Abstract
This study examined the prevalence of substance use disorders (SUDs) among psychiatric patients aged 2–17 years in an electronic health records database (N=11,457) and determined patterns of comorbid diagnoses among patients with a SUD to inform emerging comparative effectiveness research (CER) efforts. DSM-IV diagnoses of all inpatients and outpatients at a large university-based hospital and its associated psychiatric clinics were systematically captured between 2000 and 2010: SUD, anxiety (AD), mood (MD), conduct (CD), attention deficit/hyperactivity (ADHD), personality (PD), adjustment, eating, impulse-control, psychotic, learning, mental retardation, and relational disorders. The prevalence of SUD in the 2–12-year age group (n=6,210) was 1.6% and increased to 25% in the 13–17-year age group (n=5,247). Cannabis diagnosis was the most prevalent SUD, accounting for more than 80% of all SUD cases. Among patients with a SUD (n=1,423), children aged 2–12 years (95%) and females (75–100%) showed high rates of comorbidities; blacks were more likely than whites to be diagnosed with CD, impulse-control, and psychotic diagnoses, while whites had elevated odds of having AD, ADHD, MD, PD, relational, and eating diagnoses. Patients with a SUD used more inpatient treatment than patients without a SUD (43% vs. 21%); children, females, and blacks had elevated odds of inpatient psychiatric treatment. Collectively, results add clinical evidence on treatment needs and diagnostic patterns for understudied diagnoses.
Keywords: Attention deficit/hyperactivity disorder, comorbidity, comparative effectiveness research, electronic health records, mood disorder, personality disorder, relational disorder, substance use disorder
1. Introduction
Psychiatric disorders take a heavy toll on children and adolescents, especially those affected by comorbid substance use disorders (SUDs: abuse or dependence) and other psychiatric diagnoses (Riggs, 2003; Libby and Riggs, 2005). National data estimate that about 50% of Americans with a history of psychiatric disorders, including SUD, mood (MD), anxiety (AD), conduct (CD), attention deficit hyperactivity (ADHD), oppositional-defiant (ODD), and intermittent-explosive disorders, experienced their first symptoms by 14 years of age (Kessler et al., 2005). The use of electronic health record (EHR) data to discern the type and quality of health care delivery for children and adolescents with psychiatric disorders is now recognized as a priority for comparative effectiveness research (CER) (Institute of Medicine [IOM], 2009; Jha et al., 2010). This study seeks to characterize comorbid patterns of SUDs and other psychiatric disorders among psychiatric inpatients and outpatients aged 2–17 years in a large EHR database to differentiate patterns of comorbidities by patients’ age, sex, race/ethnicity, and treatment setting to inform emerging CER efforts.
Adolescence is the period with the highest risk for initiating substance use, and adolescent-onset SUDs confer a particularly high risk for prolonged addiction, treatment need, psychiatric disorders, and mortality (Brook et al., 2000; Clark et al., 2008; Kandel et al., 1999; Roberts et al., 2007; Shrier et al., 2003; Zeitlin, 1999). Unfortunately, because many psychiatric disorders have their origins in childhood or adolescence, SUDs often co-exist with other disorders (e.g., MD, AD, CD, ADHD) that further intensify clinical courses and complicate treatment options (Libby and Riggs, 2005; Lubman et al., 2007; Najt et al., 2011; Roberts et al., 2007; Shrier et al., 2003). As shown from the National Comorbidity Survey Replication-Adolescent Supplement, lifetime psychiatric disorders are prevalent among adolescents aged 13–18 years (SUDs, 11%; AD, 32%; MD, 14%; CD, 7%; ODD, 13%; ADHD, 9%), and about 40% of adolescents with one disorder also have another one (Merikangas et al., 2010).
Adolescents with a SUD are reported have a high rate of comorbidities. In a large community survey, Kandel et al. (1999) found that 76% of American adolescents aged 14–18 years with a current SUD had another current psychiatric disorder (MD, AD, CD, ADHD, ODD, antisocial personality disorders [PD]) (Kandel et al., 1999). Findings from various studies suggest that disruptive behavioral disorders (CD, ADHD, ODD) are the most prevalent disorders among adolescents with substance use problems (25–50%), particularly among males, followed by depression and AD, particularly among females (Armstrong and Costello, 2002). Rates of co-occurring depression and SUDs are thought to range from 11–32% across studies; rates of co-occurring AD and SUDs vary greatly from 7% to 40% (Armstrong and Costello, 2002; O'Neil et al., 2011). Rates of comorbidities by race/ethnicity, however, are infrequently reported (Armstrong and Costello, 2002; O'Neil et al., 2011). Available data suggest that black children or adolescents with a psychiatric disorder are more likely than their white counterparts to receive inpatient psychiatric treatment and be diagnosed with disruptive behavioral or psychotic disorders, while whites are more likely to be diagnosed with internalizing disorders and SUDs (Muroff et al., 2008).
Further, much less is known about the extent of SUDs and their comorbid conditions among children and young adolescents (Armstrong and Costello, 2002; O'Neil et al., 2011). Studies of children or adolescents with bipolar spectrum disorders indicate rarity of SUDs among children, but the rate of SUDs (16–39%) escalates in adolescence (Goldstein and Bukstein, 2010). Findings from adolescents who used mental health treatment suggest a prevalent rate of SUDs (17–39%), particularly among psychiatric inpatients (Deas-Nesmith et al., 1998; Kramer et al., 2003; Weaver et al., 2007). Collectively, these findings highlight a need to describe a more comprehensive pattern of comorbid disorders in different treatment settings, especially among children and nonwhites (IOM, 2009).
To date, major studies of comorbidities are based mainly on surveys of non-institutionalized adolescents. While they provide crucial statistics for informing health policy and resource allocation, children and severe subsets (e.g., psychiatric patients, inpatients) often are not included, and surveys tends to restrict their coverage to certain disorders (e.g., alcohol, drug, MD, AD, CD, ADHD). Therefore, there are limited data about some Diagnostic and Statistical Manual of Mental Disorder-IV (DSM-IV) diagnoses not routinely assessed by survey research (e.g., PD, adjustment, learning problems, relational problems, mental retardation, and psychosis/schizophrenia). Similarly, clinical trial samples provide another source of valuable data for determining comorbid rates. Their results about comorbid patterns, however, are constrained by the inclusion and exclusion criteria used to select participants, and children as well as adolescents with current SUDs or severe conditions are often excluded from clinical studies of mental disorders (O'Neil et al., 2011). Such constraints could impede the progress of clinical research and development of treatment options, as individuals with more severe problems or poor prognosis often have an earlier onset or a comorbid SUD (Brook et al., 2000; Cohen, 2008; Clark et al., 2008; Roberts et al., 2007; Shrier et al., 2003; Zeitlin, 1999).
Of note, because of the very high cost of conducting randomized trials and the limitations in generalizing their results to patients in the real world due to inclusion/exclusion criteria and study attrition, use of EHR has become a priority for research, including research on children and adolescents with psychiatric disorders (IOM, 2009; Jha et al., 2010). To elucidate a more comprehensive pattern of disorders and comorbidities not routinely available in survey or clinical research, and to generate evidence to inform designs of CER in “real-world” clinical settings, this study capitalizes on a large EHR database collected during treatment of psychiatric patients to examine comorbidities among children and adolescents with a SUD. It addresses an important gap by examining all available psychiatric conditions (i.e., diagnoses are not constrained by survey questions used) and by including all psychiatric patients aged ≤ 17 years with a SUD, patients were not excluded due to institutional status, severity, or inclusion/exclusion criteria.
By capturing longitudinal healthcare information, the large EHR database constitutes a source from which to gauge the extent of psychiatric conditions and guide CER efforts aimed at evaluating the types of treatments used in usual practices—as well as patients’ response and safety profiles—with the ultimate goal of improving practice and patient quality of life. By capturing all patients’ medical records longitudinally, the EHR provides health professionals with an effective means by which to monitor patients’ progress, identify problems in linkage of care, reduce repetitive procedures, and enhance the treatment efficiency. It also can serve as an anonymized, HIPAA-compliant data repository for conducting research to establish comprehensive comorbid patterns by patients’ age, sex, and racial/ethnic backgrounds; identify diagnostic profiles of patients seen in (costly) inpatient settings; and evaluate specific treatments (e.g., evidence-based or not) for diverse groups of patients (e.g., using practical clinical trials to compare responses to different treatment options). As such, diagnostic patterns and comorbid conditions disproportionally affecting certain age, sex, or, racial/ethnic groups in different treatment settings can be identified for etiological or outcome research (retrospectively or prospectively) that can enhance knowledge about treatment options and practices, and help improve service delivery and reduce health disparities.
