Abstract
Prostate cancer remains the most common noncutaneous cancer among American men. Although most patients can be cured by surgery and radiotherapy, 32,050 patients still died of the disease in 2010. Many patients receive radiotherapy either as a primary therapy, salvage therapy, or in combination with surgery or hormonal therapy. Despite initial treatment, several studies suggest that approximately 10% of low-risk prostate cancer patients and up to 30-60% with more advanced cancer patients experience biochemical recurrence within five years after radiotherapy. Thus, elucidating the molecular mechanisms underlying radioresistance and tumor recurrence has the potential to significantly reduce prostate cancer mortality. We previously demonstrated that fractionated ionizing radiation (IR) can induce the prostate cancer cell line LNCaP to undergo neuroendocrine differentiation (NED) by activation of cAMP response element binding protein (CREB) and cytoplasmic sequestration of ATF2, two CRE-binding transcription factors that oppose each other to regulate NED. Importantly, IR-induced NED is reversible and de-differentiated cells are cross-resistant to IR, androgen depletion and docetaxel treatments. These findings suggest that radiation-induced NED may allow prostate cancer cells to survive treatment and contribute to tumor recurrence. In the present study, we further demonstrated that IR also induces NED in a subset of DU-145 and PC-3 cells. In addition, we confirmed that IR induces NED in LNCaP xenograft tumors in nude mice, and observed that the plasma chro-mogranin A (CgA) level, a biomarker for NED, is increased by 2- to 5-fold in tumor-bearing mice after fractionated radiation doses of 20 and 40 Gy, respectively. Consistent with these in vivo findings, a pilot study in prostate cancer patients showed that the serum CgA level is elevated in 4 out of 9 patients after radiotherapy. Taken together, these findings provide evidence that radiation-induced NED is a general therapeutic response in a subset of prostate cancer patients. Thus, a large scale analysis of radiotherapy-induced NED in prostate cancer patients and its correlation to clinical outcomes will likely provide new insight into the role of NED in prostate cancer radiotherapy and prognosis.
Keywords: Ionizing radiation, prostate cancer, neuroendocrine differentiation, ATF2, CREB, radiotherapy
Introduction
Prostate cancer is the second leading cause of cancer death in men in the US [1]. Despite progress over the last two decades, the only curative treatments for localized prostate cancer are surgery and radiotherapy (RT). Although most patients can be cured, several large scale studies suggest that 10% of patients with low-risk prostate cancer and up to 30-60% of patients with high-risk prostate cancer experience biochemical recurrence within 5 years after RT.
Among patients with recurrent disease, 20-30% die within 10 years [2-6]. Given that only 2.4% of prostate cancer patients in the US initially present with metastatic disease, the majority of prostate cancer deaths occur in patients who underwent the primary treatment of localized cancer, local recurrence, salvage therapy, and eventually distant recurrence, hormonal therapy, and death. Because RT is: 1) one of the primary treatments for low-risk localized prostate cancer, 2) a major treatment for high-risk prostate cancer when combined with hormonal therapy, 3) a major salvage therapy for local recurrence, and 4) a recommended adjuvant therapy for patients undergoing surgery [7-13], enhancing the sensitivity of prostate cancer cells to RT will likely reduce, or even prevent, tumor recurrence and impact the management of advanced prostate cancer.
The prostate gland contains three types of epithelial cells including luminal, basal and neuroendocrine cells. While luminal and basal cells are the majority of the prostatic epithelial cells, NE cells are less than 1% of total epithelial cells. Although the physiological role of NE cells remains ill-defined, increased number of NE-like cells is observed in advanced prostate cancer patients [14, 15]. NE-like cells are androgen receptor (AR) negative and they do not proliferate [16]. However, NE-like cells secrete a number of peptide hormones and growth factors to support the growth of surrounding tumor cells [17]. In addition, NE-like cells are reversible and can de-differentiate back to a proliferating state, which may contribute to tumor recurrence [18, 19]. Further, NE-like cells express high levels of Bcl-2 and are highly apoptosis resistant [7, 20, 21]. Because the quantification of NED by identifying chromogranin A (CgA) or neuron specific enolase (NSE) positive cells from prostate cancer tissues is affected by several factors such as location of sampling and tumor volume, controversial results regarding the clinical correlation of NED to disease progression have been reported [15, 17, 22-25]. To overcome this challenge, several studies measured serum bio-markers of NED and demonstrated that the serum CgA level is the best biomarker to reflect the extent of NED in prostate cancer tissues [26-28]. Importantly, an increase in the serum CgA level correlates with disease progression and the acquisition of castration-resistant prostate cancer [25, 27, 29-32], suggesting that NED may represent a novel mechanism by which prostate cancer cells survive treatment and contribute to recurrence. Thus, targeting NE-like cells has recently been proposed and developed as a treatment for prostate cancer [7, 8, 14, 17, 22, 33].
A number of stimuli, such as cAMP [18, 34-36], IL-6 [23, 36-42], androgen ablation therapy [43-48], and EGF [21], have been reported to induce prostate cancer cells to undergo NED. We recently observed that the prostate cancer LNCaP cells also underwent NED after receiving a clinically relevant dose of fractionated ionizing radiation (IR) [19]. Upon IR doses spanning four weeks, the remaining viable cells (∼20%) all differentiated into NE-like cells and expressed higher levels of CgA and NSE [19]. Furthermore, we observed that two transcription factors, cAMP response element binding protein (CREB) and activating transcription factor 2 (ATF2), oppose each other to regulate NED. Consistent with this notion, IR induced cytoplasmic sequestration of ATF2 and increased phosphorylation of nuclear CREB. In the present study, we extend these findings to two other prostate cancer cell lines, and provide evidence that radiation also induces NED in LNCaP xenograft tumors in nude mice and in human prostate cancer patients.
Materials and methods
Cell culture and analysis of NED
Cell culture and NED analysis were exactly the same as previously reported [19] except that LNCaP cells were cultured in RPMI1640, DU-145 in MEM, and PC-3 cells in F-12K media. The irradiation protocol (2 Gy/day, 5 days/ week), VP16-bCREB, and an ATF2 short-hairpin RNA (shRNA) plasmid used in the present work to determine the effect of IR, VP16-bCREB and ATF2 knockdown on NED in DU-145 and PC-3 cells were also described before [19]. Likewise, immunofluorescence and subcellular fractionation methods were similarly used to determine the effect of IR on the phosphorylation of CREB and subcellular localization of ATF2.
