Abstract
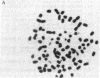
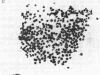
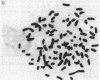
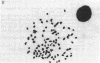
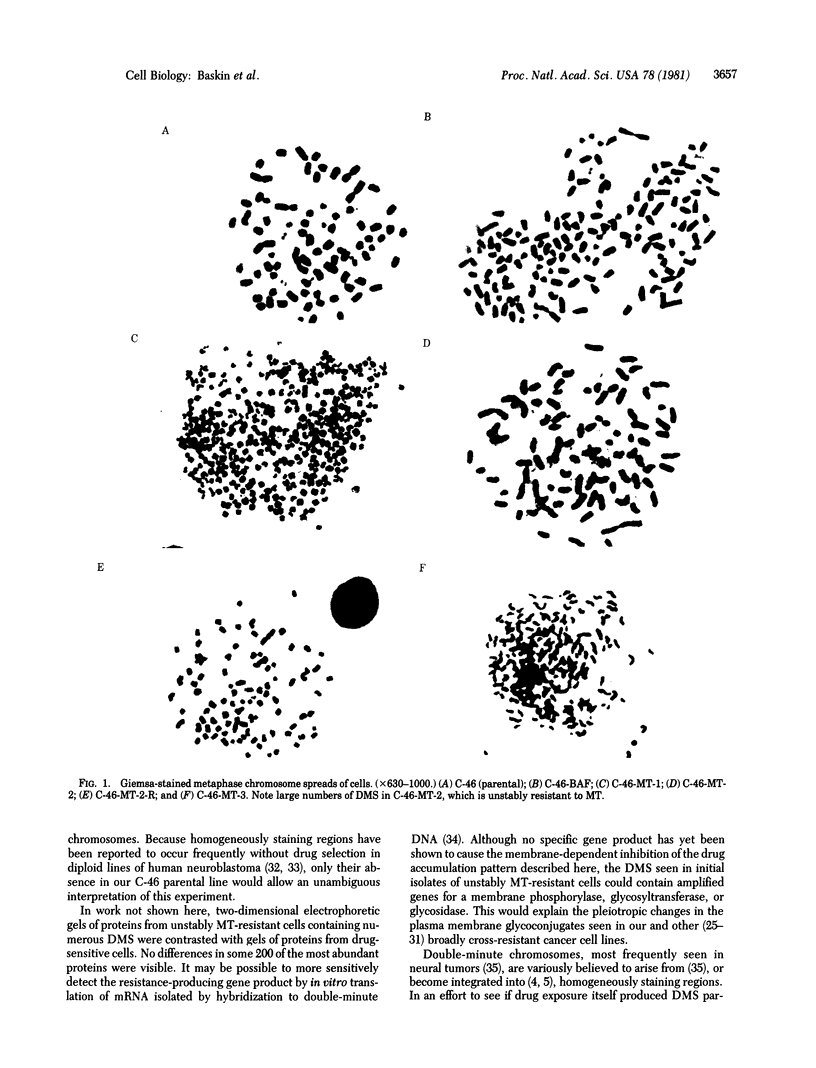
A series of increasingly drug-resistant cell populations were selected and cloned from C-46 murine neuroblastoma with the chemotherapeutic drugs maytansine, vincristine, adriamycin, or Baker's antifol. All clones demonstrated reciprocal cross-resistance to these structurally and functionally diverse drugs and failed to accumulate radiolabeled vincristine, colchicine, or Baker's antifol despite normal drug binding to cell homogenates. Initial isolates of drug-resistant populations were genetically unstable, rapidly reverting to a drug-sensitive phenotype when grown without drug, at 0.05 reversion per cell division. After prolonged growth in drug, this drug-resistant genotype stabilized. Mean chromosome number increased 300% in an initially isolated 20-fold maytansine-resistant clone, which also displayed numerous double-minute chromosomes. Descendants 240-fold more resistant than the parent, also unstable, possessed the wild-type complement of 80 chromosomes, but 45% of these cells possessed 24 double-minute chromosomes per cell; such chromosomes were absent from the drug-sensitive parental clone. Only 1.0 and 1.2 double-minute chromosomes per cell were seen in a 7-fold stably resistant revertant or 1200-fold stably resistant descendants, respectively. Double-minute chromosomes containing amplified genes for the drug target dihydrofolate reductase (tetrahydrofolate dehydrogenase; 5,6,7,8-tetrahydrofolate:NADP+ oxidoreductase, EC 1.5.1.3) have been reported in an unstable methotrexate-resistant R1-A sarcoma. These extrachromosomal gene copies were absent in stably resistant progeny. The presence of similar particles in unstably drug-resistant uptake mutants of neuroblastoma and their diminution in stably resistant descendants supports and extends their possible role in the rapid onset and instability of epigenetic drug resistance in cancer chemotherapy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich C. D. Pleiotropic phenotype of cultured murine cells resistant to maytansine, vincristine, colchicine, and adriamycin. J Natl Cancer Inst. 1979 Sep;63(3):751–757. doi: 10.1093/jnci/63.3.751. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Baker B. R., Ashton W. T. Irreversible enzyme inhibitors. 196. Active-site-directed irreversible inhibitors of dihydrofolate reductase derived from 1-(4-benzyloxy-3-chlorophenyl)-4,6-diamino-1,2-dihydro-2,2-dimethyl-s-triazine and bearing a terminal phenyl sulfonate group. J Med Chem. 1972 Sep;15(9):945–947. doi: 10.1021/jm00279a017. [DOI] [PubMed] [Google Scholar]
- Baskin F., Carlin S. C., Kraus P., Friedkin M., Rosenberg R. N. Experimental chemotherapy of neuroblastoma. II. Increased thymidylate synthetase activity in a 5-fluorodeoxyuridine-resistant variant of mouse neuroblastoma. Mol Pharmacol. 1975 Jan;11(1):105–117. [PubMed] [Google Scholar]