As an initial step for informing CER research related to SUDs, this study (1) examines the prevalence of all available SUDs among psychiatric patients aged ≤ 17 years by age at first psychiatric visit, sex, race/ethnicity, and treatment setting; (2) determines the extent and patterns of comorbid psychiatric disorders among patients with a SUD by age, sex, race/ethnicity, and treatment setting; (3) estimates associations of age, sex, and race/ethnicity with each comorbid diagnosis using logistic regression procedures to control for treatment setting and calendar year; and (4) determines demographic and diagnostic characteristics associated with psychiatric inpatient treatment.
2. Methods
2.1. Data source
Since 1998, the Duke University Medical Center (DUMC) Department of Psychiatry has used an EHR system (MindLinc) in all of its clinics and inpatient units to capture data on each patient’s medical record, ranging from social and developmental history to physical exams and discharge summaries. This system supplies health care providers with a readily available means of monitoring their patients’ courses of treatment (Gersing & Krishnan, 2002, 2003; Gersing et al., 2007; Beyer et al., 2005, 2007). To address the issue of completeness of the data, the EHR system includes a quality check that requires the attending clinicians (psychiatrists, psychiatry residents) to complete certain fields for a qualified clinical visit (diagnosis, clinician, services, medications, side effects, billing codes, and allergies). As such, a longitudinal data repository is built, comprising all qualified visit data for each patient. To comply with HIPAA requirements, the data are anonymized such that all indications that might suggest a patient's identity are removed.
As a large tertiary-care academic health care center, the longitudinal data repository includes diverse psychiatric patients from all possible sources (physician referrals, emergency departments, self-referrals). As of December 31, 2010, the sample included 53,824 unique patients. The analytic sample for this study included 11,457 unique patients aged 2–17 years that accessed any psychiatric treatment between January 1, 2000, and December 31, 2010. The analysis of comorbid disorders focused on 1,423 patients who received a DSM-IV SUD diagnosis (abuse of or dependence on alcohol or other psychoactive drugs, nicotine dependence; substance-induced disorders); this sample represented 12% of all psychiatric patients aged 2–17 years.
2.2. Psychiatric diagnoses
All psychiatric diagnoses listed in the medical record were noted at each visit and coded according to the DSM-IV code (American Psychiatric Association [APA], 2000). The 412 DSM-IV diagnoses were classified according to 66 subcategories, which were then further classified according to 18 categories. To provide a complete pattern of comorbidities, all available psychiatric diagnoses were examined, including SUDs; AD; ADHD, CD, other childhood disruptive; MD; PD (mainly borderline); eating; impulse-control; adjustment (a psychological response to an identifiable stressor[s] resulting in the development of clinically significant emotional or behavioral symptoms characterized by anxiety, depression, or disturbance in conduct); relational (mainly parent-child relational problems that are associated with clinically significant impairment in family or individual functioning); psychotic (including schizophrenia, schizoaffective); learning; and mental retardation diagnoses. All diagnoses were based on treatment visits and assigned by the evaluating clinicians (psychiatrists, psychiatric residents, licensed PhD-level psychologists).
2.3. Demographics and treatment setting
Demographic characteristics included age (2–17 years) at first psychiatric visit logged in the database, sex, and race/ethnicity (non-Hispanic white, non-Hispanic black, other [Hispanic, Asian], and missing data on race/ethnicity). To examine comorbidities by setting, psychiatric treatment was coded according to: outpatient treatment only vs. any inpatient treatment.
2.4. Data analyses
χ2 tests were conducted initially to determine differences in demographic characteristics and treatment setting by SUD status. Patterns of SUD diagnoses by age (at first psychiatric visit), sex, race, and treatment setting (outpatient only vs. any inpatient) were determined, as were patterns of comorbid diagnoses by age, sex, race/ethnicity, and treatment setting among patients with a SUD (n=1,423). To ease interpretation, 95% confidence intervals (CI) for each estimate are reported. To control for potential confounding effects of treatment setting and calendar year, logistic regression analyses were performed among patients with a SUD to determine associations of age, sex, and race/ethnicity with each comorbid diagnosis (AD, MD, ADHD, CD, PD, relational, adjustment, eating, impulse-control, psychotic diagnosis). Logistic regression analyses also were conducted to identify demographic correlates of inpatient relative to outpatient treatment while also controlling for calendar year. Finally, the association between each comorbid diagnosis and inpatient treatment was determined using logistic regression procedures to control for age, sex, race/ethnicity, and calendar year. All analyses were conducted by SAS 9.2 (SAS, 2010).
3. Results
3.1. Characteristics of young patients
Of all psychiatric patients aged 2–17 years (N=11,457), 54% were aged 2–12, 58% were males, 35% were black, and 24% received any inpatient treatment (Table 1). Patients with a SUD differed from patients without a SUD in age, sex, and treatment setting (P<0.001). Compared with patients without a SUD, patients with a SUD had higher proportions of older patients aged 13–17 years (93% vs. 39%), males (69% vs. 56%), and inpatient treatment (43% vs. 21%).
Table 1.
Characteristics of psychiatric patients aged 2–17 years in a large electronic health records database, by SUD status: 2000–2010 (N=11,457)
| Selected characteristics, N (%) |
Total | Patients with a SUD diagnosis |
Patients without a SUD diagnosis |
χ2 (df) P-value |
|---|---|---|---|---|
| Sample size | N=11,457 | N=1,423 | N=10,034 | |
| Age at first treatment visit | ||||
| 2–12 years | 6210 (54.2) | 104 (7.3) | 6106 (60.9) | 1439.4 (1) <0.001 |
| 13–17 years | 5247 (45.8) | 1319 (92.7) | 3928 (39.2) | |
| Sex | ||||
| Male | 6643 (58.0) | 982 (69.0) | 5661 (56.4) | 81.1 (1) <0.001 |
| Female | 4814 (42.0) | 441 (31.0) | 4373 (43.6) | |
| Race/ethnicity | ||||
| White, non-Hispanic | 5649 (49.3) | 716 (50.3) | 4933 (49.2) | 8.0 (3) 0.045 |
| Black, non-Hispanic | 4042 (35.3) | 488 (34.3) | 3554 (35.4) | |
| Other | 568 (5.0) | 53 (3.7) | 515 (5.1) | |
| Missing data on race/ethnicity | 1198 (10.5) | 166 (11.7) | 1032 (10.3) | |
| Treatment setting | ||||
| Inpatient | 2768 (24.2) | 617 (43.4) | 2151 (21.4) | 326.9(1) <0.001 |
| Outpatient only | 8689 (75.8) | 806 (56.6) | 7883 (78.6) | |
Df: degree of freedom; SUD: substance use disorder.
3.2. Prevalence of SUDs among young patients
Among all patients aged 2–17 (N=11,457), 6.7% had one SUD only and another 5.7% had two or more SUDs (cannabis, 10.4%; alcohol, 5.3%; nicotine, 1.4%; cocaine, 1.3%, opioids/heroin, 1.0%; sedative/tranquilizer, 0.5%; polysubstance, 0.4%; other SUDs, 1.4%). To account for age-related differences in diagnosis, SUDs are presented by age group at first visit (Table 2).
Table 2.