IR-induced NED in xenograft tumors in nude mice
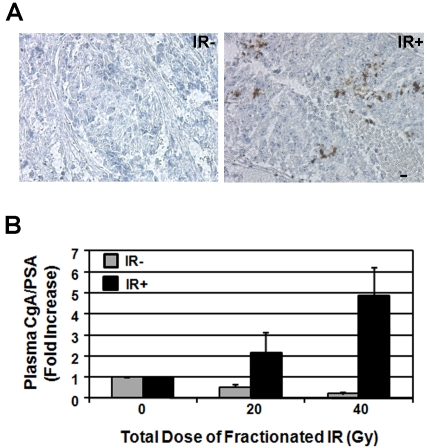
The LNCaP human prostate cancer cells were implanted subcutaneously by injecting 5×106 cells 1:1 in Matrigel into the thighs of 6-week old male athymic nude mice (BALB/c strain). Implanted tumors were allowed to grow to a volume of 300-500 mm3, prior to irradiation. All tumors were irradiated to a total dose of 40 Gy given in 8 twice weekly fractions of 5 Gy using 6 -MV x-rays from a clinical linear accelerator in the Linda and William Fleischhauer Radiation Therapy Facility at the Purdue University School of Veterinary Medicine. Tumors were treated using parallel opposed beams with dose computed manually. Tumor volumes were measured twice a week or during blood sample collections. For immunohistochemical (IHC) analysis of NED in xenograft tumors, the mice were sacrificed one day after the last treatment (40 Gy total dose), and the resected tumors were fixed in formalin and embedded in paraffin. Tissue slides were prepared at 5 μm thickness, and CgA was stained by the anti-CgA antibody (Abcam). To determine the effect of x-ray irradiation on plasma CgA and PSA levels, veinous blood samples were drawn prior to treatment (0 Gy), after 2 weeks (20 Gy) and after 4 weeks (40 Gy). The plasma CgA and PSA were measured using the CgA EIA kit (Cosmo Bio) and the PSA ELISA Kit (Calbiotech) according to the manufacturer's instructions. Plasma CgA levels were normalized to plasma PSA levels, and fold change compared with the 0 Gy time point was determined. For control mice, the 0 Gy time point was designated when xenograft tumors reached 300-500 mm3, and blood was drawn for pre-treatment time point. Blood was collected again after 2 weeks and 4 weeks, corresponding with samples collected after 20 Gy and 40 Gy in the irradiated groups. All animal experiments were approved by the Purdue Animal Care and Use Committee (PACUC No. 08-127), and all animal use followed the Assurance of Compliance with Public Health Services Policy on Human Care and Use of Laboratory Animals (Welfare Assurance #A3231-01).
Serum CgA and PSA measurement in human prostate cancer patients
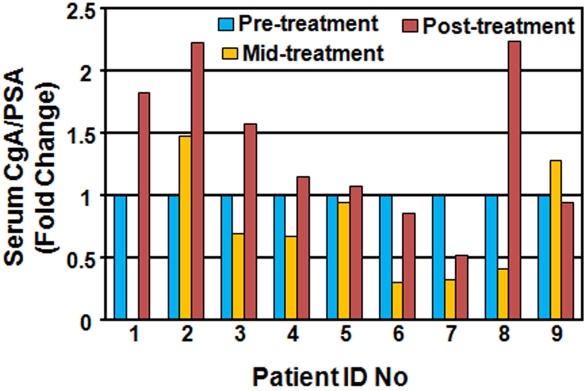
Nine patients diagnosed with localized prostate cancer (six T1c and one of each pT2b, pT2c, and pT3a) were enrolled at Indiana University School of Medicine. All patients signed the consent form and agreed to participate in the pilot study according to the approved Institutional Review Board protocol (0805-43). The average age of patients was 54.6 years old. Five patients were Gleason score 7, one patient was Gleason score 9, and three patients were Gleason score 6. All patients were treated at either the Indiana University Hospital or the Midwest Proton Radiotherapy Institute with the total dose of 70.2-79.2 Gy delivered (2 Gy/day). To determine the effect of RT on serum CgA levels, three blood samples were drawn before the start of RT treatment, in the middle of the treatment (week 4-5), and after the treatment (end of week 7 or 8), designated pre-treatment, midtreatment and post-treatment, respectively. Serum CgA and PSA levels were measured using the CgA EIA kit (Cosmo Bio) and the PSA ELISA Kit (Calbiotech) according to the manufacturer's instructions. Because some prostate cancer patients maintain a high level of serum CgA, which is likely determined by the number of preexisting NE-like cells and cancer cells that secrete CgA, serum CgA levels were normalized to serum PSA levels for the calculation of fold change.
Results
IR induces morphological changes and expression of NED markers to various extents in prostate cancer cells
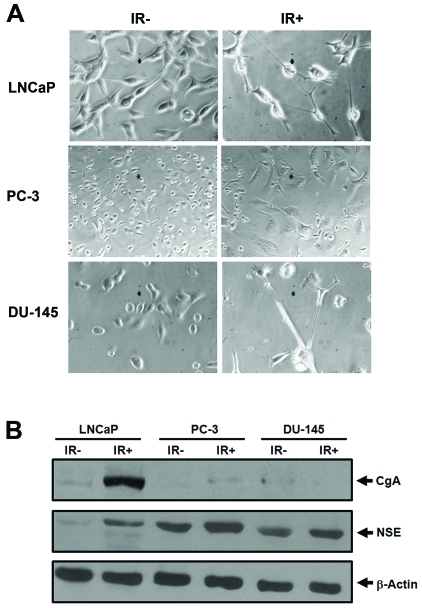
To determine whether our findings with LNCaP cells can be extended to other prostate cancer cells, we performed similar experiments on DU-145 and PC-3 cells as we did with LNCaP cells [19]. In LNCaP cells, cell bodies became smaller and the majority of cells were connected via longer neurites (Figure 1A) [19]. In contrast, enlarged and flat cell bodies were observed for both DU-145 and PC-3 cells. Unlike LNCaP cells in which almost all surviving cells showed extended neurites, only a subset of irradiated DU-145 cells (32%) showed neurite outgrowth whereas non-irradiated DU-145 cells did not show any neurite outgrowth (Figure 1A). Interestingly, approximately 6% of non-irradiated PC-3 cells already displayed neurite outgrowth whereas IR increased the number of cells with neurite outgrowth to 35%. Consistent with the morphological changes, NSE was also induced in DU-145 and PC-3, albeit to a lesser extent (Figure 1B). However, no significant induction of CgA in DU-145 and PC-3 was observed when compared with LNCaP cells. While these results confirm that IR can induce DU-145 and PC-3 cells to differentiate into NE-like cells, they also suggest that a subset of DU-145 and PC-3 cells is refractory to IR. This is also consistent with the differential responses of prostate cancer cell lines to androgen depletion, IL-6, cAMP and EGF treatments [21, 23].