- Baskin F., Davis R., Rosenberg R. N. Altered thymidine kinase or thymidylate synthetase activities in 5-fluoro deoxyuridine resistant variants of mouse neuroblastoma. J Neurochem. 1977 Dec;29(6):1031–1037. doi: 10.1111/j.1471-4159.1977.tb06506.x. [DOI] [PubMed] [Google Scholar]
- Baskin F., Rosenberg R. N. A comparison of thymidylate synthetase activities from 5-fluorodeoxyuridine sensitive and resistant variants of mouse neuroblastoma. J Neurochem. 1975 Sep;25(3):233–238. doi: 10.1111/j.1471-4159.1975.tb06958.x. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Spengler B. A. A novel chromosome abnormality in human neuroblastoma and antifolate-resistant Chinese hamster cell lives in culture. J Natl Cancer Inst. 1976 Sep;57(3):683–695. doi: 10.1093/jnci/57.3.683. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Mechanism of cellular drug resistance. Nature. 1971 Oct 22;233(5321):566–569. doi: 10.1038/233566a0. [DOI] [PubMed] [Google Scholar]
- Carlin S. C., Rosenberg R. N., VandeVenter L., Friedkin M. Quinazoline antifolates as inhibitors of growth, dihydrofolate reductase, and thymidylate synthetase of mouse neuroblastoma cells in culture. Mol Pharmacol. 1974 Mar;10(2):194–203. [PubMed] [Google Scholar]
- Carlsen S. A., Till J. E., Ling V. Modulation of membrane drug permeability in Chinese hamster ovary cells. Biochim Biophys Acta. 1976 Dec 14;455(3):900–912. doi: 10.1016/0005-2736(76)90059-6. [DOI] [PubMed] [Google Scholar]
- Carlsen S. V., Till J. E., Ling V. Modulation of drug permeability in Chinese hamster ovary cells. Possible role for phosphorylation of surface glycoproteins. Biochim Biophys Acta. 1977 Jun 2;467(2):238–250. doi: 10.1016/0005-2736(77)90199-7. [DOI] [PubMed] [Google Scholar]
- Chang S. E., Littlefield J. W. Elevated dihydrofolate reductase messenger RNA levels in methotrexate-resistant BHK cells. Cell. 1976 Mar;7(3):391–396. doi: 10.1016/0092-8674(76)90168-9. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D., Robins A. B. Loss of resistance to methotrexate in L5178Y mouse leukemia grown in vitro. J Natl Cancer Inst. 1972 Jul;49(1):45–53. [PubMed] [Google Scholar]
- Cremisi C., Sonenshein G. E., Tournier P. Studies on the mechanism of actinomycin D resistance of an SV40-transformed hamster cell line. Exp Cell Res. 1974 Nov;89(1):89–94. doi: 10.1016/0014-4827(74)90190-6. [DOI] [PubMed] [Google Scholar]
- Dano K. Cross resistance between vinca alkaloids and anthracyclines in Ehrlich ascites tumor in vivo. Cancer Chemother Rep. 1972 Dec;56(6):701–708. [PubMed] [Google Scholar]
- Degnen G. E., Miller I. L., Adelberg E. A., Eisenstadt J. M. Overexpression of an unstably inherited gene in cultured mouse cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3956–3959. doi: 10.1073/pnas.74.9.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flintoff W. F., Davidson S. V., Siminovitch L. Isolation and partial characterization of three methotrexate-resistant phenotypes from Chinese hamster ovary cells. Somatic Cell Genet. 1976 May;2(3):245–261. doi: 10.1007/BF01538963. [DOI] [PubMed] [Google Scholar]