Prevalence of SUDs among psychiatric patients aged 2–17 years by sex, race, and age group (N=11,457)
| Ages 2–12 yrs Mean age1 |
Overall N=6210 |
Male 8.1 yrs |
Female 8.4 yrs* |
White 8.2 yrs |
Black 8.2 yrs |
Inpatient 9.7 yrs |
Outpatient 8.0 yrs* |
|---|---|---|---|---|---|---|---|
| Proportion | %, 95% CI | %, 95% CI | %, 95% CI | %, 95% CI | %, 95% CI | %, 95% CI | %, 95% CI |
| Cannabis | 1.3, 1.0–1.6 | 1.6, 1.2–2.0 | 0.8, 0.4–1.1* | 1.2, 0.8–1.5 | 1.9, 1.3–2.5 | 7.1, 5.3–8.9 | 0.4, 0.3–0.6* |
| Alcohol | 0.6, 0.4–0.8 | 0.7, 0.4–0.9 | 0.4, 0.1–0.7 | 0.7, 0.4–1.0 | 0.5, 0.2–0.7 | 2.9, 1.7–4.1 | 0.2, 0.1–0.4* |
| Number of SUDs | |||||||
| 1 | 1.0, 0.8–1.3 | 1.2, 0.9–1.6 | 0.7, 0.4–1.1 | 0.7, 0.4–1.0 | 1.7, 1.1–2.2* | 5.8, 4.2–7.5 | 0.4, 0.2–0.5* |
| 2+ | 0.6, 0.4–0.8 | 0.8, 0.5–1.0 | 0.4, 0.1–0.7 | 0.9, 0.5–1.2 | 0.5, 0.2–0.8 | 3.6, 2.3–4.9 | 0.2, 0.1–0.3* |
|
Ages 13–17 yrs Mean age1 |
Overall N=5247 |
Male 15.1 yrs |
Female 15.2 yrs |
White 15.2 yrs |
Black 15.0 yrs |
Inpatient 15.1 yrs |
Outpatient 15.2 yrs |
| Cannabis | 21.2, 20.1–22.3 | 30.1, 28.4–31.8 | 12.0, 11.0–13.0* | 20.6, 19.1–22.2 | 21.0, 19.2–22.9 | 22.5, 20.6–24.3 | 20.5, 19.1–21.9 |
| Alcohol | 10.9, 10.1–11.8 | 14.4, 13.1–15.7 | 7.3, 6.3–8.3* | 14.2, 12.8–15.5 | 5.3, 4.3–6.3* | 9.8, 8.5–11.1 | 11.6, 10.5–12.7 |
| Nicotine | 2.9, 2.4–3.3 | 4.2, 3.4–4.9 | 1.5, 1.0–1.9* | 4.3, 3.5–5.1 | 0.8, 0.4–1.2* | 2.1, 1.5–2.8 | 3.3, 2.7–3.9 |
| Cocaine | 2.7, 2.3–3.2 | 2.8, 2.2–3.5 | 2.6, 2.0–3.3 | 3.4, 2.7–4.1 | 1.8, 1.2–2.4* | 4.0, 3.2–4.9 | 2.0, 1.5–2.4* |
| Opioid/heroin | 2.1, 1.7–2.5 | 3.1, 2.5–3.8 | 1.1, 0.7–1.4* | 3.3, 2.6–4.0 | 0.4, 0.1–0.6* | 2.1, 1.5–2.8 | 2.1, 1.6–2.6 |
| Sedative2 | 0.9, 0.7–1.2 | 1.3, 0.9–1.8 | 0.5, 0.2–0.7* | 1.3, 0.9–1.8 | 0.1, 0–0.2 | 1.0, 0.5–1.4 | 0.9, 0.6–1.2 |
| Polysubstance | 0.9, 0.6–1.1 | 1.2, 0.8–1.6 | 0.5, 0.3–0.8 | 1.3, 0.8–1.7 | 0.3, 0.1–0.6* | 1.3, 0.8–1.8 | 0.6, 0.3–0.8 |
| Other substance | 2.7, 2.3–3.2 | 3.5, 2.8–4.2 | 1.9, 1.3–2.4* | 3.4, 2.7–4.1 | 1.3, 0.8–1.8* | 4.3, 3.4–5.2 | 1.8, 1.3–2.2* |
| Number of SUDs | |||||||
| 1 | 13.5, 12.6–14.4 | 17.5, 16.1–19.0 | 9.3, 8.1–10 * | 10.8, 9.6–12.0 | 17.6, 15.9–19.3* | 15.1, 13.5–16.7 | 12.5, 11.4–13.6 |
| 2+ | 11.7, 10.8–12.5 | 16.2, 14.8–17.6 | 6.9, 5.9–7.9 * | 14.7, 13.4–16.1 | 5.9, 4.8–6.9* | 12.3, 10.9–13.8 | 11.3, 10.2–12.4 |
CI: confidence interval; SUD: substance use disorder.
Mean age (years) at first psychiatric visit.
Sedative diagnoses included tranquilizer diagnoses.
P<0.05 between two groups by sex, race, or treatment setting status.
Ages 2–12 years
Few patients (1.6%) had a SUD. Males had a higher prevalence of cannabis diagnoses than females (1.6% vs. 0.8%); inpatients had a higher prevalence of cannabis (7.1% vs. 0.4%) and alcohol (2.9% vs. 0.2%) diagnoses than outpatients.
Ages 13–17 years
In this older group, 13.5% had one SUD only, and another 11.7% had two or more SUDs (cannabis diagnosis, 21.2%; alcohol diagnosis, 10.9%). Males had a higher prevalence of all individual SUDs than females except for cocaine and polysubstance diagnoses, which were infrequent in both groups. Whites had a higher prevalence of all SUDs than blacks except for cannabis diagnosis (21% in each). Inpatients had a higher prevalence of cocaine diagnoses than outpatients (4.0% vs. 2.0%).
Across all groups, males had the highest prevalence of any SUD (33.7%); whites had the highest prevalence of comorbid SUDs (58% of whites with a SUD having two or more SUDs compared with 25% of blacks with a SUD).
3.3. Comorbid diagnoses among patients with a SUD
Comorbid diagnoses among patients with any SUD (n=1,423) by age group at first visit were determined.
Ages 2–12 years
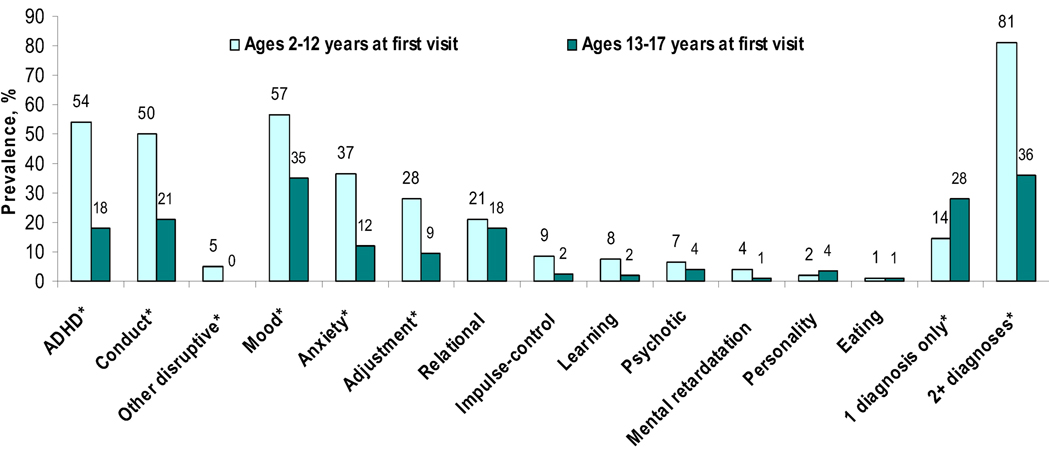
Compared with adolescents aged 13–17 years with a SUD (Figure 1), patients aged 2–12 years with a SUD had a higher prevalence of comorbid MD (56.7% vs. 35.0%), AD (36.5% vs. 12.1%), CD (50.0% vs. 21.1%), ADHD (53.8% vs. 18.0%), and adjustment diagnoses (27.9% vs. 9.4%). Of note, 95.2% of patients aged 2–12 years with a SUD also had another psychiatric diagnosis (80.8% with two or more diagnoses) compared with 64.1% of patients aged 13–17 years with a SUD (36.1% with two or more diagnoses). In the younger group (Table 3), females with a SUD had a higher prevalence of comorbid MD than males (80.0% vs. 49.4%), and all females had another psychiatric diagnosis. Inpatients also had a higher prevalence of comorbid MD (66.2% vs. 33.3%), CD (59.5% vs. 26.7%), and relational diagnoses (27.0% vs. 6.7%) than outpatients; the vast majority (97.3%) of inpatients with a SUD also had another diagnosis.
Figure 1.
Comorbid psychiatric diagnoses among psychiatric patients with a substance use diagnosis (N=1,423)
Note: ADHD: Attention deficit hyperactive disorder; *P <0.05 by age group.
Table 3.