Figure 1.
IR induces NED in prostate cancer cells. A). Representative images acquired from the indicated prostate cancer cells that were treated with 40 Gy of fractionated IR (IR+) or without IR treatment (IR-) (microscopy at 200x for LNCaP and 100x for PC-3 and DU-145). Note that enlarged and flat cell bodies were observed for irradiated PC-3 when compared with non-irradiated PC-3. B). Approximately 40 μg of total lysate from non-irradiated (IR-) and irradiated (40 Gy, IR+) cells was used for immunoblot analysis of CgA, NSE and β-actin. Similar results were reproduced from at least three independent experiments.
Effect of IR on CREB activation and ATF2 subcellar localization
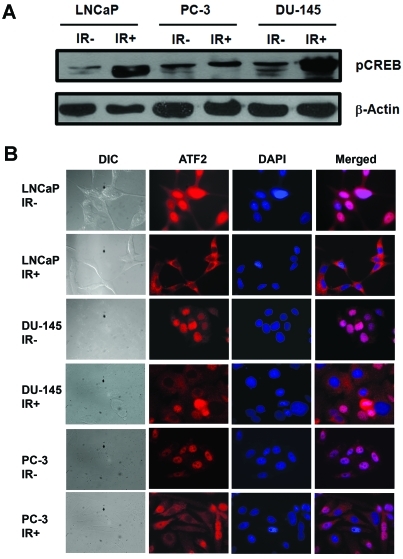
In LNCaP cells, we observed that IR-induced NED is associated with increased nuclear phospho-CREB and cytoplasmic-localized ATF2. To know whether IR also activates CREB and induces cytoplasmic localization of ATF2 in these cell lines, we performed immunofluorescence analysis and subcellular fraction. We observed that CREB was highly phosphorylated in irradiated DU-145, but to a lesser extent in PC-3 (Figure 2A). Interestingly, increased cytoplasmic localization of ATF2 was observed in both DU-145 and PC-3 cells (Figure 2B). However, only a subset of cells (∼50%) showed cytoplasmic localization of ATF2 in these two cell lines, whereas increased cytoplasmic localization was observed in almost all irradiated LNCaP cells. Consistent with immunofluorescence analysis, subcellular fractionation also showed only a slight increase of ATF2 in the cytosolic fraction in irradiated DU-145 and PC-3 cells, which is likely due to increased cytoplasmic localization of ATF2 in a subset of cells (data not shown). Thus, we conclude that IR can similarly induce CREB activation and impair ATF2 nuclear localization in a subset of DU-145 and PC-3 cells.
Figure 2.
IR induces CREB activation and cytoplasmic sequestration of ATF2 in prostate cancer cells. A). A representative im-munoblot analysis of phosphorylated CREB (pCREB) from non-irradiated cells (IR-) or from cells that received 10 Gy of fractionated IR (IR+). B). Shown are DIC and fluorescent images for ATF2 and DNA (DAPI) acquired from the indicated non-irradiated prostate cancer cells (IR-) or from cells that received 10 Gy of fractionated IR (IR+) (microscopy at 600x). These experiments were reproduced at least three times and similar results were obtained.
Overexpression of VP16-bCREB or ATF2 knockdown induces NED in DU-145 and PC-3 cells
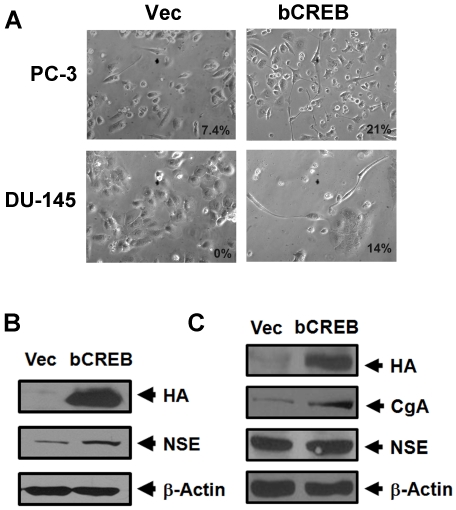
The above results suggest that DU-145 and PC-3 cell may have intrinsic defects in activating CREB or sequestering ATF2 in the cytoplasm in some cells. To know whether these cells can still be induced by VP16-bCREB, a constitutively activated CREB, we performed similar experiments in these two cell lines as we did in LNCaP cells [19]. We observed that overexpression of VP16-bCREB also induced neurite extension in a subset of DU-145 (14%) and PC-3 cells (21%) (Figure 3A). Consistent with the morphological changes, a slight induction of both CgA and NSE was observed in DU-145 transfected with the VP16-bCREB plasmid (Figure 3C). However, only a slight induction of NSE, but not CgA, was observed in PC-3 cells transfected with the VP16-CREB plasmid (Figure 3B). Because the transfection efficiency is relatively low in these two cell lines, these results suggest that expression of VP16-bCREB also induced NED in a subset of DU-145 and PC-3 cells.
Figure 3.
Activated CREB induces neurite outgrowth and the expression of CgA and NSE in PC-3 and DU-145 cells. A). Prostate cancer cells PC-3 and DU-145 were transfected with a pHA-CMV plasmid encoding a constitutively activated CREB, VP16-bCREB (bCREB), or the pHA-CMV empty vector (Vec). Shown are phase contrast images acquired five days after the transfection (microscopy at 200x). The number indicates the percentage of cells showing neurite outgrowth. B) and C). Expression of HA-VP16-bCREB (HA), CgA, NSE and β-actin in PC-3 cells (B) or DU-145 cells (C) from the experiments in A. Note that CgA was not detectable in PC-3 cells transfected with the vector control pHA-CMV or pHA-VP16-bCREB.
