Abstract
The ZEB family of transcription factors regulates key factors during embryonic development and cell differentiation but their role in cancer biology has only more recently begun to be recognized. Early evidence showed that ZEB proteins induce an epithelial-to-mesenchymal transition linking their expression with increased aggressiveness and metastasis in mice models and a wide range of primary human carcinomas. Reports over the last few years have found that ZEB proteins also play critical roles in the maintenance of cancer cell stemness, control of replicative senescence, tumor angiogenesis, overcoming of oncogenic addiction and resistance to chemotherapy. These expanding roles in tumorigenesis and tumor progression set ZEB proteins as potential diagnostic, prognostic and therapeutic targets.
Keywords: Cancer, cancer stem cells, chemotherapy resistance, E-cadherin, EMT, transcription, tumor invasiveness, ZEB1, ZEB2
Introduction
Cancer is a multistep process where normal cells evolve to cancer cells through the acquisition of a number of properties, referred as the hallmarks of cancer [1, 2] that include: self-sufficient proliferative signaling, insensitivity to tumor suppression, replicative immortality, resistance to cell death, induction of angiogenesis and invasion and metastasis. Attainment of these cancer traits could occur through overexpression, gain-of-function mutations or amplification of oncogenes and/or epigenetic/transcriptional repression, mutation or deletion of tumor suppressors. In addition to systematize our current understanding of cancer, the hallmarks of cancer have helped guiding efforts to design therapeutic targets.
One of the areas in tumor biology that has seen greater developments in recent years has been the study of how transcriptional and epigenetic alterations contribute to the acquisition of the cancer hallmark capabilities. This article reviews the role of the ZEB family of transcription factors in cancer. Early evidence about the promotion of tumor metastasis by ZEB factors has been greatly expanded in recent years by a wealth of reports involving ZEB proteins in the regulation of several other hallmarks of cancer.
Invasion of an in situ carcinoma into normal surrounding tissue requires that cancer epithelial cells lose their cell-cell adhesion and polarity characteristics in favor of a more motile fibroblast-like phenotype as part of a transdifferentiation process known as the epithelial-to-mesenchymal transition (EMT) [3]. Originally described during embryogenesis, the phenotypic and functional reprogramming associated to the EMT also takes place during the invasion of carcinoma cells from a primary tumor into normal tissues. A key initial step in the EMT is the downregulation of the E-cadherin intercellular adhesion protein, which expression could be regulated at genetic, epigenetic, transcriptional and post-translational levels [3,4]. Loss of E-cadherin often occurs through transcriptional repression, mediated by the binding of a small set of transcription factors (E-cadherin transcriptional repressors, EcTRs) to its promoter region. The EcTRs described so far include the ZEB family (ZEB1 and ZEB2, although identified under different names, see below), Snail1 (Snail), Snail2 (Slug), Twist1, Twist2 and E12/ E47. Expression of EcTRs associates with EMT and more mesenchymal and invasive properties in cancer cell lines and increased metastasis and poorer clinical prognosis in primary carcinomas [4,5]. In addition to overlapping roles and mutual regulation among EcTRs, evidence indicates that ZEB1—and, to a lesser extent, ZEB2 and Snail2—has the strongest correlation with EMT across cancer tissue origins [6,7].
A rapidly growing literature has involved ZEB1 and ZEB2 in the regulation of a large number of physiological and pathological processes [8,9]. Both ZEB proteins have recently gained special relevance in the field of molecular oncology for their roles in tumorigenesis, tumor invasiveness and metastasis, and resistance to chemotherapy drugs. The rest of this article is organized as follows. Next section reviews the structural organization of ZEB proteins, their interaction with other factors and transcriptional activities. Section three outlines the roles of ZEB proteins in tumor invasiveness, tumorigenesis, cell proliferation and senescence, and resistance to chemotherapy.
Structure and transcriptional activities of ZEB factors
Domain structure and interacting proteins of ZEB factors
In upper vertebrates, the ZEB family comprises two proteins, ZEB1 and ZEB2, known under multiple alternative names. Thus, ZEB1 was also identified as δEF1, AREB6, BZP, MEB1, Nil-2-a, TCF8, ZEB, ZEB-1, Zfhep1 and Zfhx1a [10-17]. In turn, ZEB2 is also referred as KIA0569, SIP1, SMADIP-1, ZEB-2 and Zfhx1b [18-20]. In Drosophila melanogaster, Caenorhabditis Elegans and zebrafish a single orthologue has been described, namely Zfh-1, Zag-1, and Kheper, respectively [21-23].
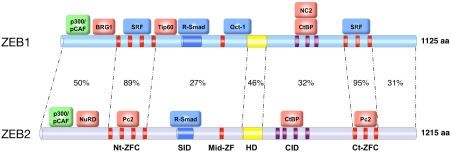
Structurally, ZEB proteins are highly modular with independent regions mediating their binding to DNA, to other transcription factors and to a number of cofactors—proteins with activator or repressor transcriptional activity but lacking a DNA binding domain on their own. All ZEB family members contain two zinc finger clusters (ZFC) located towards the N- and C-terminal ends of the protein (Nt-ZFC and Ct-ZFC, respectively) that bind to ZEB boxes (E-box and E-box-like DNA sequences) in the regulatory regions of target genes (Figure 1) [24-26]. Towards the center of ZEB proteins there is an extra zinc finger (mid-ZF, missing in human ZEB1) and a POU-like homeodomain, which has also been involved in binding to DNA [27]. Human and rodent ZEB1 and ZEB2 share a high degree of amino acid similarity in their ZFC and homeodomain, but much less elsewhere (Figure 1) [18,19].
Figure 1.
Scheme of the domain structure and main binding proteins of human ZEB1 and ZEB2. Percentages indicate identity at the amino acid level (GenBank accession numbers U12170 and AB011141, respectively). Proteins labeled in green represent coactivators, in red corepressors and in blue other transcription factors. Nt-ZFC: N-terminal zinc finger cluster. SID: Smad-interacting domain; Mid-ZFC: mid zinc finger cluster; HD: Homeodomain; CID: CtBP-interacting domain; Ct-ZFC: C-terminal zinc finger cluster. High degree of amino acid identity between ZEB1 and ZEB2 is mostly restricted to the Nt-ZFC and C-ZFC (89% and 95%, respectively), the SID (42%) and the homeodomain (46%).
ZEB1 and ZEB2 interact with other transcription factors. Downstream of the Nt-ZFC, both proteins contain a Smad Interacting Domain (SID) for binding to phosphorylated receptor-activated Smads (R-Smads), transcription factors that regulate downstream target genes in the TGFβ/ BMP signaling pathway [18, 28,29] (Figure 1). The ZFCs of ZEB1 and ZEB2 also mediate binding to transcription factors. Thus, the Nt-ZFC and Ct-ZFC of human ZEB1 have been shown to interact with SRF [30] while the mid-ZF/ homeodomain region of rat ZEB1 binds to Oct-1 [31]. Meantime, the Nt-ZFCs and Ct-ZFCs of ZEB2 interact directly with the polycomb factor Pc2[32].
Nevertheless, most of the transcriptional activities of ZEB1 and ZEB2 are mediated through their recruitment of several corepressors and coactivators (in red and green, respectively, in Figures 1 and 2). Upon activation of the TGFβ/ BMP signaling pathway and binding of ZEB1 to R-Smads, the region N-terminal to the Nt-ZFC of human ZEB1 binds to histone acetyltransferases p300 and p/CAF [28]. This interaction has also been reported for mouse and Xenopus ZEB2 although in this case independently of binding to R-Smads and TGFβ/BMP signaling [33]. The N-terminal half of human ZEB1, but not of ZEB2, also binds to another histone ace-tyltransferase, the Tat-interacting protein Tip60 [8,34]. The region N-terminal to the Nt-ZFC in both ZEB proteins recruits nucleosome remodeling factors. ZEB1 interacts with BRG1, one of the two ATPases of the SWI/SNF chromatin remodeling complex [35] while ZEB2 binds to the NuRD remodeling and deacetylase complex [36]. Between the homeodomain and the Ct-ZFC vertebrate ZEB1 and ZEB2 have a CtBP interacting domain (CID), containing multiple binding sequences for CtBP cofactors [19, 37-41], that in turn complex with histone deacetylases and methyltransferases, polycomb proteins and coREST [41-43]. In addition, it has been shown that CtBP1 could interact with the bromodomain of p300 blocking its ability to acetylate histones and activate transcription [44]. Zfh-1 and Zag-1 also interact with CtBP although their CID contains a single CtBP binding site [45,46]. Around the same region, human ZEB1 has been reported to interact with NC2α/NC2β (also referred as DRAP1/Dr1), a repressor of RNA poly-merase II and III transcription [47].