Comorbid psychiatric diagnoses among psychiatric patients aged 2–12 years with a SUD by sex, race/ethnicity, and treatment setting (N=104)
| Ages 2–12 years Mean age1 |
Male N=79 10.5 yrs |
Female N=25 10.0 yrs |
White N=48 10.2 yrs |
Black N=47 10.8 yrs |
Inpatient N=30 10.3 yrs |
Outpatient N=74 10.5 yrs |
|---|---|---|---|---|---|---|
| Comorbid diagnosis | %, 95% CI | %, 95% CI | %, 95% CI | %, 95% CI | %, 95% CI | %, 95% CI |
| Mood–any | 49.4, 38.1–60.6 | 80.0, 63.1–96.9 * | 64.6, 50.5–78.6 | 46.8, 32.0–61.6 | 66.2, 55.2–77.2 | 33.3, 15.4–51.2* |
| Anxiety–any | 32.9, 22.3–43.5 | 48.0, 26.9–69.0 | 47.9, 33.3–62.6 | 25.5, 12.6–38.5 | 36.5, 25.3–47.7 | 36.7, 18.4–55.0 |
| Conduct | 49.4, 38.1–60.6 | 52.0, 30.9–73.0 | 39.6, 25.2–53.9 | 66.0, 51.9–80.0 | 59.5, 48.0–70.9 | 26.7, 9.9–43.5* |
| Attention deficit hyperactive |
55.7, 44.5–66.9 | 48.0, 26.9–69.0 | 66.7, 52.8–80.5 | 46.8, 32.0–61.6 | 51.4, 39.7–63.0 | 60.0, 41.4–78.6 |
| Other childhood disruptive |
6.3, 0.8–11.8 | ---- | 8.3, 0.2–16.4 | 2.1, 0–6.4 | 5.4, 0.1–10.7 | 3.3, 0–10.2 |
| Relational problem | 17.7, 9.1–26.3 | 32.0, 12.3–51.6 | 12.5, 2.8–22.2 | 34.0, 20.0–48.1 | 27.0, 16.7–37.4 | 6.7, 0–16.1* |
| Adjustment | 29.1, 18.9–39.4 | 24.0, 6.0–42.0 | 33.3, 19.5–47.2 | 25.5, 12.6–38.5 | 31.1, 20.3–41.9 | 20.0, 4.8–35.2 |
| Eating | 1.3, 0–3.8 | ---- | ---- | 1.4, 0–4.0 | ---- | |
| Impulse-control | 11.4, 4.2–18.6 | ---- | 4.2, 0–10.0 | 12.8, 2.9–22.7 | 12.2, 4.5–19.8 | ---- |
| Learning | 8.9, 2.5–15.3 | 4.0, 0–12.2 | 10.4, 1.5–19.4 | 6.4, 0–13.6 | 6.8, 0.9–12.6 | 10.0, 0–21.4 |
| Mental retardation | 2.5, 0–6.1 | 8.0, 0–19.4 | 2.1, 0–6.3 | 6.4, 0–13.6 | 5.4, 0.1–10.7 | ---- |
| Psychotic | 7.6, 1.6–13.6 | 4.0, 0–12.2 | 6.3, 0–13.4 | 8.5, 0.2–16.8 | 9.5, 2.6–16.3 | ---- |
| Personality–any | 1.3, 0–3.8 | 4.0, 0–12.2 | ---- | 4.3, 0–10.2 | 2.7, 0–6.5 | ---- |
| Number of diagnoses2 | ||||||
| 0 (SUD only) | 6.3, 0.8–11.8 | 4.2, 0–10.0 | 6.4, 0–13.6 | 2.7, 0–6.5 | 10.0, 0–21.4 | |
| 1 | 15.2, 7.1–23.3 | 12.0, 0–25.6 | 10.4, 1.5–19.4 | 12.8, 2.9–22.7 | 9.5, 2.6–16.3 | 26.7, 9.9–43.5 |
| 2+ | 78.5, 69.2–87.7 | 88.0, 74.3–100 | 85.4, 75.1–95.8 | 80.9, 69.2–92.5 | 87.8, 80.2–95.5 | 63.3, 45.0–81.6 |
CI: confidence interval; SUD: substance use disorder; ----: a zero cell.
Mean age (years) at first psychiatric visit.
Number of comorbid diagnoses included: any mood, conduct, attention deficit hyperactive, relational, any anxiety, adjustment, psychotic or schizophrenia, eating, impulse-control, any personality, learning, mental retardation, and other childhood disruptive diagnoses.
P<0.05 between two groups by sex, race, or treatment setting status.
Ages 13–17 years
MD (35%) was the most prevalent comorbid diagnosis in the older group (Table 4), particularly among females (49.0% vs. 28.6% among males) and inpatients (58.6% vs. 18.6% among outpatients). Females, blacks, and inpatients generally showed a higher prevalence of comorbid diagnoses than others. Females had more AD (18.5% vs. 9.1%), CD (25.7% vs. 18.9%), adjustment (13.7% vs. 7.4%), eating (2.6% vs. 0.1%), and PD (7.0% vs. 2.2%); males had more ADHD than females (20.8% vs. 11.7%). Whites had more AD (16.0% vs. 9.1%) and relational diagnoses (23.2% vs. 14.3%) diagnoses; blacks had more CD (38.3% vs. 13.5%), impulse-control (5.0% vs. 1.0%), mental retardation (2.9% vs. 0.4%), and psychotic diagnoses (10.2% vs. 1.2%). Inpatients had more AD (18.0% vs. 7.9%), CD (40.3% vs. 7.6%), adjustment (15.8% vs. 4.9%), impulse-control (5.3% vs. 0.4%), psychotic (10.1% vs. 0.1%), and PD (8.5% vs. 0.4%) diagnoses than outpatients.
Table 4.
Comorbid psychiatric diagnoses among psychiatric patients aged 13–17 years with a SUD by age, sex, race/ethnicity, and treatment setting (N=1,319)
| Ages 13–17 years Mean age1 |
Male N=903 15.6 yrs |
Female N=416 15.5 yrs |
White N=668 15.8 yrs |
Black N=441 15.3 yrs* |
Inpatient N=543 15.3 yrs |
Outpatient N=776 15.8 yrs* |
|---|---|---|---|---|---|---|
| Comorbid diagnosis | %, 95% CI | %, 95% CI | %, 95% CI | %, 95% CI | %, 95% CI | %, 95% CI |
| Mood–any | 28.6, 25.6–31.5 | 49.0, 44.2–53.8* | 36.8, 33.2–40.5 | 37.0, 32.4–41.5 | 58.6, 54.4–62.7 | 18.6, 15.8–21.3* |
| Anxiety–any | 9.1, 7.2–11.0 | 18.5, 14.7–22.2* | 16.0, 13.2–18.8 | 9.1, 6.4–11.8* | 18.0, 14.8–21.3 | 7.9, 6.0–9.8* |
| Conduct | 18.9, 16.4–21.5 | 25.7, 21.5–29.9* | 13.5, 10.9–16.1 | 38.3, 33.8–42.9* | 40.3, 36.2–44.5 | 7.6, 5.7–9.5* |
| Attention deficit hyperactive |
20.8, 18.2–23.5 | 11.7, 8.7–14.8 * | 21.1, 18.0–24.2 | 15.4, 12.0–18.8 | 21.4, 17.9–24.8 | 15.6, 13.0–18.2 |
| Other childhood disruptive |
0.3, 0–0.7 | ---- | 0.3, 0–0.7 | 0.2, 0–0.7 | 0.4, 0–0.9 | 0.1, 0–0.4 |
| Relational problem | 18.3, 15.7–20.8 | 17.7, 14.0–21.4 | 23.2, 20.0–26.4 | 14.3, 11.0–17.6* | 19.7, 16.3–23.1 | 17.0, 14.4–19.7 |
| Adjustment | 7.4, 5.7–9.1 | 13.7, 10.3–17.0* | 9.0, 6.8–11.2 | 12.7, 9.6–15.8 | 15.8, 12.8–18.9 | 4.9, 3.4–6.4* |
| Eating | 0.1, 0–0.3 | 2.6, 1.1–4.2* | 1.6, 0.7–2.6 | 0.2, 0–0.7 | 1.3, 0.3–2.2 | 0.6, 0.1–1.2 |
| Impulse-control | 2.5, 1.5–3.6 | 2.2, 0.8–3.6 | 1.0, 0.3–1.8 | 5.0, 2.9–7.0* | 5.3, 3.4–7.2 | 0.4, 0–0.8* |
| Learning | 2.2, 1.3–3.2 | 1.2, 0.2–2.3 | 1.8, 0.8–2.8 | 2.0, 0.7–3.4 | 1.7, 0.6–2.7 | 2.1, 1.1–3.1 |
| Mental retardation | 1.2, 0.5–1.9 | 1.2, 0.2–2.3 | 0.4, 0–1.0 | 2.9, 1.4–4.5* | 2.4, 1.1–3.7 | 0.4, 0–0.8* |
| Psychotic | 4.3, 3.0–5.6 | 4.1, 2.2–6.0 | 1.2, 0.4–2.0 | 10.2, 7.4–13.0* | 10.1, 7.6–12.7 | 0.1, 0–0.4* |
| Personality–any | 2.2, 1.3–3.2 | 7.0, 4.5–9.4* | 4.6, 3.0–6.2 | 3.2, 1.5–4.8 | 8.5, 6.1–10.8 | 0.4, 0–0.8* |
| Number of diagnoses2 | ||||||
| 0 (SUD only) | 40.9, 37.7–44.1 | 25.2 21 .0–29.4* | 34.1 30.5–37.7 | 28.3 24.1–32.6 | 6.8, 4.7–8.9 | 56.3, 52.8–59.8* |
| 1 | 26.7, 23.8–29.6 | 30.7 26.3–35.2 | 29.9 26.5–33.4 | 27.9 23.7–32.1 | 34.4, 30.4–38.4 | 23.5, 20.5–26.4* |
| 2+ | 32.4, 29.4–35.5 | 43.9 39.2–48.7* | 35.9 32.3–39.6 | 43.8 39.1–48.4 | 58.7, 54.6–62.9 | 20.2, 17.4–23.1* |
CI: confidence interval; SUD: substance use disorder; ----: a zero cell.
Mean age (years) at first psychiatric visit.