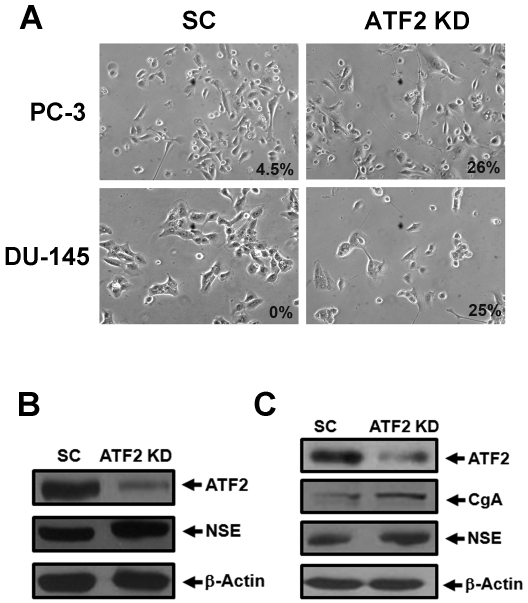
Similar results were obtained when ATF2 was knocked down in both DU-145 and PC-3 cells (Figure 4). While no extended neurites were observed in DU-145 cells transfected with scrambled control, approximately 25% of DU-145 transfected with the ATF2 shRNA plasmid for five days showed neurite outgrowth. Similarly, 26% of PC-3 cells transfected with the ATF2 shRNA plasmid for five days showed neurite outgrowth whereas 4.5% of cells transfected with the scrambled control plasmid showed neurite outgrowth.
Figure 4.
ATF2 knockdown induces neurite outgrowth and the expression of CgA and NSE in prostate cancer cells. A). Prostate cancer cells PC-3 and DU-145 were transfected with the ATF2 shRNA plasmid (ATF2 KD) or the scrambled control (SC). Shown are phase contrast images acquired five days after the transfection (Microscopy at 200x). The number indicates the percentage of cells showing neurite outgrowth. B) and C). Expression of ATF2, CgA, NSE and β-actin in PC-3 cells (B) or DU-145 cells (C) from the experiments in A. Note that CgA was not detectable in PC-3 cells transfected with either SC or ATF2 shRNA plasmids.
Radiation induces NED in LNCaP xenograft tumors in nude mice
To determine whether IR can induce NED in vivo, we employed nude mouse xenograft models. For this purpose, we used LNCaP cells as they can be better induced by IR to undergo NED. We performed x-ray irradiation to xenograft tumors at 10 Gy/week (5 Gy/fraction). Our preliminary results with this irradiation protocol also induced NED in vitro (unpublished observation). At the end of four weeks, mice were sacrificed and residual tumor nodules were resected for IHC analysis of CgA expression. Compared to non-irradiated tumors (n=3), some cells in all irradiated tumors (n=10) showed higher expression of CgA, suggesting that radiation indeed induces NED in xenograft tumors (Figure 5A).
Figure 5.
Ionizing radiation induces CgA expression in LNCaP xenograft tumors and an increase of plasma CgA levels in nude mice. A). IHC analysis of CgA expression in irradiated LNCaP xenograft tumors after 40 Gy (IR+) or in non-irradiated xenograft tumors (IR-) (microscopy at 400x). Scale bar represents 10 μm. B). Average fold change of plasma CgA levels normalized to plasma PSA at the end of week 2 (20 Gy) and week 4 (40 Gy) when compared with pre-irradiation (0 Gy). Similar time points were followed for blood collection from non-irradiated tumor-bearing mice. The average fold change presented is from all 10 mice for each group.
Because serum/plasma CgA levels can be used to quantify the extent of NED in prostate cancer tissues in human patients, we next performed similar fractionated IR to xenograft tumors and measured the plasma CgA level. We collected blood samples from tumor-bearing mice (n=10) before irradiation, and at 2 and 4 weeks of irradiation. As controls blood samples from non-irradiated tumor-bearing mice (n=10) were also collected at corresponding time points (equivalent to 0, 20 and 40 Gy). Higher plasma CgA levels were observed in all mice bearing large tumors regardless of irradiation, likely due to the increased number of LNCaP cells that express basal levels of CgA. Since LNCaP cells secrete PSA, we normalized plasma CgA levels to plasma PSA levels to control for differing amounts of cells present in each tumor. Three out of 10 mice showed elevated plasma CgA levels after 20 Gy of irradiation, and 7 mice showed elevated plasma CgA levels after 40 Gy of irradiation. In contrast, none of the non-irradiated tumor-bearing mice showed any elevation of plasma CgA levels at the corresponding time points. Instead, their normalized CgA levels were lower after 2-4 weeks of observation. Because these non-irradiated xenograft tumors continued to grow and reached 1300 mm3 to 2300 mm3 at the end of the corresponding 4-week time point, the lower normalized CgA levels in non-irradiated mice are likely due to increased PSA production by LNCaP cells under hypoxic conditions in these large tumors [49]. When plasma CgA levels in all 10 irradiated mice were considered, the average plasma CgA levels increased by 2- and 5-fold at the end of 2- and 4-week irradiation, respectively, whereas the average plasma CgA levels for the control group decreased by 2-4 fold at the end of 2- to 4- weeks' observation, respectively (Figure 5B). Thus, we conclude that x-ray irradiation can induce NED in xenograft tumors.