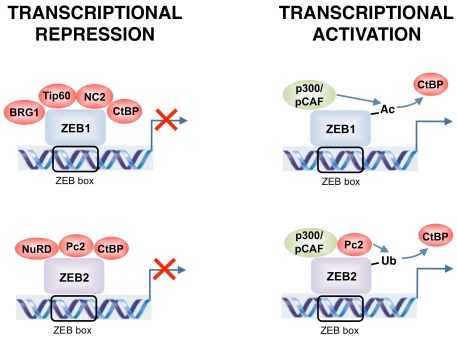
Figure 2.
ZEB1 and ZEB2 could act as either transcriptional repressors or activators depending on the target gene and tissue. Transcriptional repression and activation is achieved through differential recruitment of cofactors. Post-transcriptional modifications of ZEB1 and ZEB2 alter the set of coactivators and corepressors bound and switch both proteins from transcriptional repressors to activators.
Molecular mechanisms of transcriptional regulation by ZEB proteins
Such complexity in the number and nature of interacting factors anticipates the multiple transcriptional activities displayed by ZEB proteins. Although ZEB1 and ZEB2 were initially identified as transcriptional repressors and array screenings indicate that this is indeed their main transcriptional function (6 and below), both factors could activate or repress transcription depending on the target gene and tissue. Transcriptional repression by ZEB1 and ZEB2 could take place through either passive or active mechanisms. In the first case, ZEB proteins compete and displace transcriptional activators from their binding to E-box sequences in the DNA. Nevertheless, in some systems, the affinity of ZEB1 for DNA seems to be lower than that of other E-box binding proteins [17, 48-51]. In any case, the predominant mechanism by which ZEB1 and ZEB2 repress gene expression is through active transcriptional repression [19,45,47,51-54]. For instance, when the full-length cDNAs of ZEB1 and ZEB2 are fused to the DNA binding domain of yeast Gal4 and tested in a Gal4/UAS system, both heterologous proteins act as potent active transcriptional repressors [19,51,52]. This approach has allowed the identification of several repressor domains within the N-terminal and central regions of ZEB1 and ZEB2, each with distinct transcriptional repressor specificity, as well as an activation domain near the C-terminal end [19,45, 47,51,52,54].
It is assumed that ZEB proteins selectively recruit their corepressors in a promoter-specific manner. However, the identity of the precise corepressor(s) involved is only known for a few ZEB target genes. Tip60 mediates ZEB1 repression of CD4 [34]. A ZEB1/CtBP repressor complex regulates the growth hormone [42], interleukin-2 [55] and Bcl-6 [56]. Repression of E-cadherin by ZEB1 involves the summative activities of CtBP and BRG1 [35,40] and of CtBP and NuRD in the case of ZEB2 [36,40]. It is of note that the ATPase Mi2β/CHD4 of NuRD could also associate with BRG1 [57].
Activation of transcription by ZEB1 and ZEB2 also involves promoter-dependent recruitment of coactivators. Upon TGFβ/BMP stimulation, ZEB1 binds to R-Smads, synergizing with them in the activation of TGFβ/BMP-dependent genes through binding to p300 and p/CAF [28,29]. Binding to these histone acetylases is also assumed to mediate activation of other target genes positively regulated by ZEB1 and ZEB2. In that line, correlation between ZEB1 and Vitamin D Receptor—a gene directly activated by ZEB1— in primary colorectal carcinomas is stronger the higher the levels of p300 [58]. Although ZEB2 also binds to p/CAF and p300 [33], it remains unknown under what circumstances this mechanism is used to directly activate gene expression and for which genes, as ZEB2 inhibits R-Smad-mediated activation of TGFβ/BMP-dependent genes [28,29] (Figure 2).
The interplay among ZEB corepressors and coactivators remains to be fully defined (Figure 2). Post-translational modifications of ZEB proteins seem to contribute to switching ZEB1 and ZEB2 from repressors to activators. At least in the activation of TGFβ/BMP-dependent genes, interaction of ZEB1 with p/CAF following TGFβ/ BMP stimulation acetylates lysine residues flanking the CID, partly displacing CtBP from its binding to ZEB1 [29]. However, addition of FGF2 over TGFβ restores ZEB1-CtBP interaction [59]. Recruitment of p/CAF also blocks CtBP binding to ZEB2 [33] although, as indicated earlier, it remains to be determined what triggers p/CAF binding to ZEB2 in the first place. SUMOylation of ZEB2 by Pc2 also disrupts ZEB2 binding to CtBP and partially relieves E-cadherin repression but does not affect repression or activation of other ZEB2 target genes [32]. Independently of the binding of Pc2 to the Nt-ZFCs and Ct-ZFCs of ZEB2, CtBP also binds and is sumoylated by polycomb Pc2 [60]. Although its transcriptional significance remains unclear, phosphorylation of ZEB1 varies widely among cell types [61]. Finally, ZEB1 and ZEB2 functions are also regulated by means of their intracellular localization. Both ZEB proteins are exported to the cytoplasm in a temporal- and/or tissue-specific manner during embryonic development and in adult tissues and tumors [15, 62-64].
ZEB proteins and cancer
ZEB proteins as inducers of EMT and tumor metastasis
Since ZEB1 was originally identified in the early 1990s, several dozen target genes—including key regulatory factors during embryogenesis and cell differentiation—have been reported to be repressed or activated by ZEB proteins, mostly through direct binding to their regulatory regions [8]. But it was their ability to repress the E-cadherin and induce an EMT that set ZEB1 and ZEB2 as important regulators of tumor progression [41, 66-69].
In epithelial cells, E-cadherin locates at adherens junctions and mediates homotypic intercellular adhesion, while interacts intracellularly with catenins and the actin cytoskeleton. Intercellular adhesion via adherens junctions is critical for maintenance of epithelial cell polarity and phenotype. Consequently, loss of E-cadherin is considered a critical initial event not only in EMT, but also in tumor progression and metastasis [3,70] (Figure 3). During the EMT loss of E-cadherin is accompanied by reorganization of other intercellular complexes and synthesis of some extracellular matrix components (e.g. fibronectin, collagen I and III) and metallo-proteases (MMPs), normally produced by mesenchymal stromal cells [71]. In addition, the EMT is associated with the acquisition of a stem cell phenotype and increased resistance to apoptosis [70,72,73 and see below). Consequently, the EMT endows tumor cells with greater motility, self-renewal capacity and resistance to drugs, at the same time that degradation of the extracellular matrix facilitates their invasion into the surrounding stroma and eventual metastasis to distant organs.
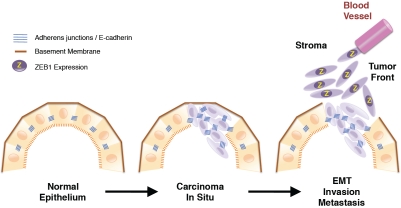
Figure 3.
Schematic representation of the evolution of a carcinoma in situ into an invasive carcinoma. Invasion of cancer cells into the surrounding stroma involves a complex process by which they lose their epithelial characteristics and acquire instead a mesenchymal phenotype as part of the EMT. ZEB1 is not present in normal epithelial cells or at the center of well-differentiated carcinomas but is expressed by invasive cancer stem cells undergoing an active EMT at the tumor front of several types of carcinomas. ZEB1 also regulates several components of the basement membrane, which breakdown is required for cancer cells to invade the surrounding stroma.
Overexpression of ZEB1 or ZEB2 in epithelial cells induces a full EMT. ZEB1 and ZEB2 not only repress E-cadherin but also P- and R-cadherins and other epithelial markers involved in cell polarity (e.g. CRB3, HUGL2, PATJ), components of tight junctions (e.g. occludin, claudin 7, JAM1, ZO3), gap junctions (e.g. connexins 26 and 31) and desmosomes (e.g. desmoplakin, plakophilin 3) [6,74,75] (Table 1). In turn, ZEB1 and ZEB2 activate the expression of mesenchymal genes such as vimentin and N-cadherin [69,75,76]. Overexpression of ZEB2 has been shown to induce several MMPs, namely MMP1, MMP2 and MMP14 [77]. In colorectal carcinomas, ZEB1 also regulates components of the epithelial basement membrane, which disruption is a key step in tumor invasiveness—e.g., knock down of ZEB1 upregulates the α3 chain of laminin 5 (LAMA3) and the α2 chain of collagen IV (COL4A2) while reduces the levels of the γ2 chain of laminin 5 (LAMC2) [78].
