Abstract
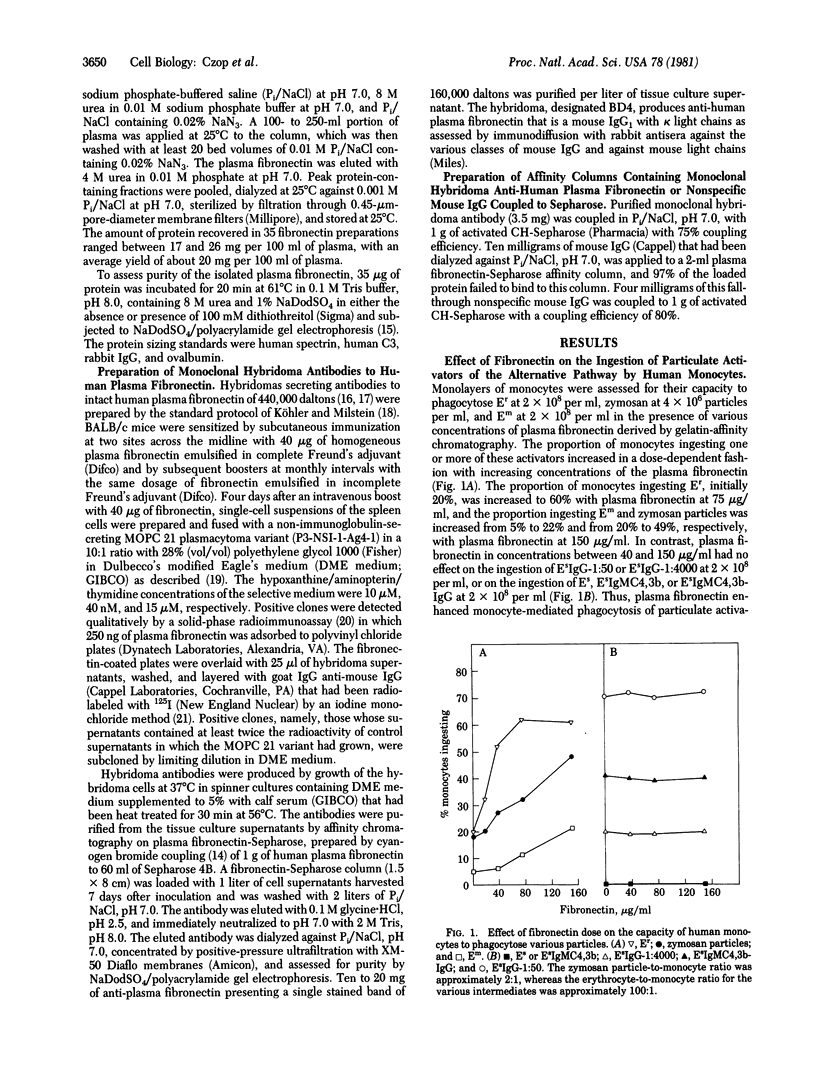
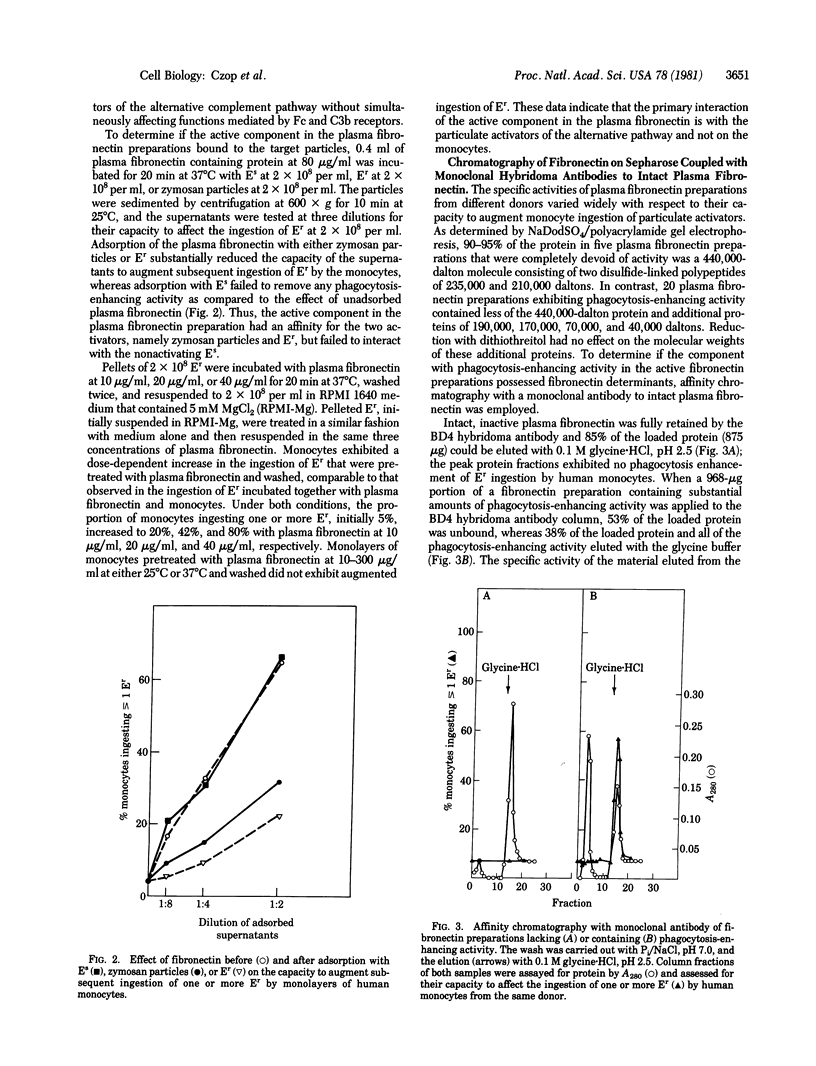
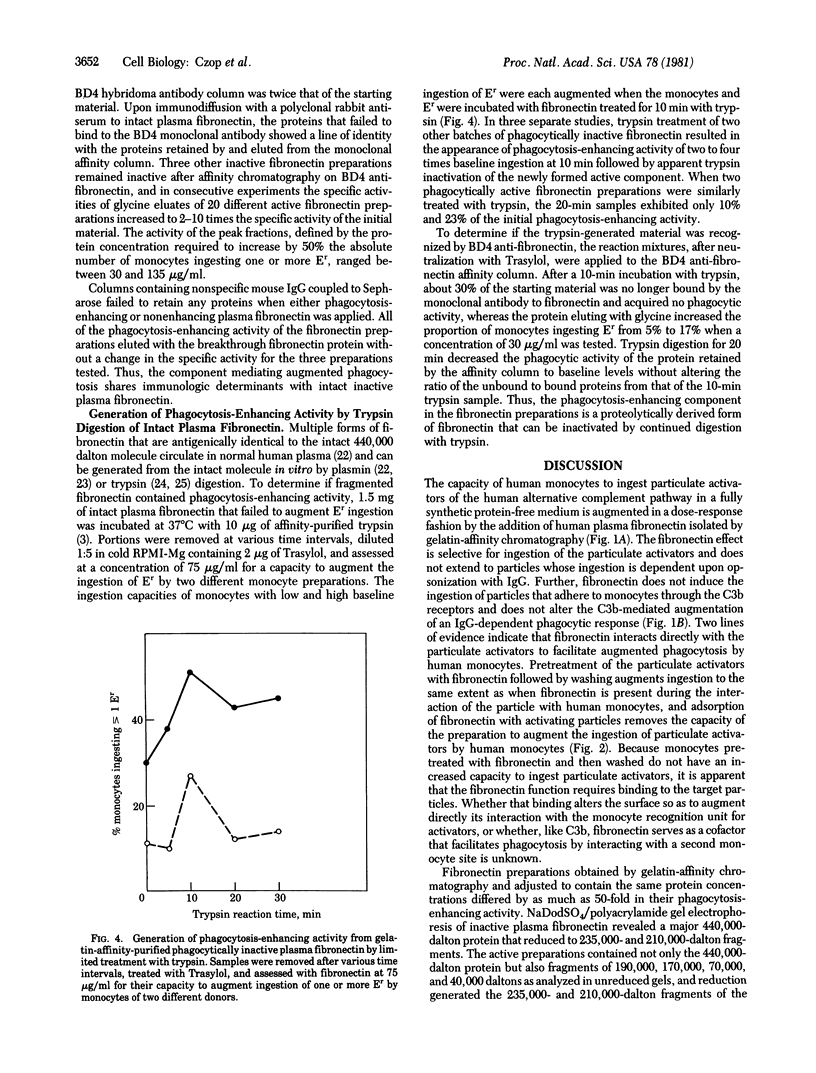
Human plasma fibronectin isolated by gelatin-affinity chromatography increases in a dose-dependent fashion the number of human monocytes that ingest particulate activators of the human alternative complement pathway in a fully synthetic medium. The fibronectin effect is selective for these particulate activators, does not extend to particles whose ingestion is dependent upon opsonization with IgG, and is not observed with pretreatment of the monocytes. Affinity chromatography with monoclonal antibody to plasma fibronectin of 440,000 daltons reveals that only 12-53% of the protein in a phagocytically active gelatin-affinity-purified fibronectin preparations is bound to the antibody. The protein eluted after affinity chromatography with monoclonal antibody of active preparations, which represented 10-43% of the protein applied, exhibits a 2- to 10-fold increment of activity per microgram of protein above the starting gelatin-affinity-purified material. Thus, the activity that augments the percent of human monocytes ingesting particulate activators of the alternative pathway is antigenically defined as plasma fibronectin. Preparations containing only intact 440,000-dalton fibronectin are also bound to and eluted from the monoclonal antibody, but they fail to augment phagocytosis. When inactive 440,000-dalton plasma fibronectin is subjected to limited trypsin cleavage, phagocytosis-enhancing activity develops that is bound to and elutes from the affinity column prepared with monoclonal antibody, thereby indicating that the enhancing activity of plasma fibronectin resides in cleavage fragments.