Prostate cancer patients show elevated levels of serum CgA after radiotherapy
Because serum CgA has been used as a biomarker to monitor hormonal therapy-induced NED in prostate cancer patients [25, 27, 29-32], the above observations that x-ray irradiation to xenograft tumors increased plasma CgA levels in nude mice prompted us to test if RT also induces serum CgA elevation in human prostate cancer patients. To this end, we collected blood samples before RT, in the middle of RT, and immediately after RT from prostate cancer patients enrolled at Indiana University School of Medicine, and measured serum CgA and PSA levels. Except for one patient from whom we missed the collection of his blood sample at the middle time point, we collected blood samples at all three time points from the other 8 patients. Among these 8 patients, 2 showed an increase in the serum CgA level at the middle of the RT treatment, and interestingly, 6 patients showed a decrease in the serum CgA level. However, the CgA level at the end of the RT treatment in these 6 patients rebounded to the pre-treatment level or higher (Figure 6). When compared with the pre-treatment CgA level, 4 out of 9 patients showed 1.5-2.2 fold increase in serum CgA levels, 2 were unchanged, and 2 had a slight decrease (less than 2 fold) after RT treatment. Thus, approximately 44% (4 out of 9) of patients showed serum CgA elevation after RT.
Figure 6.
Radiotherapy increases serum CgA levels in prostate cancer patients. All 9 prostate cancer patients were diagnosed with localized tumors and treated at the Indiana University Hospital or the Midwest Proton Radiotherapy Institute with 70.2-79.2 Gy (2 Gy/fraction). Blood samples were collected for pre-treatment, mid-treatment, and post-treatment. The serum CgA levels were normalized to the serum PSA levels, and the fold change at mid- and post-treatment time points is presented for each patient.
Discussion
Based on our recent findings that IR can induce NED in LNCaP cells, we provide evidence here that IR also induces NED in DU-145 and PC-3 cells, albeit to a lesser extent. Consistent with this, IR treatment induced cytoplasmic localization of ATF2 and CREB phosphorylation in a subset of cells. Likewise, expression of a constitutively activated CREB or ATF2 knockdown also induced the expression of NSE and/or CgA, and neurite extension in these two cell lines. Thus, it is likely that radiation-induced NED is a general phenomenon. Furthermore, we showed that IR also induced NED in LNCaP xenograft tumors in nude mice and that RT also induced elevation of serum CgA levels in 4 out of 9 prostate cancer patients. Our findings here together suggest that radiation-induced NED may represent a therapeutic response in a subset of prostate cancer patients undergoing radiotherapy.
Difference between LNCaP, DU-145 and PC-3 cells
The LNCaP cell line was established from a local metastasized lymph node whereas DU-145 and PC-3 were established from metastasized tumors in brain and bone, respectively [50]. Although the treatment history of these patients is not clear, it is possible that DU1-45 and PC-3 cells were established after extensive exposures to treatments. In addition, DU-145 and PC-3 cells do not express detectable levels of AR and other genetic, or possibly epigenetic, changes may be involved. These intrinsic differences may be responsible for the differential induction of NED by other stimuli as well [23, 34]. Consistent with these observations, three clones isolated from regrowing cells after IR-induced NED are poorly responsive to IR and ADT [19]. Interestingly, we observed that CREB activation and ATF2 cytoplasmic sequestration only occurred in a subset of DU-145 and PC-3 cells after fractionated IR whereas almost all LNCaP cells after 10 Gy showed increased pCREB in the nucleus and an increase of cytoplasmic localized ATF2. These observations suggest that while DU-145 and PC-3 cells do contain a subset of cells that are inducible by IR to undergo NED, there are also some cells that are refractory to NED. Further analysis of these intrinsic differences among these three cell lines may shed new light on the molecular mechanisms underlying IR-induced NED.
CgA as a biomarker to monitor RT-induced NED
IHC staining of CgA and NSE has been widely used to identify NE-like cells in prostate cancer tissues. Because of the difficulty in quantifying NED using the IHC method, controversial results have been reported [15, 17, 22-25]. To resolve these controversies, several groups examined serum NED biomarkers and demonstrated that serum CgA is the best biomarker that can reflect NED in tissues [26-28]. In our xenograft nude mouse model, we not only observed increased numbers of tumor cells expressing higher levels of CgA in irradiated xenograft tumors, but we also observed an increase in the plasma CgA level in a dose-dependent manner in the majority of mice bearing irradiated xenograft tumors. In contrast, no increase in plasma CgA levels was observed in any of the non-irradiated tumor-bearing mice. Our results suggest that plasma or serum CgA levels can be used to monitor NED during treatment. In fact, results from a preliminary test with 9 prostate cancer patients suggest that RT increases serum CgA levels in 4 out of 9 patients after RT. It is worth noting that a previous study measured serum CgA levels in 100 prostate cancer patients before RT and three months after RT, and observed that 10 patients also showed elevated serum CgA levels three months after RT [51]. Since the number of prostate cancer cells has an impact on serum CgA levels, it is possible that many patients who underwent NED during treatment may eventually show lower CgA levels due to the decrease in tumor cells. Because prostate cancer patients also often have preexisting NE-like cells, it is therefore important to monitor patient's responses during RT by measuring serum CgA at multiple time points and compare with the serum CgA level before treatment. Indeed, we observed that 6 patients showed an initial decrease in serum CgA levels by the middle of RT, but rebound to levels comparable to or higher than before RT. Because tumors start to shrink once treatment begins, an initial decrease in serum CgA levels and subsequent rebound after completion of the treatment may provide an interesting pattern to monitor RT-induced NED. Though one limitation of this pilot study is the small sample size, the preliminary finding warrants a detailed analysis of RT-induced NED and its correlation to clinical outcomes.
Can targeting NED be explored as a novel radio-sensitization approach?
The extent of pre-existing NE-like cells and hormonal therapy-induced NED appear to contribute to disease progression and poor prognosis [25, 27, 29-32]. It is therefore proposed that targeting NED can be explored as a novel therapeutic approach [7, 8, 14, 17, 22, 33]. We have observed that CREB activation can be induced by radiation doses as low as 10 Gy in prostate cancer cells. In the case of LNCaP cells, approximately 80% of cells are killed by IR during the second week, after a total dose of 20 Gy, and the remaining 20% of cells surviving the treatment undergo NED by the end of 4 weeks after a total dose of 40 Gy [19]. After that, no cell death occurs after total doses of up to 72 Gy. These observations suggest an interesting model that radiation-induced NED likely includes at least two important phases. The first phase is the selection and enrichment of radio-resistant cells during the first two weeks, and the second phase is the NED phase during the second two weeks. Since increased CREB phoshphorylation was observed in a dose-dependent manner during the course of treatment, it is likely that CREB activation is not only involved in radioresistance but also involved in IR-induced NED. Thus, targeting CREB signaling, in principle, may sensitize prostate cancer cells to IR. In fact, targeting of CREB upstream signaling molecules such as PKA and CaMKII in prostate cancer cells can induce cell death or sensitize cells to RT or ADT [52-57]. Because CREB can be phosphorylated and activated by more than 15 different protein kinases such as MAPKs, AKT, PKA, CaMKII, ATM [58] and because many of these protein kinases can be activated by IR [59, 60], future identification of upstream protein kinases involved in radiation-induced CREB activation and NED may enable development of effective radiosensitizers for prostate cancer treatment.