Table 1.
Selected targets of ZEB1 and ZEB2 during EMT*
| ZEB1 | ZEB2 | |
|---|---|---|
| Direct Transcriptional Targets | Genes downregulated | Genes downregulated |
| E-cadherin [41,66,68,69] Crumbs3, HUGL2, PATJ [6] Paklophilin 3 [74] MMP1 [102] miR-200 family [88, 89] | E-cadherin [67] P-cadherin [75] Claudin 4 [75] Connexin 26 [75] miR-200 family [89] | |
| Genes upregulated | ||
| Vimentin [76] | ||
| Indirect Targets or Unknown Mechanism | Genes downregulated | Genes downregulated |
| P- & R-Cadherin [6] Occludin & JAM1 [6] Desmocollin-2 [6] Gap junction protein β2 & β3 [6] Epiplakin & Periplakin [6] Mucin [6] EpCAM [6,65] ESRP1/2 & ST14 [65] | ZO-3 [75] Plakophilin 2 & Desmoplakin 1/2 [75] Connexin 31 [75] Gap junction protein β2 & β3 [75] EpCAM [65] ESRP1/2 & ST14 [65] | |
| Genes upregulated | Genes upregulated | |
| Vimentin [68,69] | Fibronectin (77) MMP1, MMP2, MMP14 [77] |
Note that being a direct transcriptional target of ZEB1 and/or ZEB2 does not exclude indirect regulation as well. Genes under the label indirect target/unknown mechanism refer to those that are either known to be regulated by ZEB1 and/or ZEB2 exclusively through indirect mechanisms or where direct binding of ZEB1 and/or ZEB2 to their regulatory regions has not been demonstrated yet. Numbers in brackets refer to the references where these targets were identified.
ZEB1 and ZEB2 mediate the EMT triggered by key signaling cascades such as TGFβ/BMP, NFκB, Ras-ERK2, and HIF-1, often activated in tumors [79-82]. On the other hand, ZEB1 and ZEB2 are repressed by non-coding microRNAs of the miR-200 family, which are important in maintaining an epithelial phenotype and preventing an EMT [83-87]. Interestingly, miR-200 members are, in turn, transcriptionally repressed by ZEB1 and ZEB2, thus forming regulatory loops that maintain cells in either an epithelial or mesenchymal state [9,88,89].
Large areas of many carcinomas, including colorectal, are relatively well-differentiated, with tumor cells maintaining their polarity and E-cadherin associated at the membrane with β-catenin [90,91]. By contrast, at their invasive edge, tumor cells undergo an active EMT with loss of E-cadherin and nuclear translocation of β-catenin [90]. These dedifferentiated, fibroblastic-like cells at the tumor front have been referred as “migrating cancer stem cells” because of their stem cell-like phenotype [78,91]. As inhibitor of E-cadherin and epithelial phenotype, ZEB1 is not expressed in normal epithelium but is found in isolated cells at the stroma. ZEB1 is neither expressed by tumor cells in well-differentiated areas of carcinomas, but is expressed at high levels in invading tumor cells of endometrial, colorectal, lung, breast, prostate, gallbladder, and pancreatic carcinomas among others [35,78,92-97] (Figures 3 and 4). Increased numbers of ZEB1-positive cells in the stroma are found in colorectal, breast, lung and bladder carcinomas [6,65,98] and it has been suggested that ZEB1-dependent paracrine signaling from the stroma could mediate E-cadherin repression in other parts of the tumor [65].
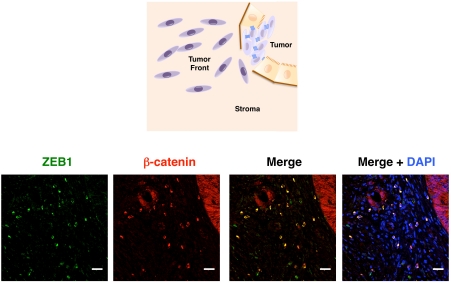
Figure 4.
ZEB1 and β-catenin colocalize at the nucleus of invasive cancer cells at the tumor front of colorectal carcinomas. While in epithelial cells of well-differentiated areas of colorectal carcinomas β-catenin has a membranous/ cytoplasmic distribution it becomes mostly nuclear in these invasive cancer cells. ZEB1 (green, E-20 antibody, Santa Cruz Biotechnology) and β-catenin (red, Ab6032 antibody, Abcam) colocalize (yellow, merge panel) in invasive cancer cells of a sporadic colorectal carcinoma [35,78]. Labeling with 4',6-diamidino-2-phenylindole (DAPI) is also shown. Scale bar represents 25 μm.
Although stronger than for most other EcTRs, inverse correlation between ZEB2 and E-cadherin across cancer cell lines is not as evident as in the case of ZEB1 [7]. Interestingly, ZEB2 is detected at high levels in the cytoplasm of normal E-cadherin-positive epithelial cells of several tissues (e.g. esophagus, stomach, colon and rectum, hepatocytes, renal tubules), but is downregulated when these epithelia evolve towards carcinomas [63,64]. Since ZEB2 is only known as a transcription factor, the functional significance of its cytoplasmic expression in epithelial cells remains to be elucidated.
Expression of ZEB proteins at the invasive front of carcinomas translates into increased tumor metastasis in ZEB1-positive tumors. Thus, in mouse xenograft models, expression of ZEB1 promotes metastasis of colorectal carcinoma cells [99]. Over the last few years, a wealth of reports have linked ZEB1 and/or ZEB2 expression to increased aggressiveness and higher metastatic capacity in a wide range of primary human carcinomas, including ovarian, breast, endometrium, lung, prostate, colon, gallbladder, pancreas and bladder [92-98,100].
The transition from an avascular hyperplasia into a larger hypervascularized tumor mass requires the formation of new vessels, the so-called “angiogenic switch”, which results from the production by the tumor of angiogenic factors and proteases, including MMPs [101]. Evidence in the literature indicates that ZEB1 and ZEB2 play opposing roles in this regard. On the one hand, and contrary to its tumor progression role discussed so far, ZEB1 inhibits tumor angiogenesis in vivo. Subcutaneous injection of melanoma cells leads to larger tumors with more tumor vessels—and higher ZEB1 endothelial expression—in heterozygous ZEB1 -/+ mice than in wild type animals [102]. On the other hand, ZEB2 has a pro-angiogenic effect with ZEB2 -/- embryos displaying defective vessel maturation [103].
ZEB proteins in stemness maintenance and tumorigenesis
In the classic stochastic model of tumorigenesis, all cells are homogenous and have the same potential to initiate a tumor in response to intrinsic or extrinsic factors. However, oncologists have been puzzled for decades by the great level of heterogeneity displayed by tumors— both hematologic and solid—in terms of morphology, surface markers, genetic mutations and sensitivity to treatment [104,105]. Evidence built in the last two decades have revived an alternative model of tumorigenesis, the “cancer stem cell” (CSC) model [105,106]. Seminal studies in the 1990s demonstrated that some variants of acute myeloid leukemia originate from a subset of cells, which phenotype resembles that of normal hematopoietic stem cells—thus coined as “leukemia stem cells”—and that retain the capacity to reproduce leukemias in xenotransplanted recipient mice [105]. Using similar approaches researchers have also found CSCs in colon, pancreas, breast and brain cancers. Contrary to cells in the bulk of the tumor, CSCs have the capacity for self-renewal, differentiation and initiation of tumorigenesis [104,106]. A recent report has showed that oncogenic transformation enhances the conversion of non-stem cancer cells into cancer stem cells. Oncogenic transformation of mammary stem-like cells also produces more aggressive tumors that transformation of differentiated mammary epithelial cells [107].
Induction of EMT by TGFβ or overexpression of EcTRs—is able to reprogram differentiated populations of normal mammary epithelial cells and breast carcinoma cells into undifferentiated cells with stem cell-like phenotype and functional properties (e.g. generation of spheres in culture, increased turmorigenecity in xenotransplants) [72]. A subpopulation of cancer cells within breast tumors display a phenotype similar to normal stem cells, including lower levels of miR-200c-141, miR-200b-200a-429, and miR-183-96-182 [108]. Expression of miR-200c in normal and cancerous breast stem cells reduces the expression of self-renewal factor Bmi1 and these cells' ability to form mammary ducts and tumors, respectively.