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenstock F. A., Saba T. M., Weber P., Laffin R. Biochemical and immunological characterization of human opsonic alpha2SB glycoprotein: its identity with cold-insoluble globulin. J Biol Chem. 1978 Jun 25;253(12):4287–4291. [PubMed] [Google Scholar]
- Blumenstock F. A., Saba T. M., Weber P. Purification of alpha-2-opsonic protein from human serum and its measurement by immunoassay. J Reticuloendothel Soc. 1978 Feb;23(2):119–134. [PubMed] [Google Scholar]
- Brown C. A., Carey K., Colvin R. B. Inhibition of autoimmune tubulointerstitial nephritis in guinea pigs by heterologous antisera containing anti-idiotype antibodies. J Immunol. 1979 Nov;123(5):2102–2107. [PubMed] [Google Scholar]
- Chen A. B., Amrani D. L., Mosesson M. W. Heterogeneity of the cold-insoluble globulin of human plasma (CIg), a circulating cell surface protein. Biochim Biophys Acta. 1977 Aug 23;493(2):310–322. doi: 10.1016/0005-2795(77)90187-8. [DOI] [PubMed] [Google Scholar]
- Chen A. B., Mosesson M. W. An improved method for purification of the cold-insoluble globulin of human plasma (CLg). Anal Biochem. 1977 May 1;79(1-2):144–151. doi: 10.1016/0003-2697(77)90388-8. [DOI] [PubMed] [Google Scholar]
- Czop J. K., Austen K. F. Functional discrimination by human monocytes between their C3b receptors and their recognition units for particulate activators of the alternative complement pathway. J Immunol. 1980 Jul;125(1):124–128. [PubMed] [Google Scholar]