Acknowledgments
We appreciate the input from the Hu lab, and we also thank Drs. Sarah Parson, Evan Keller, Jiaoti Huang, Liang Cheng, Peter Johnstone, and Michael Koch for the support and consultation during the course of this work. Thanks to Sandra Torregrosa-Allen in the Molecular Discovery and Evaluation Shared Resource for the establishment of xenograft tumors, blood collections, and animal care. Thanks to Caroline Bolyard and Jill Huenemann in the Purdue Veterinary Teaching Hospital for technical assistance with mouse irradiation. This work was supported, in part, by grants from the U.S. Army Medical Research Acquisition Activity, Prostate Cancer Research Program grant PC073098, Purdue University Center for Cancer Research Small Grants Program, and the Indiana Clinical and Translational Science Institute funded, in part, by RR025761 from the National Institutes of Health, National Center for Research Resources, Clinical and Translational Sciences Award. DNA sequencing and animal experiments were conducted in the Purdue University Center for Cancer Research Genomic Core Facility and the Molecular Discovery and Evaluation Shared Resource supported by NCI CCSG CA23168 to Purdue University Center for Cancer Research.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Kuban DA, Thames HD, Levy LB, Horwitz EM, Kupelian PA, Martinez AA, Michalski JM, Pisansky TM, Sandler HM, Shipley WU, Zelefsky MJ, Zietman AL. Long-term multi-institutional analysis of stage T1-T2 prostate cancer treated with radiotherapy in the PSA era. Int J Radiat Oncol Biol Phys. 2003;57:915–28. doi: 10.1016/s0360-3016(03)00632-1. [DOI] [PubMed] [Google Scholar]
- 3.Zietman AL, DeSilvio ML, Slater JD, Rossi CJ, Jr, Miller DW, Adams JA, Shipley WU. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294:1233–9. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 4.D'Amico AV, Chen MH, Renshaw AA, Loffredo B, Kantoff PW. Risk of prostate cancer recurrence in men treated with radiation alone or in conjunction with combined or less than combined androgen suppression therapy. J Clin Oncol. 2008;26:2979–83. doi: 10.1200/JCO.2007.15.9699. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR. Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer. 2008;112:307–14. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 6.Kimura M, Mouraviev V, Tsivian M, Mayes JM, Satoh T, Polascik TJ. Current salvage methods for recurrent prostate cancer after failure of primary radiotherapy. BJU Int. 2009;105:191–201. doi: 10.1111/j.1464-410X.2009.08715.x. [DOI] [PubMed] [Google Scholar]
- 7.Raldow A, Hamstra DA, Kim SN, Yu JB. Adjuvant radiotherapy after radical prostatectomy: evidence and analysis. Cancer Treat Rev. 2011;37:89–96. doi: 10.1016/j.ctrv.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal SA, Sandler HM. Treatment strategies for high-risk locally advanced prostate cancer. Nat Rev Urol. 2010;7:31–8. doi: 10.1038/nrurol.2009.237. [DOI] [PubMed] [Google Scholar]
- 9.Choe KS, Liauw SL. Radiotherapeutic strategies in the management of low-risk prostate cancer. ScientificWorldJournal. 2010;10:1854–69. doi: 10.1100/tsw.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooperberg MR, Broering JM, Litwin MS, Lubeck DP, Mehta SS, Henning JM, Carroll PR. The contemporary management of prostate cancer in the United States: lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. J Urol. 2004;171:1393–401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 11.Trock BJ, Han M, Freedland SJ, Humphreys EB, DeWeese TL, Partin AW, Walsh PC. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–9. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chin JL. Post-radical prostatectomy management options for the positive surgical margin: argument for adjuvant radiotherapy. Urol Oncol. 2009;27:87–8. doi: 10.1016/j.urolonc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Billiet I, Torecilla JL, Pfeffer R, Cutajar CL, Van der Kwast T, Collette L. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–73. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 14.Daneshmand S, Quek ML, Pinski J. Neuroendocrine differentiation in prostate cancer. Cancer Therapy. 2005;3:383–396. [Google Scholar]
- 15.Nelson EC, Cambio AJ, Yang JC, Ok JH, Lara PN, Jr, Evans CP. Clinical implications of neuroendocrine differentiation in prostate cancer. Prostate Cancer Prostatic Dis. 2007;10:6–14. doi: 10.1038/sj.pcan.4500922. [DOI] [PubMed] [Google Scholar]
- 16.Bonkhoff H. Neuroendocrine differentiation in human prostate cancer. Morphogenesis, proliferation and androgen receptor status. Ann Oncol. 2001;12(Suppl 2):S141–4. doi: 10.1093/annonc/12.suppl_2.s141. [DOI] [PubMed] [Google Scholar]
- 17.Amorino GP, Parsons SJ. Neuroendocrine cells in prostate cancer. Crit Rev Eukaryot Gene Expr. 2004;14:287–300. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.40. [DOI] [PubMed] [Google Scholar]
- 18.Cox ME, Deeble PD, Lakhani S, Parsons SJ. Acquisition of neuroendocrine characteristics by prostate tumor cells is reversible: implications for prostate cancer progression. Cancer Res. 1999;59:3821–30. [PubMed] [Google Scholar]
- 19.Deng X, Liu H, Huang J, Cheng L, Keller ET, Parsons SJ, Hu CD. Ionizing radiation induces prostate cancer neuroendocrine differentiation through interplay of CREB and ATF2: Implications for disease progression. Cancer Res. 2008;68:9663–9670. doi: 10.1158/0008-5472.CAN-08-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JC. Finding primary targets of transcriptional regulators. Cell Cycle. 2005;4:356–8. doi: 10.4161/cc.4.3.1521. [DOI] [PubMed] [Google Scholar]