Number of comorbid diagnoses included: any mood, conduct, attention deficit hyperactive, relational, any anxiety, adjustment, psychotic or schizophrenia, eating, impulse-control, any personality, learning, mental retardation, and other childhood disruptive diagnoses.
P<0.05 between two groups by sex, race, or treatment setting status.
3.4. Logistic regression analyses of correlates of comorbid psychiatric diagnoses
Given that comorbid diagnoses varied by age, sex, race/ethnicity, and treatment setting, adjusted logistic regression analyses were performed among patients with a SUD to estimate the strength of associations of age at first visit, sex, and race/ethnicity with each comorbid diagnosis while controlling for treatment setting and calendar year (Table 5).
Table 5.
Logistic regression of comorbid diagnoses among psychiatric patients aged 2–17 years with a SUD (N=1,423)
| Adjusted logistic regression model of comorbid diagnosis1 |
Age | Sex | Race/ethnicity | ||
|---|---|---|---|---|---|
| Age 2–12 years vs. 13–17 years |
Female vs. male | Black vs. white | Other vs. white | Missing vs. white | |
| Diagnosis | AOR, 95% CI | AOR, 95% CI | AOR, 95% CI | AOR, 95% CI | AOR, 95% CI |
| Adjustment | 2.02, 1.19–3.45 | 1.35, 0.93–1.95 | 1.04, 0.71–1.53 | 0.43, 0.13–1.47 | 0.45, 0.19–1.08 |
| Anxiety–any | 2.09, 1.25–3.51 | 1.79, 1.27–2.52 | 0.41, 0.28–0.61 | 0.63, 0.26–1.55 | 0.34, 0.16–0.73 |
| Attention deficit hyperactive | 2.77, 1.73–4.44 | 0.39, 0.27–0.55 | 0.65, 0.47–0.91 | 0.21, 0.06–0.71 | 0.91, 0.56–1.46 |
| Conduct | 1.96, 1.20–3.21 | 0.89, 0.65–1.20 | 3.02, 2.23–4.10 | 1.11, 0.51–2.42 | 0.62, 0.31–1.23 |
| Impulse-control | 1.79, 0.72–4.44 | 0.36, 0.16–0.78 | 3.04, 1.39–6.67 | 2.70, 0.53–13.64 | 1.84, 0.37–9.14 |
| Mood–any | 0.86, 0.52–1.43 | 1.82, 1.40–2.38 | 0.58, 0.43–0.78 | 1.19, 0.61–2.32 | 0.76, 0.48–1.19 |
| Personality–any | 0.10, 0.02–0.49 | 2.12, 1.13–3.97 | 0.45, 0.23–0.90 | 0.40, 0.05–3.18 | 1.48, 0.40–5.45 |
| Psychotic | 0.31, 0.11–0.90 | 0.42, 0.22–0.78 | 6.11, 2.90–12.90 | 3.28, 0.65–16.57 | 1.18, 0.14–10.00 |
| Relational | 0.66, 0.37–1.20 | 0.82, 0.59–1.15 | 0.69, 0.49–0.98 | 0.57, 0.22–1.49 | 0.54, 0.30–0.98 |
| Eating | 0.25, 0.19–0.34 | 12.70, 8.55–18.86 | 0.14, 0.09–0.21 | 0.68, 0.39–1.17 | ----2 |
| Personality–borderline3 | 0.51, 0.31–0.85a | 8.54, 3.65–19.96 | 0.51, 0.29–0.91 | 0.82, 0.28–2.41 | 0.71, 0.21–2.40 |
AOR: adjusted odds ratio; CI: confidence interval; SUD: substance use disorder.
Bold: P<0.05.
Each model included age, sex, race/ethnicity, treatment setting, and calendar year.
---: not included due to a small cell size.
The analysis was based on adolescents aged 13–17 years due to a small number of cases aged 2–12 years.
Ages 13–15 years vs. 16–17 years.
Adjusted analyses showed that (a) early psychiatric treatment (ages 2–12 years) was associated with having an adjustment, AD, ADHD, or CD diagnosis, while adolescent treatment (ages 13–17 years) was associated with having a PD, psychotic, or eating diagnosis; (b) female sex was associated with having an AD, MD, PD (mainly borderline), and eating diagnosis, while male sex was associated with having an ADHD, impulse-control, and psychotic diagnosis; (c) black race was associated with having a CD, impulse-control, and psychotic diagnosis, while white race was associated with having an AD, ADHD, MD, PD, relational, and eating diagnosis.
3.5. Logistic regression analyses of inpatient treatment use
Adjusted logistic regression analyses then were performed among patients with a SUD (n=1,423) to determine the associations of age at first visit, sex, and race/ethnicity with inpatient treatment (relative to outpatient treatment) adjusted for calendar year. The adjusted model indicated that ages 2–12 years at first treatment relative to ages 13–17 years (AOR 3.50, 95% CI 2.11–5.80), female sex (AOR 2.48, 95% CI 1.92–3.21), black race relative to white race (AOR 3.26, 95% CI 2.51–4.24), and “other race” relative to white race (AOR 2.44; 95% CI 1.34–4.44) were associated with greater odds of inpatient treatment.
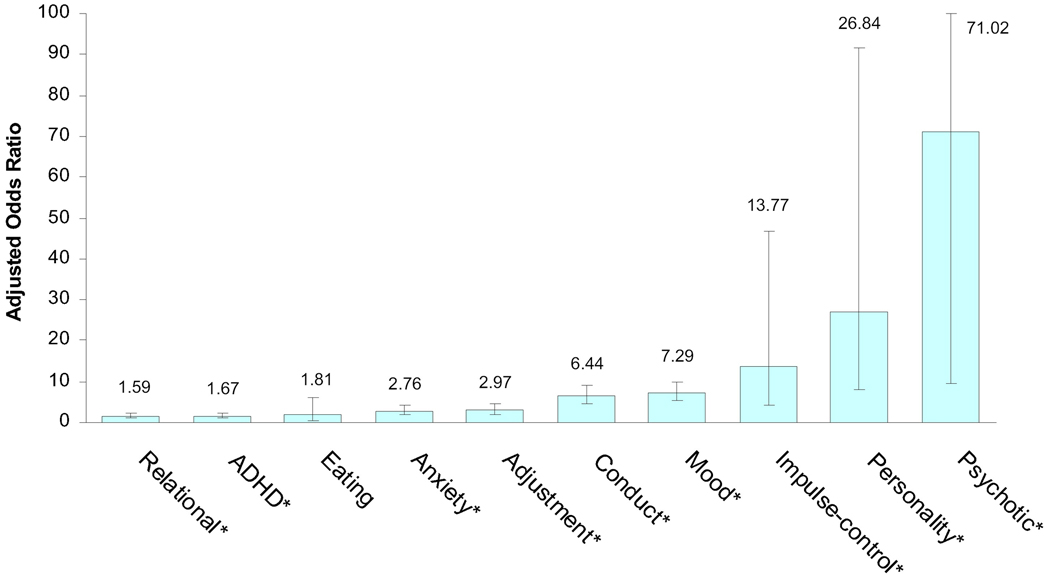
Finally, diagnostic correlates of inpatient treatment (relative to outpatient treatment) among patients with a SUD were determined by logistic regression analyses that controlled for patients’ age at first treatment, sex, race/ethnicity, and calendar year. As shown in Figure 2, while having an AD, ADHD, adjustment, or relational diagnosis moderately increased odds of inpatient treatment among patients with a SUD, having a CD, MD, impulse-control, and especially PD or psychotic diagnosis substantially increased odds of inpatient treatment.
Figure 2.
Adjusted odds ratios of inpatient treatment use relative to outpatient treatment in relation to specific DSM-IV diagnosis among psychiatric patients aged 2–17 years with a substance use diagnosis (N=1,423)
Note: ADHD: Attention deficit hyperactive disorder.
Each logistic regression model adjusted for age, sex, race/ethnicity, and calendar year; *P <0.05.
4. Discussion
In this study, we examined EHRs of a large, tertiary-care, university-based hospital and its associated psychiatric clinics, which systematically captured clinical data on all psychiatric patients between 2000 and 2010. Patients were referred for any psychiatric treatment from various sources regardless of whether the SUD antedated or post-dated the onset of other psychiatric illnesses. This large EHR database captured an unusually broad range of co-occurring DSM-IV SUDs and other disorders, providing a unique opportunity to comprehensively characterize the pattern of comorbidities in the real-world setting for children and adolescents of different racial/ethnic backgrounds in outpatient and inpatient settings. Several findings are important for health care administrators, clinicians, and researchers.