- Czop J. K., Fearon D. T., Austen K. F. Membrane sialic acid on target particles modulates their phagocytosis by a trypsin-sensitive mechanism on human monocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3831–3835. doi: 10.1073/pnas.75.8.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czop J. K., Fearon D. T., Austen K. F. Opsonin-independent phagocytosis of activators of the alternative complement pathway by human monocytes. J Immunol. 1978 Apr;120(4):1132–1138. [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- HELMKAMP R. W., GOODLAND R. L., BALE W. F., SPAR I. L., MUTSCHLER L. E. High specific activity iodination of gamma-globulin with iodine-131 monochloride. Cancer Res. 1960 Nov;20:1495–1500. [PubMed] [Google Scholar]
- Huber H., Fudenberg H. H. Receptor sites of human monocytes for IgG. Int Arch Allergy Appl Immunol. 1968;34(1):18–31. doi: 10.1159/000230091. [DOI] [PubMed] [Google Scholar]
- Huber H., Polley M. J., Linscott W. D., Fudenberg H. H., Müller-Eberhard H. J. Human monocytes: distinct receptor sites for the third component of complement and for immunoglobulin G. Science. 1968 Dec 13;162(3859):1281–1283. doi: 10.1126/science.162.3859.1281. [DOI] [PubMed] [Google Scholar]
- Jilek F., Hörmann H. Cold-insoluble globulin, II. Plasminolysis of cold-insoluble globulin. Hoppe Seylers Z Physiol Chem. 1977 Jan;358(1):133–136. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LoBuglio A. F., Cotran R. S., Jandl J. H. Red cells coated with immunoglobulin G: binding and sphering by mononuclear cells in man. Science. 1967 Dec 22;158(3808):1582–1585. doi: 10.1126/science.158.3808.1582. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Molnar J., Gelder F. B., Lai M. Z., Siefring G. E., Jr, Credo R. B., Lorand L. Purification of opsonically active human and rat cold-insoluble globulin (plasma fibronectin). Biochemistry. 