- 21.Humez S, Monet M, Legrand G, Lepage G, Delcourt P, Prevarskaya N. Epidermal growth factor-induced neuroendocrine differentiation and apoptotic resistance of androgenindependent human prostate cancer cells. Endocr Relat Cancer. 2006;13:181–95. doi: 10.1677/erc.1.01079. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Wu C, di Sant'Agnese PA, Yao JL, Cheng L, Na Y. Function and molecular mechanisms of neuroendocrine cells in prostate cancer. Anal Quant Cytol Histol. 2007;29:128–38. [PubMed] [Google Scholar]
- 23.Zelivianski S, Verni M, Moore C, Kondrikov D, Taylor R, Lin MF. Multipathways for transdifferentiation of human prostate cancer cells into neuroendocrine-like phenotype. Biochim Biophys Acta. 2001;1539:28–43. doi: 10.1016/s0167-4889(01)00087-8. [DOI] [PubMed] [Google Scholar]
- 24.di Sant'Agnese PA. Neuroendocrine differentiation in prostatic carcinoma: an update on recent developments. Ann Oncol. 2001;12(Suppl 2):S135–40. [PubMed] [Google Scholar]
- 25.Komiya A, Suzuki H, Imamoto T, Kamiya N, Nihei N, Naya Y, Ichikawa T, Fuse H. Neuroendocrine differentiation in the progression of prostate cancer. Int J Urol. 2009;16:37–44. doi: 10.1111/j.1442-2042.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- 26.Kamiya N, Suzuki H, Kawamura K, Imamoto T, Naya Y, Tochigi N, Kakuta Y, Yamaguchi K, Ishikura H, Ichikawa T. Neuroendocrine differentiation in stage D2 prostate cancers. Int J Urol. 2008;15:423–8. doi: 10.1111/j.1442-2042.2008.02015.x. [DOI] [PubMed] [Google Scholar]
- 27.Berruti A, Mosca A, Porpiglia F, Bollito E, Tucci M, Vana F, Cracco C, Torta M, Russo L, Cappia S, Saini A, Angeli A, Papotti M, Scarpa RM, Dogliotti L. Chromogranin A expression in patients with hormone naive prostate cancer predicts the development of hormone refractory disease. J Urol. 2007;178:838–43. doi: 10.1016/j.juro.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Angelsen A, Syversen U, Haugen OA, Stridsberg M, Mjolnerod OK, Waldum HL. Neuroendocrine differentiation in carcinomas of the prostate: do neuroendocrine serum markers reflect immunohistochemical findings? Prostate. 1997;30:1–6. doi: 10.1002/(sici)1097-0045(19970101)30:1<1::aid-pros1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Berruti A, Dogliotti L, Mosca A, Bellina M, Mari M, Torta M, Tarabuzzi R, Bollito E, Fontana D, Angeli A. Circulating neuroendocrine markers in patients with prostate carcinoma. Cancer. 2000;88:2590–7. doi: 10.1002/1097-0142(20000601)88:11<2590::aid-cncr23>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Taplin ME, George DJ, Halabi S, Sanford B, Febbo PG, Hennessy KT, Mihos CG, Vogelzang NJ, Small EJ, Kantoff PW. Prognostic significance of plasma chromogranin a levels in patients with hormone-refractory prostate cancer treated in Cancer and Leukemia Group B 9480 study. Urology. 2005;66:386–91. doi: 10.1016/j.urology.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 31.Sciarra A, Monti S, Gentile V, Mariotti G, Cardi A, Voria G, Lucera R, Di Silverio F. Variation in chromogranin A serum levels during intermittent versus continuous androgen deprivation therapy for prostate adenocarcinoma. Prostate. 2003;55:168–79. doi: 10.1002/pros.10222. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki T, Komiya A, Suzuki H, Shimbo M, Ueda T, Akakura K, Ichikawa T. Changes in chromogranin a serum levels during endocrine therapy in metastatic prostate cancer patients. Eur Urol. 2005;48:224–9. doi: 10.1016/j.eururo.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Vashchenko N, Abrahamsson PA. Neuroendocrine differentiation in prostate cancer: implications for new treatment modalities. Eur Urol. 2005;47:147–55. doi: 10.1016/j.eururo.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Bang YJ, Pirnia F, Fang WG, Kang WK, Sartor O, Whitesell L, Ha MJ, Tsokos M, Sheahan MD, Nguyen P, et al. Terminal neuroendocrine differentiation of human prostate carcinoma cells in response to increased intracellular cyclic AMP. Proc Natl Acad Sci U S A. 1994;91:5330–4. doi: 10.1073/pnas.91.12.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farini D, Puglianiello A, Mammi C, Siracusa G, Moretti C. Dual effect of pituitary adenylate cyclase activating polypeptide on prostate tumor LNCaP cells: short- and long-term exposure affect proliferation and neuroendocrine differentiation. Endocrinology. 2003;144:1631–43. doi: 10.1210/en.2002-221009. [DOI] [PubMed] [Google Scholar]
- 36.Deeble PD, Murphy DJ, Parsons SJ, Cox ME. Interleukin-6- and cyclic AMP-mediated signaling potentiates neuroendocrine differentiation of LNCaP prostate tumor cells. Mol Cell Biol. 2001;21:8471–82. doi: 10.1128/MCB.21.24.8471-8482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SO, Chun JY, Nadiminty N, Lou W, Gao AC. Interleukin-6 undergoes transition from growth inhibitor associated with neuroendocrine differentiation to stimulator accompanied by androgen receptor activation during LNCaP prostate cancer cell progression. Prostate. 2007;67:764–73. doi: 10.1002/pros.20553. [DOI] [PubMed] [Google Scholar]