- HAKALA M. T., ZAKRZEWSKI S. F., NICHOL C. A. Relation of folic acid reductase to amethopterin resistance in cultured mammalian cells. J Biol Chem. 1961 Mar;236:952–958. [PubMed] [Google Scholar]
- Inaba M., Johnson R. K. Uptake and retention of adriamycin and daunorubicin by sensitive and anthracycline-resistant sublines of P388 leukemia. Biochem Pharmacol. 1978;27(17):2123–2130. doi: 10.1016/0006-2952(78)90284-8. [DOI] [PubMed] [Google Scholar]
- Johnson R. K., Chitnis M. P., Embrey W. M., Gregory E. B. In vivo characteristics of resistance and cross-resistance of an adriamycin-resistant subline of P388 leukemia. Cancer Treat Rep. 1978 Oct;62(10):1535–1547. [PubMed] [Google Scholar]
- Kaufman R. J., Brown P. C., Schimke R. T. Amplified dihydrofolate reductase genes in unstably methotrexate-resistant cells are associated with double minute chromosomes. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5669–5673. doi: 10.1073/pnas.76.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye S. B., Boden J. A. Cross resistance between actinomycin-D, adriamycin and vincristine in a murine solid tumour in vivo. Biochem Pharmacol. 1980 Apr 1;29(7):1081–1084. doi: 10.1016/0006-2952(80)90176-8. [DOI] [PubMed] [Google Scholar]
- Kessel D. Enhanced glycosylation induced by adriamycin. Mol Pharmacol. 1979 Jul;16(1):306–312. [PubMed] [Google Scholar]
- Peterson R. H., Beutler W. J., Biedler J. L. Ganglioside composition of malignant and actinomycin D-resistant nonmalignant Chinese hamster cells. Biochem Pharmacol. 1979 Mar 1;28(5):579–582. doi: 10.1016/0006-2952(79)90138-2. [DOI] [PubMed] [Google Scholar]
- Rank G. H., Robertson A. J. The viscosity and lipid composition of the plasma membrane of multiple drug resistant and sensitive yeast strains. Can J Biochem. 1978 Nov;56(11):1036–1041. doi: 10.1139/o78-163. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Kaufman R. J., Alt F. W., Kellems R. F. Gene amplification and drug resistance in cultured murine cells. Science. 1978 Dec 8;202(4372):1051–1055. doi: 10.1126/science.715457. [DOI] [PubMed] [Google Scholar]
- Sherline P., Bodwin C. K., Kipnis D. M. A new colchicine binding assay for tubulin. Anal Biochem. 1974 Dec;62(2):400–407. doi: 10.1016/0003-2697(74)90172-9. [DOI] [PubMed] [Google Scholar]
- Sinha B. K., Sik R. H. Binding of [14C]-adriamycin to cellular macromolecules in vivo. Biochem Pharmacol. 1980 Jun 15;29(12):1867–1868. doi: 10.1016/0006-2952(80)90156-2. [DOI] [PubMed] [Google Scholar]
- Skovsgaard T. Transport and binding of daunorubicin, adriamycin, and rubidazone in Ehrlich ascites tumour cells. Biochem Pharmacol. 1977 Feb 1;26(3):215–222. doi: 10.1016/0006-2952(77)90306-9. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Wilson L., Bamburg J. R., Mizel S. B., Grisham L. M., Creswell K. M. Interaction of drugs with microtubule proteins. Fed Proc. 1974 Feb;33(2):158–166. [PubMed] [Google Scholar]
- Woodcock D. M., Cooper I. A. Aberrant double replication of segments of chromosomal DNA following DNA synthesis inhibition by cytosine arabinoside. Exp Cell Res. 1979 Oct 1;123(1):157–166. doi: 10.1016/0014-4827(79)90432-4. [DOI] [PubMed] [Google Scholar]