The salience of ZEB proteins in cancer biology was further enhanced by work from Brabletz's group elegantly showing that ZEB1 maintains an stemness phenotype in pancreatic cancer cells and increases their tumorigenic capacity in nude mice [97]. Regulation of stemness by ZEB1 occurs through transcriptional repression of miR-200, miR-183 and miR-203, which in turn repress Bmi1, Sox2 and KLF4. In this line, breast stem cells express higher levels of both ZEB1 and ZEB2 than differentiated cells [108]. Formation of spheres in mouse embryo fibroblasts (MEFs) from Rb family and Rb1 -/- mice is accompanied by the generation of stem-like cells, which phenotype and viability requires of ZEB1 expression [109]. Recent reports have showed that p53 suppresses EMT, stemness and reduces ZEB1 levels through direct transcriptional activation of miR-200c [110]. Conversely, loss of p53—or overexpression of oncogenic p53 mutants—induces a downregulation of miR-200c and increased expression of ZEB1 and stem cells markers (including Bmi1 and KLF4) in mammary and pancreatic acinar epithelial cells and associates with higher tumor grading in breast carcinomas [110, 111]. The connection between ZEB proteins, EMT and stemness may play an important role not only during tumorigenesis but also in embryonic development [9,112].
ZEB proteins in the regulation of cell cycle and senescence
The involvement of ZEB proteins in the regulation of cell cycle and proliferation varies depending on the cell type and model used. Overexpression of either ZEB1 or ZEB2 in lung epithelial cells does not have by itself any effect in cell cycle. However, when combined with low doses of TGFβ—suboptimal to induce a full growth arrest—ZEB1 increases the percentage of cells in G1 phase [28]. This synergistic effect between ZEB1 and TGFβ depends on the direct interaction of ZEB1 with R-Smads. By contrast, ZEB1 -/- mice display decreased proliferation in tissues affected by developmental defects (e.g. palate, skeleton, nervous system) and MEFs from ZEB1 -/+ and ZEB1 -/- mice arrest at much earlier passage than wild type MEFs [69].
Overexpression of ZEB2 in lung epithelial cells raises the concentration of TGFβ needed to trigger growth arrest [28]. Likewise, specific targeted deletion of ZEB2 in the mice developing cerebral cortex results in decreased proliferation of precursor cells in the hippocampus and dentate gyrus [113]. However, in epidermoid and bladder carcinoma cell lines the reverse is observed, overexpression of ZEB2 induces a G1 arrest by direct transcriptional repression of cyclin D1 [98,114].
ZEB1, but not ZEB2 or Snail1, is repressed by the p16INK4a/Rb1 tumor suppressor pathway. Rb1/E2F1-HDAC1 repressor complexes regulate ZEB1 transcription by direct binding to its promoter and, compared to MEFs from wild type mice, Rb1 -/- and E2F1 -/- MEFs display higher levels of ZEB1 [115]. Loss of Rb1 has been involved in both tumor initiation and progression and may contribute to explain overexpression of ZEB1 in proliferating cells and many primary tumors.
Replicative senescence is considered an important tumor-suppressor mechanism and, for instance, knock down of Rb1 inhibits the ability of cells to undergo oncogene-induced senescence. ZEB1 helps cancer cells to overcome replicative senescence and its elimination (e.g. ZEB1 -/+ and ZEB1 -/- MEFs) triggers premature senescence in a dose-dependent manner [69]. Circumvention of senescence by ZEB1 occurs independent of p16INK4a but rather through direct transcriptional repression of p15INK4b and p21CDKN1a [28,69]. By contrast, ZEB2 induces senescence arrest in hepatic and breast carcinoma cells through repression of hTERT [116].
ZEB proteins in oncogenic addiction and resistance to chemotherapy and radiotherapy
Some tumors require the expression of one or more genes for the maintenance of their malignant phenotype and viability, in what is known as “oncogenic addiction” [117,118]. The success of antibodies and drugs targeting specific oncogenes (e.g. K-Ras, Her2/Neu, Bcr/Abl, EGFR, c-kit) in the treatment of a number of solid and hematologic cancers in mice models and humans has helped to reinforce the oncogenic addiction concept. Given the potential therapeutic applications, the identification of the specific oncogene(s) on which particular types of human cancers are dependent on has attracted a great deal of interest in recent years.
A survey of K-ras mutant lung and pancreatic cancer cell lines found that their epithelial status determines their dependency on K-ras for survival [70,73]. Thus, cancer cells that are independent on K-Ras for their growth and viability have a mesenchymal phenotype while K-Ras-dependent cell lines exhibit epithelial characteristics [73]. Furthermore, induction of EMT protects lung cancer cells from apoptosis following K-ras knock down. The same study found that ZEB1 expression overrides K-ras oncogenic addiction while its elimination reverses K-Ras dependency. In the same line, the phenotype and histology of tumors formed from ZEB1-dependent CSCs in Rb1 -/- MEFs are similar to those observed by expression of activated Ras [109]. The presence of ZEB1 within the “K-ras dependency gene signature” highlights its role as a potential therapeutic target. Other EcTRs seem to play parallel roles—Twist1 and Twist2 override ErbB2-dependent senescence and cooperate with Ras to induce malignant transformation, EMT and metastasis [119]. It has been therefore suggested that the induction of ZEB1 and other EcTRs during tumor progression—and the parallel acquisition of a dedifferentiated stem cell phenotype—may substitute for constitutive Ras activation [120].
EMT is also accompanied by increased resistance to apoptosis by DNA damaging drugs [70,73], and mounting evidence indicates that expression of different EcTRs by cancer cell lines and primary tumors confers tumor cells resistance to chemotherapy and radiotherapy. In lung cancer cell lines, expression of E-cadherin has been associated to higher sensitivity to the EGFR inhibitor gefitinib while expression of ZEB1—but not of ZEB2, Snail1 and Snail2—correlates to higher resistance [121] (Table 2). Conversely, knock down of ZEB1 increases the sensitivity of colorectal and pancreatic cancer cell lines to gemcitabine, fluorouracil and cisplatin while gemcitabine-resistant clones of these cell lines exhibit high levels of ZEB1 [97,122,123]. Likewise, elimination of ZEB1 in non-small cell lung and head and neck cancer squamous carcinoma cell lines reduced cell death in response to the EGFR inhibitor erlotinib in an E-cadherin-dependent manner [124]. Interestingly, simultaneous knockdown of ZEB1 and E-cadherin reverses the sensitization to erlotinib induced by ZEB1 knockdown, suggesting that sensitivity to this EGFR inhibitor requires E-cadherin re-expression. Levels of ZEB1, but not ZEB2, also inversely correlate with sensitivity of breast cancer cells to doxorubicin [125].
Table 2.
ZEB1 and ZEB2 confer resistance to chemotherapy and radiotherapy
| Evidence | Resistance | Reference | |
|---|---|---|---|
| ZEB1 | Pancreatic carcinoma cell lines | Gemcitabine 5-Fluorouracil Cisplatin | [97, 120, 121] |
| ZEB1 | Breast carcinoma cell lines | Doxorubicin | [123] |
| ZEB1 | Non-small cell lung carcinoma cell lines | Gefitinib | [119] |
| ZEB1 | Head and neck squamous carcinoma cell lines | Ertotinib | [122] |
| ZEB2 | Primary transitional cell carcinomas of the bladder (clinical evidence) | Radiotherapy | [98] |
| ZEB2 | Bladder carcinoma cell lines | Cisplatin UV radiation | [98] |
| ZEB2 | Squamous carcinoma cell lines | Cisplatin UV radiation | [98] |
Parallelly, ZEB2 has been shown to have an independent prognostic value in transitional cell carcinomas of the bladder [98]. Irrespective of ZEB1 levels, patients with ZEB2-negative bladder carcinomas exhibit better survival outcomes and response to radiotherapy [98]. In addition, expression of ZEB2 protects bladder and squamous carcinoma cell lines against DNA damage-induced cell death (e.g. UV radiation, cisplatin).