1979 Sep 4;18(18):3909–3916. doi: 10.1021/bi00585a010. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Chen A. B., Huseby R. M. The cold-insoluble globulin of human plasma: studies of its essential structural features. Biochim Biophys Acta. 1975 Apr 29;386(2):509–524. doi: 10.1016/0005-2795(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Kuusela P., Shively J. E., Engvall E. Isolation of a tryptic fragment containing the collagen-binding site of plasma fibronectin. J Biol Chem. 1979 Jul 10;254(13):6054–6059. [PubMed] [Google Scholar]
- Saba T. M., Blumenstock F. A., Scovill W. A., Bernard H. Cryoprecipitate reversal of opsonic alpha2-surface binding glycoprotein deficiency in septic surgical and trauma patients. Science. 1978 Aug 18;201(4356):622–624. doi: 10.1126/science.675246. [DOI] [PubMed] [Google Scholar]
- Scovill W. A., Saba T. M., Blumenstock F. A., Bernard H., Powers S. R., Jr Opsonic alpha2 surface binding glycoprotein therapy during sepsis. Ann Surg. 1978 Oct;188(4):521–529. doi: 10.1097/00000658-197810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi K., Hakomori S. Functional domain structure of fibronectin. Proc Natl Acad Sci U S A. 1980 May;77(5):2661–2665. doi: 10.1073/pnas.77.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathakis N. E., Mosesson M. W. Interactions among heparin, cold-insoluble globulin, and fibrinogen in formation of the heparin-precipitable fraction of plasma. J Clin Invest. 1977 Oct;60(4):855–865. doi: 10.1172/JCI108840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



