-004-2028-y. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hsieh CH, Li HY, Chen JC. Nitric oxide and interleukin-1beta mediate noradrenergic induced corticotrophin-releasing hormone release in organotypic cultures of rat paraventricular nucleus. Neuroscience. 2010;165:1191–202. doi: 10.1016/j.neuroscience.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Huang TL, Lee CT. T-helper 1/T-helper 2 cytokine imbalance and clinical phenotypes of acute-phase major depression. Psychiatry Clin Neurosci. 2007;61:415–20. doi: 10.1111/j.1440-1819.2007.01686.x. [DOI] [PubMed] [Google Scholar]
- Jones SA, Challis JR. Local stimulation of prostaglandin production by corticotropin-releasing hormone in human fetal membranes and placenta. Biochem Biophys Res Commun. 1989;159:192–9. doi: 10.1016/0006-291x(89)92422-4. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Suda T. Regulatory mechanisms underlying corticotropin-releasing factor gene expression in the hypothalamus. Endocr J. 2009;56:335–44. doi: 10.1507/endocrj.k09e-075. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci. 1992;89:9117–9120. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Veeneman J, Combe C, Poole S, Luheshi GN, Dantzer R. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur J Neurosci. 2008;28:2499–2510. doi: 10.1111/j.1460-9568.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- Lehto SM, Niskanen L, Miettola J, Tolmunen T, Viinamaki H, Mantyselka P. Serum anti-inflammatory markers in general population subjects with elevated depressive symptoms. Neurosci Lett. 2010;484:201–205. doi: 10.1016/j.neulet.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Lelas S, Wong H, Li YW, Heman KL, Ward KA, Zeller KL, Sieracki KK, Polino JL, Godonis HE, Ren SX, Yan XX, Arneric SP, Robertson DW, Hartig PR, Grossman S, Trainor GL, Taub RA, Zaczek R, Gilligan PJ, McElroy JF. Anxiolytic-like effects of the corticotropin-releasing factor1 (CRF1) antagonist DMP904 [4-(3-pentylamino)-2,7-dimethyl-8-(2-methyl-4-methoxyphenyl)-pyrazolo-[1,5-a]-pyrimidine] administered acutely or chronically at doses occupying central CRF1 receptors in rats. J Pharmacol Exp Ther. 2004;309:293–302. doi: 10.1124/jpet.103.058784. [DOI] [PubMed] [Google Scholar]
- Leo R, Di Lorenzo G, Tesauro M, Razzini C, Forleo GB, Chiricolo G, Cola C, Zanasi M, Troisi A, Siracusano A, Lauro R, Romeo F. Association between enhanced soluble CD40 ligand and proinflammatory and prothrombotic states in major depressive disorder: pilot observations on the effects of selective serotonin reuptake inhibitor therapy. J Clin Psychiatry. 2006;67:1760–6. doi: 10.4088/jcp.v67n1114. [DOI] [PubMed] [Google Scholar]
- Leonard BE, Myint A. The psychoneuroimmunology of depression. Hum Psychopharmacol. 2009;24:165–175. doi: 10.1002/hup.1011. [DOI] [PubMed] [Google Scholar]
- Li YW, Fitzgerald L, Wong H, Lelas S, Zhang G, Lindner MD, Wallace T, McElroy J, Lodge NJ, Gilligan P, Zaczek R. The pharmacology of DMP696 and DMP904, non-peptidergic CRF1 receptor antagonists. CNS Drug Rev. 2005;11:21–52. doi: 10.1111/j.1527-3458.2005.tb00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd RB, Nemeroff CB. The role of corticotropin-releasing hormone in the pathophysiology of depression: therapeutic implications. Curr Top Med Chem. 2011;11:609–17. doi: 10.2174/1568026611109060609. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Hauser P. The phenomenology and treatment of interferon-induced depression. J Affect Disord. 2004;82:175–190. doi: 10.1016/j.jad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Huckans M, Ruimy S, Hinrichs DJ, Hauser P. Depressive symptoms in patients with chronic hepatitis C are correlated with elevated plasma levels of interleukin-1beta and tumor necrosis factor-alpha. Neurosci Lett. 2008;430:264–268. doi: 10.1016/j.neulet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- Mesquita AR, Correia-Neves M, Roque S, Castro AG, Vieira P, Pedrosa J, Palha JA, Sousa N. IL-10 modulates depressive-like behavior. J Psychiatr Res. 2006;43:89–97. doi: 10.1016/j.jpsychires.2008.02.004. 2008. [DOI] [PubMed] [Google Scholar]
- Minor TR, Huang Q, Foley EA. Cytokine-purine interactions in behavioral depression in rats. Integr Physiol Behav Sci. 2003;38:189–202. doi: 10.1007/BF02688853. [DOI] [PubMed] [Google Scholar]
- Nielsen D. Corticotropin-releasing factor type-1 receptor antagonists: The next class of antidepressants? Life Sci. 2006;78:909–919. doi: 10.1016/j.lfs.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Makino S, Matsumoto R, Nakayama S, Nishiyama M, Terada Y, Hashimoto K. Regulation of glucocorticoid receptor transcription and nuclear translocation during single and repeated immobilization stress. Endocrinology. 2010;151:4344–4355. doi: 10.1210/en.2010-0266. [DOI] [PubMed] [Google Scholar]