- 38.Qiu Y, Robinson D, Pretlow TG, Kung HJ. Etk/Bmx, a tyrosine kinase with a pleckstrinhomology domain, is an effector of phosphatidylinositol 3'-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci U S A. 1998;95:3644–9. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiotto MT, Chung TD. STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate. 2000;42:186–95. doi: 10.1002/(sici)1097-0045(20000215)42:3<186::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q, Horiatis D, Pinski J. Inhibitory effect of IL-6-induced neuroendocrine cells on prostate cancer cell proliferation. Prostate. 2004;61:253–9. doi: 10.1002/pros.20106. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Horiatis D, Pinski J. Interleukin-6 inhibits the growth of prostate cancer xenografts in mice by the process of neuroendocrine differentiation. Int J Cancer. 2004;111:508–13. doi: 10.1002/ijc.20286. [DOI] [PubMed] [Google Scholar]
- 42.Xie S, Lin HK, Ni J, Yang L, Wang L, di Sant'Agnese PA, Chang C. Regulation of interleukin-6-mediated PI3K activation and neuroendocrine differentiation by androgen signaling in prostate cancer LNCaP cells. Prostate. 2004;60:61–7. doi: 10.1002/pros.20048. [DOI] [PubMed] [Google Scholar]
- 43.Ismail AH, Landry F, Aprikian AG, Chevalier S. Androgen ablation promotes neuroendocrine cell differentiation in dog and human prostate. Prostate. 2002;51:117–25. doi: 10.1002/pros.10066. [DOI] [PubMed] [Google Scholar]
- 44.Jiborn T, Bjartell A, Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma during hormonal treatment. Urology. 1998;51:585–9. doi: 10.1016/s0090-4295(97)00684-5. [DOI] [PubMed] [Google Scholar]
- 45.Jin RJ, Wang Y, Masumori N, Ishii K, Tsukamoto T, Shappell SB, Hayward SW, Kasper S, Matusik RJ. NE-10 neuroendocrine cancer promotes the LNCaP xenograft growth in castrated mice. Cancer Res. 2004;64:5489–95. doi: 10.1158/0008-5472.CAN-03-3117. [DOI] [PubMed] [Google Scholar]
- 46.Wright ME, Tsai MJ, Aebersold R. Androgen receptor represses the neuroendocrine transdifferentiation process in prostate cancer cells. Mol Endocrinol. 2003;17:1726–37. doi: 10.1210/me.2003-0031. [DOI] [PubMed] [Google Scholar]
- 47.Yuan TC, Veeramani S, Lin FF, Kondrikou D, Zelivianski S, Igawa T, Karan D, Batra SK, Lin MF. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocr Relat Cancer. 2006;13:151–67. doi: 10.1677/erc.1.01043. [DOI] [PubMed] [Google Scholar]
- 48.Zhang XQ, Kondrikov D, Yuan TC, Lin FF, Hansen J, Lin MF. Receptor protein tyrosine phosphatase alpha signaling is involved in androgen depletion-induced neuroendocrine differentiation of androgen-sensitive LNCaP human prostate cancer cells. Oncogene. 2003;22:6704–16. doi: 10.1038/sj.onc.1206764. [DOI] [PubMed] [Google Scholar]
- 49.Horii K, Suzuki Y, Kondo Y, Akimoto M, Nishimura T, Yamabe Y, Sakaue M, Sano T, Kitagawa T, Himeno S, Imura N, Hara S. Androgendependent gene expression of prostatespecific antigen is enhanced synergistically by hypoxia in human prostate cancer cells. Mol Cancer Res. 2007;5:383–91. doi: 10.1158/1541-7786.MCR-06-0226. [DOI] [PubMed] [Google Scholar]
- 50.Sobel RE, Sadar MD. Cell lines used in prostate cancer research: a compendium of old and new lines–part 1. J Urol. 2005;173:342–59. doi: 10.1097/01.ju.0000141580.30910.57. [DOI] [PubMed] [Google Scholar]
- 51.Lilleby W, Paus E, Skovlund E, Fossa SD. Prognostic value of neuroendocrine serum markers and PSA in irradiated patients with pN0 localized prostate cancer. Prostate. 2001;46:126–33. doi: 10.1002/1097-0045(20010201)46:2<126::aid-pros1016>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 52.Deeble PD, Cox ME, Frierson HF, Jr, Sikes RA, Palmer JB, Davidson RJ, Casarez EV, Amorino GP, Parsons SJ. Androgen-independent growth and tumorigenesis of prostate cancer cells are enhanced by the presence of PKAdifferentiated neuroendocrine cells. Cancer Res. 2007;67:3663–72. doi: 10.1158/0008-5472.CAN-06-2616. [DOI] [PubMed] [Google Scholar]
- 53.Rokhlin OW, Taghiyev AF, Bayer KU, Bumcrot D, Koteliansk VE, Glover RA, Cohen MB. Calcium/calmodulin-dependent kinase II plays an important role in prostate cancer cell survival. Cancer Biol Ther. 2007;6:732–42. doi: 10.4161/cbt.6.5.3975. [DOI] [PubMed] [Google Scholar]
- 54.Mamaeva OA, Kim J, Feng G, McDonald JM. Calcium/calmodulin-dependent kinase II regulates notch-1 signaling in prostate cancer cells. J Cell Biochem. 2009;106:25–32. doi: 10.1002/jcb.21973. [DOI] [PubMed] [Google Scholar]
- 55.Merkle D, Hoffmann R. Roles of cAMP and cAMP-dependent protein kinase in the progression of prostate cancer: cross-talk with the androgen receptor. Cell Signal. 2011;23:507–15. doi: 10.1016/j.cellsig.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 56.Hensley HH, Hannoun-Levi JM, Hachem P, Mu Z, Stoyanova R, Khor LY, Agrawal S, Pollack A. PKA knockdown enhances cell killing in response to radiation and androgen deprivation. Int J Cancer. 2011;128:962–73. doi: 10.1002/ijc.25634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rokhlin OW, Guseva NV, Taghiyev AF, Glover RA, Cohen MB. KN-93 inhibits androgen receptor activity and induces cell death irrespective of p53 and Akt status in prostate cancer. Cancer Biol Ther. 2010;9:224–35. doi: 10.4161/cbt.9.3.10747. [DOI] [PubMed] [Google Scholar]
- 58.Johannessen M, Moens U. Multisite phosphorylation of the cAMP response elementbinding protein (CREB) by a diversity of protein kinases. Front Biosci. 2007;12:1814–32. doi: 10.2741/2190. [DOI] [PubMed] [Google Scholar]
- 59.Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, Hagan MP, Grant S, Schmidt-Ullrich R. Stress and radiationinduced activation of multiple intracellular signaling pathways. Radiation Research. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 60.Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885–96. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]