The mechanisms involved in ZEB1- and ZEB2-mediated drug resistance are still being investigated. In different cell systems, ZEB1 has been shown to directly inhibit TAp73, which triggers apoptosis, but also ΔNp73 and ΔNp63, that function as anti-apoptotic factors [48, 126, 127]. The pro-survival effect of ZEB2 is independent of cell cycle arrest and intercellular adhesion and is mediated through inhibition of cleavage of PARP and pro-caspase 3 and phosphorylation of ATM/ATR substrates [98].
Interestingly, other EcTRs such as Snail and Twist proteins also protect cells from apoptosis and contribute to drug resistance in cancer cells, in part through regulation of p53/p63/ p73 family members. Snail-mediated resistance to cell death is also critical for migration of embryonic cells during development [128]. Snail1 and Snail2 confer resistance to chemotherapy and radiotherapy through two mechanisms: repression of genes involved in p53-mediated apoptosis (e.g. ATM, PUMA, PTEN) and derepression of genes associated to stemness (e.g. Nanog, claudin 3, KFL4) [129]. Snail1 inhibition of ΔNp63α induces increased invasiveness in human squamous cell carcinomas independent of its effect over E-cadherin [130]. Meantime, Twist1 inhibits p53-mediated apoptosis by direct interaction with the DNA binding domain of p53 [131,132].
Concluding remarks
The literature here reviewed involves ZEB1 and/ or ZEB2 in the control of several cancer cell capabilities, namely, cell proliferation, senescence, apoptosis, angiogenesis, resistance to chemotherapy and radiotherapy and tumor invasiveness and metastasis. Targeting of a single cancer cell trait often provides limited success in cancer treatment and, thus, simultaneous approach to several of them may be a more appropriate strategy. The contribution of ZEB1 and ZEB2 to multiple cancer hallmarks, along with their highly modular structure and complex transcriptional activities, offers translational researchers with an attractive therapeutic target. Examination of the levels of ZEB1 and ZEB2 expression (or lack of) in primary tumors may help to prospectively identify their resistance (or sensitivity) to particular chemotherapy treatments. In addition, the inhibition of ZEB1, ZEB2 or its cofactors could be used to reverse drug resistance in cancer patients. Altogether, ZEB1 and ZEB2 are thus poised to become important diagnostic, prognostic and/or therapeutic cancer targets in the near future.
Acknowledgments
Experimental work in AP's laboratory was conducted by EST, LS and OdB. MC, EV and AC identified patients, prepared tissue samples and/or advised in the interpretation of immunostaining experiments. AP wrote the article and all authors contributed to its critical revision. We apologize to those researchers whose relevant work was cited only indirectly through reviews because of space limitations. Work in AP's lab is funded by grants from Olga Torres Foundation, Spanish Ministry of Science and Innovation (BFU2007-60302, BFU2010-15163), Spanish Association Against Cancer, La Caixa Foundation and the European Commission. EST's salary was party funded by La Caixa Foundation and the European Commission.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 4.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 5.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 6.Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist P, Sommergruber W, Schweifer N, Wernitznig A, Beug H, Foisner R, Eger A. The transcription factor ZEB1 (δEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinhold WC, Reimers MA, Lorenzi P, Ho J, Shankavaram UT, Ziegler MS, Bussey KJ, Nishizuka S, Ikediobi O, Pommier YG, Weinstein JN. Multifactorial regulation of E-cadherin expression: An integrative study. Mol Cancer Ther. 2010;9:1–16. doi: 10.1158/1535-7163.MCT-09-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66:773–787. doi: 10.1007/s00018-008-8465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop–a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams TM, Moolten D, Burlein J, Romano J, Bhaerman R, Godillot A, Mellon M, Rauscher FJ, Kant JA. Identification of a zinc finger protein that inhibits IL-2 gene expression. Science. 1991;254:1791–1794. doi: 10.1126/science.1840704. [DOI] [PubMed] [Google Scholar]
- 11.Williams TM, Montoya G, Wu Y, Eddy RL, Byers MG, Shows TB. The TCF8 gene encoding a zinc finger protein (Nil-2-a) resides on human chromosome 10p11.2. Genomics. 1992;14:194–196. doi: 10.1016/s0888-7543(05)80307-6. [DOI] [PubMed] [Google Scholar]
- 12.Funahashi J, Sekido R, Murai K, Kamachi Y, Kondoh H. δ-crystallin enhancer binding protein δEF1 is a zinc finger-homeodomain protein implicated in postgastrulation embryogenesis. Development. 1993;119:433–446. doi: 10.1242/dev.119.2.433. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe Y, Kawakami K, Hirayama Y, Nagano K. Transcription factors positively and negatively regulating the Na,K-ATPase α 1 subunit gene. J Biochem. 1993;114:849–855. doi: 10.1093/oxfordjournals.jbchem.a124267. [DOI] [PubMed] [Google Scholar]
- 14.Genetta T, Ruezinsky D, Kadesch T. Displacement of an E-box-binding repressor by basic helix-loop-helix proteins: implications for B-cell specificity of the immunoglobulin heavy-chain enhancer. Mol Cell Biol. 1994;14:6153–6163. doi: 10.1128/mcb.14.9.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franklin AJ, Jetton TL, Shelton KD, Magnuson MA. BZP, a novel serum-responsive zinc finger protein that inhibits gene transcription. Mol Cell Biol. 1994;14:6773–6788. doi: 10.1128/mcb.14.10.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabanillas AM, Darling DS. Alternative splicing gives rise to two isoforms of Zfhep, a zinc finger/homeodomain protein that binds T3-response elements. DNA Cell Biol. 1996;15:643–651. doi: 10.1089/dna.1996.15.643. [DOI] [PubMed] [Google Scholar]
- 17.Genetta T, Kadesch T. Cloning of a cDNA encoding a mouse transcriptional repressor displaying striking sequence conservation across vertebrates. Gene. 1996;169:289–290. doi: 10.1016/0378-1119(95)00824-1. [DOI] [PubMed] [Google Scholar]
- 18.Verschueren K, Remacle JE, Collart C, Kraft H, Baker BS, Tylzanowski P, Nelles L, Wuytens G, Su MT, Bodmer R, Smith JC, Huylebroeck D. SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5-CACCT sequences in candidate target genes. J. Biol. Chem. 1999;274:20489–20498. doi: 10.1074/jbc.274.29.20489. [DOI] [PubMed] [Google Scholar]
- 19.Postigo AA, Dean DC. Differential expression and function of members of the zfh-1 family of zinc finger/homeodomain repressors. Proc Natl Acad Sci USA. 2000;97:6391–6396. doi: 10.1073/pnas.97.12.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amiel J, Espinosa-Parrilla Y, Steffann J, Gosset P, Pelet A, Prieur M, Boute O, Choiset A, Lacombe D, Philip N, Le Merrer M, Tanaka H, Till M, Touraine R, Toutain A, Vekemans M, Munnich A, Lyonnet S. Large-scale deletions and SMADIP1 truncating mutations in syndromic Hirschsprung disease with involvement of mid-line structures. Am J Hum Genet. 2001;69:1370–1377. doi: 10.1086/324342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortini ME, Lai ZC, Rubin GM. The Drosophila zfh-1 and zfh-2 genes encode novel proteins containing both zinc-finger and homeodomain motifs. Mech Dev. 1991;34:113–122. doi: 10.1016/0925-4773(91)90048-b. [DOI] [PubMed] [Google Scholar]
- 22.Clark SG, Chiu C. C. elegans ZAG-1, a Zn-finger-homeodomain protein, regulates axonal development and neuronal differentiation. Development. 2003;13016:3781–3794. doi: 10.1242/dev.00571. [DOI] [PubMed] [Google Scholar]
- 23.Muraoka O, Ichikawa H, Shi H, Okumura S, Taira E, Higuchi H, Hirano T, Hibi M, Miki N. Kheper, a novel ZFH/8EF1 family member, regulates the development of the neuroectoderm of zebrafish (Danio rerio) Dev Biol. 2000;228:29–40. doi: 10.1006/dbio.2000.9909. [DOI] [PubMed] [Google Scholar]