4.1. Patients with a SUD used more inpatient treatment than patients without a SUD
The prevalence of SUD among patients increased from about 2% in the 2–12-year age group to 25% in the 13–17-year age group. Using the actual treatment data, results are consistent with findings from research-based diagnostic interviews or self-reports showing that SUDs are uncommon among children but are prevalent among adolescents, especially males (Armstrong and Costello, 2002; O'Neil et al., 2011). Although patients aged 2–12 years with a SUD constituted only 0.9% of all patients, more than one half of all patients were aged 2–12 years at their first visits, suggesting that SUDs in adolescents with comorbid diagnoses often occur later than onset of other disorders, as early internalizing or disruptive behavioral conditions, as well as shared environmental or familial risk factors for psychiatric conditions, all could increase liability to substance use or intensify SUD (O'Neil et al., 2011; Shrier et al., 2003). Thus, the high proportion (43%) of inpatient treatment among patients with a SUD, particularly younger patients, can be related to more severe childhood-onset disorders (e.g., MD, AD, CD, PD) or comorbidities than are seen in patients without a SUD. For example, 9.4% of inpatients aged 2–12 years had a SUD compared with 0.6% of outpatients aged 2–12 years; of the patients with a SUD, the vast majority (81%) of patients aged 2–12 years had two or more other diagnoses compared with 36% of the older group. However, the low prevalence of SUDs in the younger group also might be related partly to under-diagnosis, as some patients might not have been comprehensively assessed for all possible SUDs.
Another clinically important finding concerns the magnitude of cannabis diagnosis (Shapiro et al., 2010). Cannabis diagnosis was the most prevalent SUD across various age, sex, and racial/ethnic groups and treatment settings, accounting for more than 80% of all SUD cases. Data from the U.S. Treatment Episode Data Set (TEDS) also show primary cannabis diagnoses accounting for the majority of treatment admissions for patients aged under 15 years (SAMHSA, 2009b), a finding that could be associated with increased levels of cannabis potency or marijuana-related problems in the past decade (Compton et al., 2004; National Center on Addiction and Substance Abuse [NCASA], 2008). On the other hand, the relatively low rate of alcohol-related treatment when compared to a relatively high prevalence of alcohol use suggests that many adolescents with an alcohol use disorder (especially those without a comorbid condition) might ignore the problem or seek help in non-psychiatric or self-help settings (Kelly & Myers, 2007; Wu et al., 2006). Alternatively, the “illicit” nature of cannabis use might increase the likelihood of referral of cannabis users for treatment (e.g., due to involvement with the criminal justice system), or the presence of cannabis diagnosis is associated with a greater level of substance use problems (Wu et al., 2005).
Nevertheless, findings suggest a cannabis diagnosis is the SUD most likely to be encountered by clinicians at psychiatric treatment settings, and, as such, it deserves research to evaluate treatments received and identify factors moderating treatment outcomes, especially among adolescents with comorbid conditions given the scarcity of data on the quality of substance abuse care for adolescents in clinical practice (Waxmonsky and Wilens, 2005). The increased prevalence in cannabis use and cannabis-related treatment use (Compton et al., 2004; Johnston et al., 2011; NCASA, 2008; SAMHSA, 2009b), along with legalization of medicinal marijuana in 14+ states in the United States (http://medicalmarijuana.procon.org/), also point toward a need for monitoring cannabis use and elucidating its harmful effects (Baker et al., 2010; Large et al., 2011). Specifically, a growing body of research has found an association between early cannabis use and early-onset of psychotic disorder, and a recent meta-analysis has provided strong evidence for this association (Large et al., 2011). These findings highlight the need for using the longitudinal data to specify subgroups of cannabis users vulnerable for developing psychosis and discern the clinical courses of patients with psychotic symptoms by cannabis use status.
4.2. Children and females showed a particularly severe pattern of comorbidities
These treatment data also suggest a high rate of comorbidities among early treatment users who also had a SUD (O'Neil et al., 2011). Adjustment, AD, ADHD, and CD diagnoses were comparatively prevalent among patients aged 2–12 years with a SUD, suggesting that these conditions are associated with a high level of impairment in multiple domains that trigger early treatment use. This pattern is in line with survey data that CD and ADHD confer a high risk for subsequent SUDs and that AD frequently co-occurs with CD or ADHD (Armstrong and Costello, 2002; Bubier and Drabick, 2009; Costello, 2007). With the diagnostic data from all patients, the results further indicate that PD, eating, and psychotic diagnoses are less likely to be diagnosed in children than in adolescents.
There are also clinically important sex differences in comorbidities: females had greater odds having MD, AD, PD (borderline), and eating diagnoses, whereas males had greater odds of having ADHD, impulse-control, and psychotic diagnoses. This pattern also is noted in results from surveys and clinical samples (Garland et al., 2001; Leung and Chue, 2000; Lewinsohn et al., 1993; O'Neil et al., 2011; Roberts et al., 2007), demonstrating differential needs for clinical assessments and treatments. Moreover, a few studies have suggested that females with comorbid SUD and other disorders represent a particularly severe subgroup that is affected by multiple psychiatric and family problems and that manifests high risk for engaging in risky sexual behaviors resulting in adverse consequences (Armstrong and Costello, 2002; Dakof, 2000; O'Neil et al., 2011; Rao et al., 2000; Roberts et al., 2007; Rowe et al., 2004; Shrier et al., 2003; Zeitlin, 1999). Regrettably, our results also reveal a greater level of comorbidity among females with a SUD than among their male counterparts, which appears to be related to females’ high rate of MD and CD. Both are robust correlates of multiple psychiatric problems; as the level of psychopathology increases, the severity of SUDs can escalate (Costello, 2007; Armstrong and Costello, 2002; Shrier et al., 2003).
Collectively, prevalent rates of comorbidities support the rationale for in-depth research to assess specific patterns and the quality of health care received in clinical practices and to investigate whether early mental health treatments improve SUD outcomes (Kendall and Kessler, 2002). Sex differences in comorbidities underline the importance of incorporating tailored interventions, given that internalizing and externalizing conditions require different treatment approaches (Compton et al., 2002; Farmer et al., 2002).
4.3. Blacks, children, and females disproportionally used inpatient treatment
Consistent with a more pervasive pattern of comorbidities among patients aged 2–12 years and females, both groups demonstrated elevated odds for inpatient treatment. This study also highlights clinically important racial/ethnic differences in diagnosis and location of treatment (Armstrong and Costello, 2002; O'Neil et al., 2011). Blacks with a SUD had higher odds of being diagnosed with CD, impulse-control, and psychotic diagnoses, while whites with a SUD had higher odds of AD, ADHD, MD, PD, relational, and eating diagnoses. Data from other treatment-seeking samples also show a higher prevalence of disruptive behavioral and psychotic disorders but a lower prevalence of internalizing disorders, PD, and ADHD among blacks compared with whites (McGilloway et al., 2010; Muroff et al., 2008; Stevens et al., 2005).
While there are no differences in the overall prevalence of any comorbidity between the two groups, elevated odds of inpatient treatment among blacks with a SUD may be related to their higher rates of CD, impulse-control, and psychotic diagnoses (Muroff et al., 2008). These diagnoses were substantially associated with inpatient treatment, which might be related in part to their diagnostic features (impulsivity, injuries, impairments) (Halamandaris and Anderson, 1999). Alternatively, racial/ethnic differences in comorbid patterns could be related in part to various sources of bias, including race/ethnicity-related referral bias (due to financial or severity status, specialty of clinicians), differential health belief and help-seeking behaviors (due to variations in cultural backgrounds, financial resources, access to care, preferences), parental reporting bias (due to differential self-reporting tendency), or clinicians’ diagnostic bias (Garland et al., 2005; Kilgus et al., 1995; Muroff et al., 2008; Stevens et al., 2005). For example, comparatively high rates of ADHD and internalizing conditions among whites may be related to whites’ greater levels of utilizing mental health care and reporting of these symptoms to clinicians than blacks’ (Garland et al., 2005; Hillemeier et al., 2007).
Nonetheless, systematic racial/ethnic differences in diagnosis and treatment location not only warrant research to describe underlying mechanisms accounting for these variations, but also point to distinct burdens and needs for intervention. The longitudinal EHRs provide the opportunities to investigate further racial/ethnic differences in diagnosis, patterns and the quality of care received, as well as long-term prognosis to identify means by which to reduce health disparities.
4.4. Limitations and strengths
These results should be interpreted within the following context. Diagnoses were based on actual treatment data collected as a part of usual clinical practice in real-world settings, meaning that they were based on treating clinicians’ evaluations using all of the available health care information collected in the longitudinal EHR database along with medical examinations, laboratory data, treatment options available from third party payers, and information from clinical interactions among clinicians, patients, and guardians. Within this natural context, results are unlikely to be influenced by bias associated with research subjects (e.g., underreporting due to social desirability). This differs from research-based diagnostic data, which typically rely on diagnostic instruments (administered frequently by research assistants or lay interviewers; infrequently by clinicians) or participants’ self-reports on survey questions. Additionally, research-based data are confined to certain categories available in the assessments, detailed diagnostic-related medical data are often not assessed, and participants who are aware of their participation in research are selected by predetermined inclusion/exclusion criteria.