- Owen BM, Eccleston D, Ferrier IN, Young AH. Raised levels of plasma interleukin-1beta in major and postviral depression. Acta Psychiatr Scand. 2001;103:226–8. doi: 10.1034/j.1600-0447.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim HJ, Lee JH, Lee JY, Cho BK, Kang JS, Kang H, Yang Y, Cho DH. Corticotropin-releasing hormone (CRH) downregulates interleukin-18 expression in human HaCaT keratinocytes by activation of p38 mitogen-activated protein kinase (MAPK) pathway. J Invest Dermatol. 2005;124:751–755. doi: 10.1111/j.0022-202X.2005.23656.x. [DOI] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- Petty AP, Wright SE, Rewers-Felkins KA, Yenderrozos MA, Vorderstrasse BA, Lindsey JS. Targeting migration inducting gene-7 inhibits carcinoma cell invasion, early primary tumor growth and stimulates monocyte oncolytic activity. MolCancer Ther. 2009;8:2412–2423. doi: 10.1158/1535-7163.MCT-09-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitychoutis PM, Nakamura K, Tsonis PA, Papadopoulou-Daifoti Z. Neurochemical and behavioral alterations in an inflammatory model of depression: sex differences exposed. Neurocience. 2009;159:1216–1232. doi: 10.1016/j.neuroscience.2009.01.072. [DOI] [PubMed] [Google Scholar]
- Prins J, Olivier B, Korte SM. Triple reuptake inhibitors for treating subtypes of major depressive disorder: the monoamine hypothesis revisited. Expert Opin Investig Drugs. 2011 Jun 20; doi: 10.1517/13543784.2011.594039. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Sairanen TR, Lindsberg PJ, Brenner M, Siren AL. Global forebrain ischemia results in differential cellular and expression of interleukin-1beta (IL-1beta) and its receptor at mRNA and protein level. J Cereb Blood Flow Metab. 1997;17:1107–1120. doi: 10.1097/00004647-199710000-00013. [DOI] [PubMed] [Google Scholar]
- Sammut S, Goodall G, Muscat R. Acute interferon-alpha administration modulates sucrose consumption in the rat. Psychoneuroendocrinology. 2001;26:261–72. doi: 10.1016/s0306-4530(00)00051-2. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Schüle C, Baghai TC, Eser D, Häfner S, Born C, Herrmann S, Rupprecht R. The combined dexamethasone/CRH Test (DEX/CRF test) and prediction of acute treatment response in major depression. PLoS One. 2009;4:e4324. doi: 10.1371/journal.pone.0004324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR, Olsson T. Glucocorticoid hypersecretion and the age-impaired hippocampus: cause or effect. J Endocrinol. 1995;145:201–211. doi: 10.1677/joe.0.1450201. [DOI] [PubMed] [Google Scholar]
- Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, Nierenberg AA, Fava M, Wong KK. A detailed examination of cytokine abnormalities in Major Depressive Disorder. Eur Neuropsychopharmacol. 2008;18:230–233. doi: 10.1016/j.euroneuro.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Horrobin D, Leonard B. The comparison of changes in behavior, neurochemistry, endocrine, and immune functions after different routes, doses, and durations of administrations of IL-1 beta in rats. Pharmacopsychiatry. 2006;39:88–99. doi: 10.1055/s-2006-941557. [DOI] [PubMed] [Google Scholar]
- Sternberg EM. Neural response of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazi A, Dantzer R, Crestani F, Le Moal M. Interleukin-1 induces conditioned taste aversion in rats: a possible explanation for its pituitary-adrenal stimulating activity. Brain Res. 1988;473:369–371. doi: 10.1016/0006-8993(88)90868-2. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Holmes A, Postolache TT. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology. 2008;33:1038–1048. doi: 10.1038/sj.npp.1301488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR. Development of CRF1 receptor antagonists as antidepressants and anxiolytics: progess to date. CNS Drugs. 2006;20:887–96. doi: 10.2165/00023210-200620110-00002. [DOI] [PubMed] [Google Scholar]
- Valdez GR. CRF receptors as a potential target in the development of novel pharmacotherapies for depression. Curr Pharm Des. 2009;15:1587–1594. doi: 10.2174/138161209788168083. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–92. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Wang L, Martínez V, Rivier JE, Taché Y. Peripheral urocortin inhibits gastric emptying and food intake in mice: differential role of CRF receptor 2. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1401–10. doi: 10.1152/ajpregu.2001.281.5.R1401. [DOI] [PubMed] [Google Scholar]
- Woiciechowsky C, Schöning B, Lanksch WR, Volk HD, Döcke WD. Mechanisms of brain-mediated systemic anti-inflammatory syndrome causing immunodepression. J Mol Med. 1999;77:769–780. doi: 10.1007/s001099900051. [DOI] [PubMed] [Google Scholar]
- Wood LJ, Nail LM, Gilster A, Winders KA, Elsea CR. Cancer chemotherapy-related symptoms: evidence to suggest a role for proinflammatory cytokines. Oncol Nurs Forum. 2006a;33:535–542. doi: 10.1188/06.ONF.535-542. [DOI] [PubMed] [Google Scholar]
- Wood LJ, Nail LM, Perrin NA, Elsea CR, Fischer A, Druker BJ. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biol Res Nurs. 2006b;8:157–169. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 Receptor Activation by Systemic Lipopolysaccharide Induces Behavioral Despair Linked to MAPK Regulation of CNS Serotonin Transporters. Neuropsychopharmacology. doi: 10.1038/npp.2010.116. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Künzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]