- 24.Sekido R, Murai K, Funahashi J, Kamachi Y, Fujisawa-Sehara A, Nabeshima Y, Kondoh H. The δ-crystallin enhancer-binding protein δEF1 is a repressor of E2-box-mediated gene activation. Mol Cell Biol. 1994;14:5692–5700. doi: 10.1128/mcb.14.9.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda K, Kawakami K. DNA binding through distinct domains of zinc-finger-homeodomain protein AREB6 has different effects on gene transcription. Eur J Biochem. 1995;233:73–82. doi: 10.1111/j.1432-1033.1995.073_1.x. [DOI] [PubMed] [Google Scholar]
- 26.Remacle JE, Kraft H, Lerchner W, Wuytens G, Collart C, Verschueren K, Smith JC, Huylebroeck D. New mode of DNA binding of multi-zinc finger transcription factors : δEF1 family members bind with two hands to two target sites. EMBO J. 1999;18:5073–5084. doi: 10.1093/emboj/18.18.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su MT, Fujioka M, Goto T, Bodmer R. The Drosophila homeobox genes zfh-1 and even-skipped are required for cardiac-specific differentiation of a numb-dependent lineage decision. Development. 1999;126:3241–3251. doi: 10.1242/dev.126.14.3241. [DOI] [PubMed] [Google Scholar]
- 28.Postigo AA. Opposing functions of ZEB proteins in the regulation of the TGFβ/BMP signaling pathway. EMBOJ. 2003;22:2443–2452. doi: 10.1093/emboj/cdg225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Postigo AA, Depp JL, Taylor JJ, Kroll KL. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 2003;22:2453–2462. doi: 10.1093/emboj/cdg226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimura G, Manabe I, Tsushima K, Fujiu K, Oishi Y, Imai Y, Maemura K, Miyagishi M, Higashi Y, Kondoh H, Nagai R. δEF1 mediates TGF-β signaling in vascular smooth muscle cell differentiation. Dev Cell. 2006;11:93–104. doi: 10.1016/j.devcel.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Smith GE, Darling DS. Combination of a zinc finger and homeodomain required for protein-interaction. Mol Biol Rep. 2003;30:199–206. doi: 10.1023/a:1026330907065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long J, Zuo D, Park M. Pc2-mediated sumoylation of Smad-interacting protein 1 attenuates transcriptional repression of E-cadherin. J Biol Chem. 2005;280:35477–35489. doi: 10.1074/jbc.M504477200. [DOI] [PubMed] [Google Scholar]
- 33.Van Grunsven LA, Taelman V, Michiels C, Opdecamp K, Huylebroeck D, Bellefroid EJ. δEF1 and SIP1 are differentially expressed and have overlapping activities during Xenopus embryogenesis. Dev Dyn. 2006;235:1491–1500. doi: 10.1002/dvdy.20727. [DOI] [PubMed] [Google Scholar]
- 34.Hlubek F, Löhberg C, Meiler J, Jung A, Kirchner T, Brabletz T. Tip60 is a cell-type-specific transcriptional regulator. J Biochem. 2001;129:635–641. doi: 10.1093/oxfordjournals.jbchem.a002901. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Tillo E, Lazaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, Engel P, Postigo A. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;29:3490–3500. doi: 10.1038/onc.2010.102. [DOI] [PubMed] [Google Scholar]
- 36.Verstappen G, van Grunsven LA, Michiels C, Van de Putte T, Souopgui J, Van Damme J, Bellefroid E, Vandekerckhove J, Huylebroeck D. Atypical Mowat-Wilson patient confirms the importance of the novel association between ZFHX1B/SIP1 and NuRD corepressor complex. Hum Mol Genet. 2008;17:1175–1183. doi: 10.1093/hmg/ddn007. [DOI] [PubMed] [Google Scholar]
- 37.Postigo AA, Dean DC. ZEB represses transcription through interaction with the corepressor CtBP. Proc Natl Acad Sci USA. 1999;96:6683–6688. doi: 10.1073/pnas.96.12.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furusawa T, Moribe H, Kondoh H, Higashi Y. Identification of CtBP1 and CtBP2 as corepressors of zinc finger-homeodomain factor δEF1. Mol Cell Biol. 1999;19:8581–8590. doi: 10.1128/mcb.19.12.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Grunsven LA, Taelman V, Michiels C, Verstappen G, Souopgui J, Nichane M, Moens E, Opdecamp K, Vanhomwegen J, Kricha S, Huylebroeck D, Bellefroid EJ. XSip1 neuralizing activity involves the co-repressor CtBP and occurs through BMP dependent and independent mechanisms. Dev Biol. 2007;306:34–49. doi: 10.1016/j.ydbio.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 40.van Grunsven LA, Michiels C, Van de Putte T, Nelles L, Wuytens G, Verschueren K, Huylebroeck D. Interaction between Smad-interacting protein-1 and the corepressor C-terminal binding protein is dispensable for transcriptional repression of E-cadherin. J Biol Chem. 2003;278:26135–26145. doi: 10.1074/jbc.M300597200. [DOI] [PubMed] [Google Scholar]
- 41.Shi Y, Sawada J, Sui G, Affar el B, Whetstine JR, Lan F, Ogawa H, Luke MP, Nakatani Y, Shi Y. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature. 2003;422:735–738. doi: 10.1038/nature01550. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Scully K, Zhu X, Cai L, Zhang J, Prefontaine GG, Krones A, Ohgi KA, Zhu P, Garcia-Bassets I, Liu F, Taylor H, Lozach J, Jayes FL, Korach KS, Glass CK, Fu XD, Rosenfeld MG. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature. 2007;446:882–887. doi: 10.1038/nature05671. [DOI] [PubMed] [Google Scholar]
- 43.Chinnadurai G. The transcriptional corepressor CtBP: a foe of multiple tumor suppressors. Cancer Res. 2009;69:731–734. doi: 10.1158/0008-5472.CAN-08-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JH, Cho EJ, Kim ST, Youn HD. CtBP represses p300-mediated transcriptional activation by direct association with its bromodomain. Nat Struct Mol Biol. 2005;12:423–428. doi: 10.1038/nsmb924. [DOI] [PubMed] [Google Scholar]
- 45.Postigo AA, Ward E, Skeath JB, Dean DC. zfh-1, the Drosophila homologue of ZEB, is a transcriptional repressor that regulates somatic myogenesis. Mol Cell Biol. 1999;19:7255–7263. doi: 10.1128/mcb.19.10.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wacker I, Schwarz V, Hedgecock EM, Hutter H. zag-1, a Zn-finger homeodomain transcription factor controlling neuronal differentiation and axon outgrowth in C. elegans. Development. 2003;130:3795–3805. doi: 10.1242/dev.00570. [DOI] [PubMed] [Google Scholar]
- 47.Ikeda K, Halle JP, Stelzer G, Meisterernst M, Kawakami K. Involvement of negative cofactor NC2 in active repression by zinc finger-homeodomain transcription factor AREB6. Mol Cell Biol. 1998;18:10–18. doi: 10.1128/mcb.18.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fontemaggi G, Gurtner A, Strano S, Higashi Y, Sacchi A, Piaggio G, Blandino G. The transcriptional repressor ZEB regulates p73 expression at the crossroad between proliferation and differentiation. Mol Cell Biol. 2001;21:8461–8470. doi: 10.1128/MCB.21.24.8461-8470.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ponticos M, Partridge T, Black CM, Abraham DJ, Bou-Gharios G. Regulation of collagen type I in vascular smooth muscle cells by competition between Nkx2.5 and δEF1/ZEB1. Mol Cell Biol. 2004;24:6151–6161. doi: 10.1128/MCB.24.14.6151-6161.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jethanandani P, Kramer RH. α7 integrin expression is negatively regulated by δEF1 during skeletal myogenesis. J Biol Chem. 2005;280:36037–36046. doi: 10.1074/jbc.M508698200. [DOI] [PubMed] [Google Scholar]
- 51.Postigo AA, Dean DC. ZEB, a vertebrate homolog of Drosophila Zfh-1, is a negative regulator of muscle differentiation. EMBO J. 1997;16:3935–3943. doi: 10.1093/emboj/16.13.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sekido R, Murai K, Kamachi Y, Kondoh H. Two mechanisms in the action of repressor δEF1: binding site competition with an activator and active repression. Genes Cells. 1997;2:771–783. doi: 10.1046/j.1365-2443.1997.1570355.x. [DOI] [PubMed] [Google Scholar]
- 53.Postigo AA, Sheppard AM, Mucenski ML, Dean DC. c-Myb and Ets proteins synergize to overcome transcriptional repression by ZEB. EMBO J. 1997;16:3924–3934. doi: 10.1093/emboj/16.13.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Postigo AA, Dean DC. Independent repressor domains in ZEB regulate muscle and T-cell differentiation. Mol Cell Biol. 1999;19:7961–7971. doi: 10.1128/mcb.19.12.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Lee S, Teh CE, Bunting K, Ma L, Shannon MF. The transcription repressor, ZEB1, cooperates with CtBP2 and HDAC1 to suppress IL-2 gene activation in T cells. Int Immunol. 2009;21:227–325. doi: 10.1093/intimm/dxn143. [DOI] [PubMed] [Google Scholar]