In this regard, the large retrospective data warehouse collected by an EHR constitutes a valuable source of clinical data for evaluating an unusually broad range of DSM-IV Axis I and II diagnoses in real-world clinical practice because they serve a wider range of populations (not restricted by diagnostic questions and inclusion/exclusion criteria). However, actual treatment data are based on non-standardized clinical evaluations, as are typical of usual care settings, which can be influenced by variations in clinicians’ specialties and treatment coverage from third party payers (e.g., billable diagnoses). These results also may underestimate SUDs and comorbidities as some patients might not have been systematically evaluated for all psychiatric diagnoses, and variations in clinical detection and practices over time also could affect diagnostic patterns (e.g., billable diagnoses). Nonetheless, other similar issues also affect research-based or survey data: diagnostic assessments are restricted to available research questions, participants are chosen by study-specific inclusion/exclusion criteria, and variations in the quality and content of diagnostic assessments often complicates comparisons of diagnostic data across studies and over time.
Although patients who entered psychiatric treatment in a large tertiary-care, academic medical center (DUMC) included patients from all possible referral sources, patterns of comorbidities also may be influenced by specific faculty or departmental interests and expertise. DUMC might attract some severe patients into treatment. These young patients may not be representative of children and adolescents with a SUD. Further, data on family history of psychiatric conditions, parents’ socioeconomic status, or attitudes toward treatment, as well as treatment use outside of DUMC, were not available, and the sample size for Hispanics was too small to make comparisons.
Nonetheless, this study has unique strengths not available from small-scale studies or survey research. DUMC serves a diverse population consisting of rural, urban, and suburban residents, as shown by the finding that 35% of the patients are black, and all young patients with a SUD were included. This diversity in demographics and the inclusion of all available diagnoses provide an opportunity to examine more wide-ranging patterns of comorbid diagnoses among young patients in the real world and to document clinically important diagnoses that have been omitted or overlooked in major surveys (e.g., adjustment, relational, PD, psychotic/schizophrenic diagnoses). Findings showed a low rate of learning or mental retardation diagnoses; however, adjustment and relational diagnoses were not uncommon, indicating the clinical importance of assessing for these conditions and evaluating their impact on the treatment course of substance-using adolescents. Lastly, results add clinically important data for diagnostic profiles among children, females, and blacks, who are vulnerable groups emphasized by the National Institutes of Health and the Institute of Medicine.
4.5. Conclusions
These results on patterns of comorbid diagnoses are remarkably consistent with findings from national, community-based, and clinical studies (Costello, 2007; Kessler et al., 2005; Merikangas et al., 2010; O'Neil et al., 2011). The high rate of cannabis use diagnoses adds to the findings that cannabis is used by adolescents more frequently than alcohol or other drugs and should call physicians' attention to the importance of screening and intervention for cannabis use problems in adolescents (Wu et al., 2011). Additionally, they reveal clinical evidence on treatment locations and diagnostic patterns for understudied diagnoses among children, females, and blacks. Psychiatric patients with a SUD use more costly inpatient treatment than patients without a SUD, especially children, females, and blacks. These patients require intensive and coordinated mental and substance abuse care for multiple diagnoses. Therefore, patients with any SUD require comprehensive psychiatric assessments for treatment planning, and for using CER efforts to inform the quality of health care and assessment of long-term outcomes. Together, these findings underscore the need to investigate how comorbidity affects interventions for either SUD or other psychiatric diagnoses and to address sex and racial/ethnic differences in psychiatric profiles to optimize treatment response and reduce health disparities (O’Neil et al., 2011).
Acknowledgments
This study was approved by the Duke University Institutional Review Board. The authors thank A. Deo Garlock and staff of the MindLinc for their contribution to the data depository and Amanda McMillan for her editorial assistance.
Role of Funding Source: This work was made possible by Department of Psychiatry of Duke University and research grants from the U.S. National Institute on Drug Abuse of the National Institutes of Health (R33DA027503, R01DA019623, and R01DA019901 to Li-Tzy Wu). Dan G. Blazer was supported by HHSN271200522071C; George E. Woody was supported by K05DA017009 and U10DA013043. The National Institutes of Health had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health and the Department of Psychiatry of Duke University Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: LT Wu developed research aims and questions and wrote the initial draft of the paper. K Gersing designed the electronic health record database system. B Burchett managed the database and conducted statistical analyses. All authors contributed to critical revisions and interpretations of the findings to result in the final manuscript.
Conflicts of interest: George E. Woody is a member of the RADARS post-marketing study scientific advisory group, whose job is to assess abuse of prescription medications. Denver Health administers RADARS, and pharmaceutical companies support its work. The other authors have no conflicts of interest to disclose.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revision. Washington, DC: American Psychiatric Publishing; 2000. [Google Scholar]
- Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. Journal of Consulting and Clinical Psychology. 2002;70:1224–1239. doi: 10.1037//0022-006x.70.6.1224. [DOI] [PubMed] [Google Scholar]
- Baker AL, Hides L, Lubman DI. Treatment of cannabis use among people with psychotic or depressive disorders: a systematic review. Journal of Clinical Psychiatry. 2010;71:247–254. doi: 10.4088/JCP.09r05119gry. [DOI] [PubMed] [Google Scholar]
- Beyer J, Kuchibhatla M, Gersing K, Krishnan KR. Medical comorbidity in a bipolar outpatient clinical population. Neuropsychopharmacology. 2005;30:401–404. doi: 10.1038/sj.npp.1300608. [DOI] [PubMed] [Google Scholar]
- Beyer JL, Taylor L, Gersing KR, Krishnan KR. Prevalence of HIV infection in a general psychiatric outpatient population. Psychosomatics. 2007;48:31–37. doi: 10.1176/appi.psy.48.1.31. [DOI] [PubMed] [Google Scholar]
- Brook JS, Richter L, Rubenston E. Consequences of adolescent drug use on psychiatric disorders in early adulthood. Annals of Medicine. 2000;32:401–407. doi: 10.3109/07853890008995947. [DOI] [PubMed] [Google Scholar]
- Bubier JL, Drabick DA. Co-occurring anxiety and disruptive behavior disorders: the roles of anxious symptoms, reactive aggression, and shared risk processes. Clinical Psychology Review. 2009;29:658–669. doi: 10.1016/j.cpr.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Martin CS, Cornelius JR. Adolescent-onset substance use disorders predict young adult mortality. Journal of Adolescent Health. 2008;42:637–639. doi: 10.1016/j.jadohealth.2007.11.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. Child development and personality disorder. Psychiatric Clinics of North America. 2008;31:477–493. doi: 10.1016/j.psc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Compton SN, Burns BJ, Helen LE, Robertson E. Review of the evidence base for treatment of childhood psychopathology: internalizing disorders. Journal of Consulting and Clinical Psychology. 2002;70:1240–1266. doi: 10.1037//0022-006x.70.6.1240. [DOI] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. JAMA. 2004;291:2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Costello EJ. Psychiatric predictors of adolescent and young adult drug use and abuse. Drug and Alcohol Dependence. 2007;88 Suppl 1:S1–S3. doi: 10.1016/j.drugalcdep.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Dakof GA. Understanding gender differences in adolescent drug abuse: issues of comorbidity and family functioning. Journal of Psychoactive Drugs. 2000;32:25–32. doi: 10.1080/02791072.2000.10400209. [DOI] [PubMed] [Google Scholar]
- Deas-Nesmith D, Campbell S, Brady KT. Substance use disorders in an adolescent inpatient psychiatric population. Journal of the National Medical Association. 1998;90:233–238. [PMC free article] [PubMed] [Google Scholar]
- Farmer EM, Compton SN, Bums BJ, Robertson E. Review of the evidence base for treatment of childhood psychopathology: externalizing disorders. Journal of Consulting and Clinical Psychology. 2002;70:1267–1302. doi: 10.1037//0022-006x.70.6.1267. [DOI] [PubMed] [Google Scholar]
- Garland AF, Hough RL, McCabe KM, Yeh M, Wood PA, Aarons GA. Prevalence of psychiatric disorders in youths across five sectors of care. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:409–418. doi: 10.1097/00004583-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Garland AF, Lau AS, Yeh M, McCabe KM, Hough RL, Landsverk JA. Racial and ethnic differences in utilization of mental health services among high-risk youths. American Journal of Psychiatry. 2005;162:1336–1343. doi: 10.1176/appi.ajp.162.7.1336. [DOI] [PubMed] [Google Scholar]