- 56.Papadopoulou V, Postigo A, Sánchez-Tilló E, Porter AC, Wagner SD. ZEB1 and CtBP form a repressive complex at a distal promoter element of the BCL6 locus. Biochem J. 2010;427:541–550. doi: 10.1042/BJ20091578. [DOI] [PubMed] [Google Scholar]
- 57.Shimono Y, Murakami H, Kawai K, Wade PA, Shimokata K, Takahashi M. Mi-2 beta associates with BRG1 and RET finger protein at the distinct regions with transcriptional activating and repressing abilities. J Biol Chem. 2003;278:51638–51645. doi: 10.1074/jbc.M309198200. [DOI] [PubMed] [Google Scholar]
- 58.Peña C, Garcia JM, Garcia V, Silva J, Dominguez G, Rodriguez R, Maximiano C, Garcia de Herreros A, Munoz A, Bonilla F. The expression levels of the transcriptional regulators p300 and CtBP modulate the correlations between SNAIL, ZEB1, E-cadherin and vitamin D receptor in human colon carcinomas. Int J Cancer. 2006;119:2098–2104. doi: 10.1002/ijc.22083. [DOI] [PubMed] [Google Scholar]
- 59.Shirakihara T, Horiguchi K, Miyazawa K, Ehata S, Shibata T, Morita I, Miyazono K, Saitoh M. TGF-β regulates isoform switching of FGF receptors and epithelial-mesenchymal transition. EMBO J. 2011;30:783–795. doi: 10.1038/emboj.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kagey MH, Melhuish TA, Wotton D. The polycomb protein Pc2 is a SUMO E3. Cell. 2003;113:127–137. doi: 10.1016/s0092-8674(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 61.Costantino ME, Stearman RP, Smith GE, Darling DS. Cell-specific phosphorylation of Zfhep transcription factor. Biochem Biophys Res Commun. 2002;296:368–373. doi: 10.1016/s0006-291x(02)00880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graham TR, Yacoub R, Taliaferro-Smith L, Osunkoya AO, Odero-Marah VA, Liu T, Kimbro KS, Sharma D, O'Regan RM. Reciprocal regulation of ZEB1 and AR in triple negative breast cancer cells. Breast Cancer Res Treat. 2010;123:139–147. doi: 10.1007/s10549-009-0623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oztas E, Avci ME, Ozcan A, Sayan AE, Tulchinsky E, Yagci T. Novel monoclonal antibodies detect Smad-interacting protein 1 (SIP1) in the cytoplasm of human cells from multiple tumor tissue arrays. Exp Mol Pathol. 2010;89:182–189. doi: 10.1016/j.yexmp.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Acun T, Oztas E, Yagci T, Yakicier MC. SIP1 is downregulated in hepatocellular carcinoma by promoter hypermethylation. BMC Cancer. 2011;11:223. doi: 10.1186/1471-2407-11-223. doi: 10.1186/1471-2407-11-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gemmill RM, Roche J, Potiron VA, Nasarre P, Mitas M, Coldren CD, Helfrich BA, Garrett-Mayer E, Bunn PA, Drabkin HA. ZEB1-responsive genes in non-small cell lung cancer. Cancer Lett. 2011;300:66–78. doi: 10.1016/j.canlet.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grooteclaes ML, Frisch SM. Evidence for a function of CtBP in epithelial gene regulation and anoikis. Oncogene. 2000;19:3823–3828. doi: 10.1038/sj.onc.1203721. [DOI] [PubMed] [Google Scholar]
- 67.Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 68.Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. δEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 69.Liu Y, El-Naggar S, Darling DS, Higashi Y, Dean DC. Zeb1 links epithelial-mesenchymal transition and cellular senescence. Development. 2008;135:579–588. doi: 10.1242/dev.007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 72.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, Settleman J. A gene expression signature associated with ‘K-Ras addiction’ reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15:489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aigner K, Descovich L, Mikula M, Sultan A, Dampier B, Bonné S, van Roy F, Mikulits W, Schreiber M, Brabletz T, Sommergruber W, Schweifer N, Wernitznig A, Beug H, Foisner R, Eger A. The transcription factor ZEB1 (δEF1) represses Plakophilin 3 during human cancer progression. FEBS Lett. 2007;581:1617–1624. doi: 10.1016/j.febslet.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vandewalle C, Comijn J, De Craene B, Vermassen P, Bruyneel E, Andersen H, Tulchinsky E, Van Roy F, Berx G. (SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res. 2005;33:6566–6578. doi: 10.1093/nar/gki965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bindels S, Mestdagt M, Vandewalle C, Jacobs N, Volders L, Noel A, van Roy F, Berx G, Foidart JM, Gilles C. Regulation of vimentin by SIP1 in human epithelial breast tumor cells. Oncogene. 2006;25:4975–4985. doi: 10.1038/sj.onc.1209511. [DOI] [PubMed] [Google Scholar]
- 77.Miyoshi A, Kitajima Y, Sumi K, Sato K, Hagiwara A, Koga Y, Miyazaki K. Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer. 2004;90:1265–1273. doi: 10.1038/sj.bjc.6601685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spaderna S, Schmalhofer O, Hlubek F, Berx G, Eger A, Merkel S, Jung A, Kirchner T, Brabletz T. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology. 2006;131:830–840. doi: 10.1053/j.gastro.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 79.Shirakihara T, Saitoh M, Miyazono K. Differential regulation of epithelial and mesenchymal markers by δEF1 proteins in epithelial mesenchymal transition in- duced by TGF-β. Mol Biol Cell. 2007;18:3533–3544. doi: 10.1091/mbc.E07-03-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-κB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 81.Shin S, Dimitri CA, Yoon SO, Dowdle W, Blenis J. ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol Cell. 2010;38:114–127. doi: 10.1016/j.molcel.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krishnamachary B, Zagzag D, Nagasawa H, Rainey K, Okuyama H, Baek JH, Semenza GL. Hypoxia-inducible factor-1-dependent repression of E-cad- herin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- 83.Christoffersen NR, Silahtaroglu A, Orom UA, Kauppinen S, Lund AH. miR-200b mediates post-transcriptional repression of ZFHX1B. RNA. 2007;13:1172–1178. doi: 10.1261/rna.586807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hurteau GJ, Carlson JA, Spivack SD, Brock GJ. Overexpression of the microRNA hsa-miR-200c leads to reduced expression of transcription factor 8 and increased expression of E-cadherin. Cancer Res. 2007;67:7972–7976. doi: 10.1158/0008-5472.CAN-07-1058. [DOI] [PubMed] [Google Scholar]
- 85.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodal Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 87.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. 2008. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68:7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 90.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Migrating cancer stem cells : an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 92.Spoelstra NS, Manning NG, Higashi Y, Darling D, Singh M, Shroyer KR, Broaddus RR, Horwitz KB, Richer JK. The transcription factor ZEB1 is aberrantly expressed in aggressive uterine cancers. Cancer Res. 2006;66:3893–3902. doi: 10.1158/0008-5472.CAN-05-2881. [DOI] [PubMed] [Google Scholar]
- 93.Dohadwala M, Yang SC, Luo J, Sharma S, Batra RK, Huang M, Lin Y, Goodglick L, Krysan K, Fishbein MC, Hong L, Lai C, Cameron RB, Gemmill RM, Drabkin HA, Dubinett SM. Cyclooxy-genase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional rep-ressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006;66:5338–5345. doi: 10.1158/0008-5472.CAN-05-3635. [DOI] [PubMed] [Google Scholar]
- 94.Singh M, Spoelstra NS, Jean A, Howe E, Torkko KC, Clark HR, Darling DS, Shroyer KR, Horwitz KB, Broaddus RR, Richer JK. ZEB1 expression in type I vs type II endometrial cancers: a marker of aggressive disease. Mod Pathol. 2008;21:912–923. doi: 10.1038/modpathol.2008.82. [DOI] [PubMed] [Google Scholar]
- 95.Graham TR, Zhau HE, Odero-Marah VA, Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW, O'Regan RM. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68:2479–2488. doi: 10.1158/0008-5472.CAN-07-2559. [DOI] [PubMed] [Google Scholar]
- 96.Adachi Y, Takeuchi T, Nagayama T, Ohtsuki Y, Furihata M. Zeb1-mediated T-cadherin repression increases the invasive potential of gallbladder cancer. FEBS Lett. 2009;583:430–436. doi: 10.1016/j.febslet.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 97.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 98.Sayan AE, Griffiths TR, Pal R, Browne GJ, Ruddick A, Yagci T, Edwards R, Mayer NJ, Qazi H, Goyal S, Fernandez S, Straatman K, Jones GD, Bowman KJ, Colquhoun A, Mellon JK, Kriajevska M, Tulchinsky E. SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer. Proc Natl Acad Sci U S A. 2009;106:14884–14889. doi: 10.1073/pnas.0902042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spaderna S, Schmalhofer O, Wahlbuhl M, Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A, Kirchner T, Behrens J, Brabletz T. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68:537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- 100.Elloul S, Elstrand MB, Nesland JM, Trope CG, Kvalheim G, Goldberg I, Reich R, Davidson B. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103:1631–1643. doi: 10.1002/cncr.20946. [DOI] [PubMed] [Google Scholar]
- 101.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 102.Inuzuka T, Tsuda M, Tanaka S, Kawaguchi H, Higashi Y, Ohba Y. Integral role of transcription factor 8 in the negative regulation of tumor angiogenesis. Cancer Res. 2009;69:1678–1684. doi: 10.1158/0008-5472.CAN-08-3620. [DOI] [PubMed] [Google Scholar]
- 103.Goossens S, Janzen V, Bartunkova S, Yokomizo T, Drogat B, Crisan M, Haigh K, Seuntjens E, Umans L, Riedt T, Bogaert P, Haenebalcke L, Berx G, Dzierzak E, Huylebroeck D, Haigh JJ. The EMT regulator Zeb2/Sip1 is essential for murine embryonic hematopoietic stem/ progenitor cell differentiation and mobilization. Blood. 2011;117:5620–5630. doi: 10.1182/blood-2010-08-300236. [DOI] [PubMed] [Google Scholar]
- 104.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 106.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 107.Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, Arendt LM, Kuperwasser C, Bierie B, Weinberg RA. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, Dirbas FM, Somlo G, Pera RA, Lao K, Clarke MF. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Y, Clem B, Zuba-Surma EK, El-Naggar S, Telang S, Jenson AB, Wang Y, Shao H, Ratajczak MZ, Chesney J, Dean DC. Mouse fibroblasts lacking RB1 function form spheres and undergo reprogramming to a cancer stem cell phenotype. Cell Stem Cell. 2009;4:336–347. doi: 10.1016/j.stem.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH, Liu M, Chen CT, Yu D, Hung MC. p53 regulates epithe-lial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol. 2011;13:317–323. doi: 10.1038/ncb2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pinho AV, Rooman I, Real FX. p53-dependent regulation of growth, epithelial-mesenchymal transition and stemness in normal pancreatic epithelial cells. Cell Cycle. 2011;10:1312–1321. doi: 10.4161/cc.10.8.15363. [DOI] [PubMed] [Google Scholar]
- 112.Peter ME. Regulating cancer stem cells the miR way. Cell Stem Cell. 2010;6:4–6. doi: 10.1016/j.stem.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 113.Miquelajauregui A, Van de Putte T, Polyakov A, Nityanandam A, Boppana S, Seuntjens E, Karabinos A, Higashi Y, Huylebroeck D, Tarabykin V. Smad-interacting protein-1 (Zfhx1b) acts upstream of Wnt signaling in the mouse hippocampus and controls its formation. Proc Natl Acad Sci USA. 2007;104:12919–12924. doi: 10.1073/pnas.0609863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mejlvang J, Kriajevska M, Vandewalle C, Chernova T, Sayan AE, Berx G, Mellon JK, Tulchinsky E. Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol Biol Cell. 2007;18:4615–4624. doi: 10.1091/mbc.E07-05-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu Y, Costantino ME, Montoya-Durango D, Higashi Y, Darling DS, Dean DC. The zinc finger transcription factor ZFHX1A is linked to cell proliferation by Rb-E2F1. Biochem J. 2007;408:79–85. doi: 10.1042/BJ20070344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ozturk N, Erdal E, Mumcuoglu M, Akcali KC, Yalcin O, Senturk S, Arslan-Ergul A, Gur B, Yulug I, Cetin-Atalay R, Yakicier C, Yagci T, Tez M, Ozturk M. Reprogramming of replicative senescence in hepatocellular carcinoma-derived cells. Proc Natl Acad Sci U S A. 2006;103:2178–2183. doi: 10.1073/pnas.0510877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68:3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. [DOI] [PubMed] [Google Scholar]
- 118.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, Maestro R, Voeltzel T, Selmi A, Valsesia-Wittmann S, Caron de Fromentel C, Puisieux A. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 120.Liu Y, Dean DC. Tumor initiation via loss of cell contact inhibition versus Ras mutation: do all roads lead to EMT? Cell Cycle. 2010;9:897–900. doi: 10.4161/cc.9.5.10933. [DOI] [PubMed] [Google Scholar]
- 121.Witta SE, Gemmill RM, Hirsch FR, Coldren CD, Hedman K, Ravdel L, Helfrich B, Dziadziuszko R, Chan DC, Sugita M, Chan Z, Baron A, Franklin W, Drabkin HA, Girard L, Gazdar AF, Minna JD, Bunn PA., Jr Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 2006;66:944–950. doi: 10.1158/0008-5472.CAN-05-1988. [DOI] [PubMed] [Google Scholar]
- 122.Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, Ali S, Abbruzzese JL, Gallick GE, Sarkar FH. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–2407. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haddad Y, Choi W, McConkey DJ. Delta-crystallin enhancer binding factor 1 controls the epithelial to mesenchymal transition phenotype and resistance to the epidermal growth factor receptor inhibitor erlotinib in human head and neck squamous cell carcinoma lines. Clin Cancer Res. 2009;15:532–542. doi: 10.1158/1078-0432.CCR-08-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tryndyak VP, Beland FA, Pogribny IP. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int J Cancer. 2010;126:2575–2583. doi: 10.1002/ijc.24972. [DOI] [PubMed] [Google Scholar]
- 126.Fontemaggi G, Gurtner A, Damalas A, Costanzo A, Higashi Y, Sacchi A, Strano S, Piaggio G, Blandino G. δEF1 repressor controls selectively p53 family members during differentiation. Oncogene. 2005;24:7273–7280. doi: 10.1038/sj.onc.1208891. [DOI] [PubMed] [Google Scholar]
- 127.Bui T, Sequeira J, Wen TC, Sola A, Higashi Y, Kondoh H, Genetta T. ZEB1 Links p63 and p73 in a Novel Neuronal Survival Pathway Rapidly Induced in Response to Cortical Ischemia. PLoS ONE. 2009;4:e4373. doi: 10.1371/journal.pone.0004373. doi: 10.1371/journal.pone.0004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vega S, Morales AS, Ocaña OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes & Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;7:2059–2068. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 130.Higashikawa K, Yoneda S, Tobiume K, Taki M, Shigeishi H, Kamata N. Snail-induced down-regulation of ΔNp63α acquires invasive phenotype of human squamous cell carcinoma. Cancer Res. 2007;67:9207–9213. doi: 10.1158/0008-5472.CAN-07-0932. [DOI] [PubMed] [Google Scholar]
- 131.Stasinopoulos IA, Mironchik Y, Raman A, Wildes F, Winnard P, Raman V. HOXA5-Twist interaction alters p53 homeostasis in breast cancer cells. J Biol Chem. 2005;280:2294–2299. doi: 10.1074/jbc.M411018200. [DOI] [PubMed] [Google Scholar]
- 132.Shiota M, Izumi H, Onitsuka T, Miyamoto N, Kashiwagi E, Kidani A, Hirano G, Takahashi M, Naito S, Kohno K. Twist and p53 reciprocally regulate target genes via direct interaction. Oncogene. 2008;27:5543–5553. doi: 10.1038/onc.2008.176. [DOI] [PubMed] [Google Scholar]