- Gersing KR, Krishnan KR. Practical evidence-based medicine. Psychopharmacology Bulletin. 2002;36:20–26. [PubMed] [Google Scholar]
- Gersing K, Krishnan R. Clinical computing: Clinical Management Research Information System (CRIS) Psychiatric Services. 2003;54:1199–1200. doi: 10.1176/appi.ps.54.9.1199. [DOI] [PubMed] [Google Scholar]
- Gersing K, Burchett B, March J, Ostbye T, Krishnan KR. Predicting antipsychotic use in children. Psychopharmacology Bulletin. 2007;40:116–124. [PubMed] [Google Scholar]
- Goldstein BI, Bukstein OG. Comorbid substance use disorders among youth with bipolar disorder: opportunities for early identification and prevention. Journal of Clinical Psychiatry. 2010;71:348–358. doi: 10.4088/JCP.09r05222gry. [DOI] [PubMed] [Google Scholar]
- Halamandaris PV, Anderson TR. Children and adolescents in the psychiatric emergency setting. The Psychiatric Clinics of North America. 1999;22:865–874. doi: 10.1016/s0193-953x(05)70130-1. [DOI] [PubMed] [Google Scholar]
- Hillemeier MM, Foster EM, Heinrichs B, Heier B. Conduct Problems Prevention Research Group. Racial differences in parental reports of attention-deficit/hyperactivity disorder behaviors. Journal of Developmental and Behavioral Pediatrics. 2007;28:353–361. doi: 10.1097/DBP.0b013e31811ff8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Initial national priorities for comparative effectiveness research. Washington, DC: Institute of Medicine; 2009. [Google Scholar]
- Jha AK, DesRoches CM, Kralovec PD, Joshi MS. A progress report on electronic health records in U.S. hospitals. Health Affairs (Millwood) 2010;29:1951–1957. doi: 10.1377/hlthaff.2010.0502. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman J, Schulenberg JE. Monitoring the Future national results on adolescent drug use: overview of key findings, 2010. Ann Arbor: Institute for Social Research, University of Michigan; 2011. [Google Scholar]
- Kandel DB, Johnson JG, Bird HR, Weissman MM, Goodman SH, Lahey BB, et al. Psychiatric comorbidity among adolescents with substance use disorders: findings from the MECA study. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:693–699. doi: 10.1097/00004583-199906000-00016. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Myers MG. Adolescents' participation in Alcoholics Anonymous and Narcotics Anonymous: review, implications and future directions. Journal of Psychoactive Drugs. 2007;39:259–269. doi: 10.1080/02791072.2007.10400612. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Kessler RC. The impact of childhood psychopathology interventions on subsequent substance abuse: policy implications, comments, and recommendations. Journal of Consulting and Clinical Psychology. 2002;70:1303–1306. [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. Erratum in: Archives of General Psychiatry 2005;62:768. [DOI] [PubMed] [Google Scholar]
- Kilgus MD, Pumariega AJ, Cuffe SP. Influence of race on diagnosis in adolescent psychiatric inpatients. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:67–72. doi: 10.1097/00004583-199501000-00016. [DOI] [PubMed] [Google Scholar]
- Kramer TL, Robbins JM, Phillips SD, Miller TL, Burns BJ. Detection and outcomes of substance use disorders in adolescents seeking mental health treatment. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:1318–1326. doi: 10.1097/01.chi.0000084833.67701.44. [DOI] [PubMed] [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Archives of General Psychiatry. 2011;68:555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatrica Scandinavica. Supplementum. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Hops H, Roberts RE, Seeley JR, Andrews JA. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III-R disorders in high school students. Journal of Abnormal Psychology. 1993;102:133–144. doi: 10.1037//0021-843x.102.1.133. [DOI] [PubMed] [Google Scholar]
- Libby AM, Riggs PD. Integrated substance use and mental health treatment for adolescents: aligning organizational and financial incentives. Journal of Child and Adolescent Psychopharmacology. 2005;15:826–834. doi: 10.1089/cap.2005.15.826. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Allen NB, Rogers N, Cementon E, Bonomo Y. The impact of co-occurring mood and anxiety disorders among substance-abusing youth. Journal of Affective Disorders. 2007;103(1–3):105–112. doi: 10.1016/j.jad.2007.01.011. [DOI] [PubMed] [Google Scholar]
- McGilloway A, Hall RE, Lee T, Bhui KS. A systematic review of personality disorder, race and ethnicity: prevalence, aetiology and treatment. BMC Psychiatry. 2010;10:33. doi: 10.1186/1471-244X-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroff J, Edelsohn GA, Joe S, Ford BC. The role of race in diagnostic and disposition decision making in a pediatric psychiatric emergency service. General Hospital Psychiatry. 2008;30:269–276. doi: 10.1016/j.genhosppsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najt P, Fusar-Poly P, Brambilla P. Co-occurring mental and substance abuse disorders: a review on the potential predictors and clinical outcomes. Psychiatry Research. 2011;186(2–3):159–164. doi: 10.1016/j.psychres.2010.07.042. [DOI] [PubMed] [Google Scholar]
- National Center on Addiction and Substance Abuse. Non-medical marijuana III: rite of passage or Russian roulette? New York, NY: The National Center on Addiction and Substance Abuse at Columbia University; 2008. [Google Scholar]
- O'Neil KA, Conner BT, Kendall PC. Internalizing disorders and substance use disorders in youth: comorbidity, risk, temporal order, and implications for intervention. Clinical Psychology Review. 2011;31(1):104–112. doi: 10.1016/j.cpr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- ProCon.org. [Accessed November 13, 2010];Should marijuana be a medical option? Available at: http://medicalmarijuana.procon.org/.
- Rao U, Daley SE, Hammen C. Relationship between depression and substance use disorders in adolescent women during the transition to adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:215–222. doi: 10.1097/00004583-200002000-00022. [DOI] [PubMed] [Google Scholar]
- Riggs PD. Treating adolescents for substance abuse and comorbid psychiatric disorders. Science & Practice Perspectives. 2003;2:18–29. doi: 10.1151/spp032118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, Xing Y. Comorbidity of substance use disorders and other psychiatric disorders among adolescents: evidence from an epidemiologic survey. Drug and Alcohol Dependence. 2007;88 Suppl 1:S4–S13. doi: 10.1016/j.drugalcdep.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CL, Liddle HA, Greenbaum PE, Henderson CE. Impact of psychiatric comorbidity on treatment of adolescent drug abusers. Journal of Substance Abuse Treatment. 2004;26:129–140. doi: 10.1016/S0740-5472(03)00166-1. [DOI] [PubMed] [Google Scholar]
- SAS version 9.2. Cary, NC, USA: SAS Institute; 2010. [Google Scholar]
- Shapiro GK, Buckley-Hunter L. What every adolescent needs to know: cannabis can cause psychosis. Journal of Psychosomatic Research. 2010;69:533–539. doi: 10.1016/j.jpsychores.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Shrier LA, Harris SK, Kurland M, Knight JR. Substance use problems and associated psychiatric symptoms among adolescents in primary care. Pediatrics. 2003;111(6 Pt 1):e699–e705. doi: 10.1542/peds.111.6.e699. [DOI] [PubMed] [Google Scholar]
- Stevens J, Harman JS, Kelleher KJ. Race/ethnicity and insurance status as factors associated with ADHD treatment patterns. Journal of Child and Adolescent Psychopharmacology. 2005;15:88–96. doi: 10.1089/cap.2005.15.88. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Treatment Episode Data Set (TEDS) highlights—2007 national admissions to substance abuse treatment services. OAS Series #S-45, HHS Publication No. (SMA) 09-4360. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009b. [Google Scholar]
- Waxmonsky JG, Wilens TE. Pharmacotherapy of adolescent substance use disorders: a review of the literature. Journal of Child and Adolescent Psychopharmacology. 2005;15:810–825. doi: 10.1089/cap.2005.15.810. [DOI] [PubMed] [Google Scholar]
- Weaver MF, Dupre MA, Cropsey KL, Koch JR, Sood BA, Wiley JL, Balster RL. Addiction epidemiology in adolescents receiving inpatient psychiatric treatment. Addictive Behaviors. 2007;32:3107–3113. doi: 10.1016/j.addbeh.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Pilowsky DJ, Schlenger WE. High prevalence of substance use disorders among adolescents who use marijuana and inhalants. Drug and Alcohol Dependence. 2005;78:23–32. doi: 10.1016/j.drugalcdep.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Wu LT, Ringwalt CL. Use of alcohol treatment and mental health services among adolescents with alcohol use disorders. Psychiatric Services. 2006;57:84–92. doi: 10.1176/appi.ps.57.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Woody GE, Yang C, Pan JJ, Blazer DG. Racial/ethnic variations in alcohol and drug use disorders among adolescents in the United States. Archives of General Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.120. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin H. Psychiatric comorbidity with substance misuse in children and teenagers. Drug and Alcohol Dependence. 1999;55:225–234. doi: 10.1016/s0376-8716(99)00018-6. [DOI] [PubMed] [Google Scholar]