Abstract
Following the onset of sensorineural hearing loss, degeneration of mechanosensitive hair cells and spiral ganglion cells (SGCs) in humans and animals occurs to variable degrees, with a trend for greater neural degeneration with greater duration of deafness. Emergence of the cochlear implant prosthesis has provided much needed aid to many hearing impaired patients and has become a well-recognized therapy worldwide. However, ongoing peripheral nerve fiber regression and subsequent degeneration of SGC bodies can reduce the neural targets of cochlear implant stimulation and diminish its function. There is increasing interest in bio-engineering approaches that aim to enhance cochlear implant efficacy by preventing SGC body degeneration and/or regenerating peripheral nerve fibers into the deaf sensory epithelium. We review the advancements inmaintaining and regeneratingnerves indamaged animal cochleae, with an emphasis on the therapeutic capacity of neurotrophic factorsdelivered to the inner ear after an insult. Additionally, we summarize the histological process of neuronal degeneration in the inner ear and describe different animal models that have been employed to study this mechanism. Research on enhancing the biological infrastructure of the deafened cochlea in order to improve cochlear implant efficacy is of immediate clinical importance.
Keywords: Nerve regeneration, Neurotrophic factors, BDNF, NTF-3, Cochlear implant, Spiral ganglion cell
1. Introduction
Injury or loss of sensory cells in the peripheral nervous system (PNS) results in detachment and withdrawal of nerve endings, also known as Wallerian degeneration. The soma of the detached neurons may survive, and at times, reconnect with a new target by regenerative growth of nerve fibers. During this process, regrowth of a single axon occurs spontaneously in the PNS at a slow rate of 1mm/day (Schnell et al., 1994). Regenerated axons or dendrites reinnervate either a new target or their original target, leading to partial to complete recovery of function (Brushart et al., 1998; Fu et al., 1997; Soileau et al., 1987). The regenerative response of nerve fibers varies between species and types of injury (Torvik et al., 1975). The molecules that participate in facilitating or inhibiting nerve fiberregeneration have been characterized in both the PNS and the central nervous system (CNS) (Bomze et al., 2001; Caroni et al., 1988a; Caroni et al., 1988b; Savio et al., 1989; Schwab et al., 2005; Schwab et al., 1985). Knowledge of these molecular players helps in designing therapeutic approaches to initiate or enhance nerve regeneration in many tissues, including the inner ear.
Several pathologies in the inner ear involve degeneration of auditory nerve fibers from the area of the sensory epithelium. This usually is due to loss of the target, the inner hair cell. In some cases, degeneration of the spiral ganglion cell (SGC) bodies ensues. With the loss of these bipolar neurons responsible of conveying the sensory signals produced by hair cells into the brain, the ability to treat deafness with a cochlear implant is diminished. One way to enhance the benefits derived from the cochlear implant is to maximize the physiological condition of remaining SGCs. An alternative therapeutic goal is to induce regeneration of nerve fibers back into the basilar membrane area (BMA). Such regeneration could have several important benefits: enhancing function of the cochlear implant due to greater proximity of the nerve endings to the electrodes, indirectly improving the physiological status of the SGCs, and providing a neural substrate for future therapies such as hair cellregeneration via transdifferentiation or stem cell implantation. This chapter discusses nerve regeneration in the traumatized cochlea with emphasis on two main strategies of improving the efficacy of the cochlear prosthesis: (a) development of preventive therapy to preserve the SGC population, and (b) bio-engineering approaches which could induce axonal regrowth of the peripheral nerve fibers and increase their proximity to the electrode. This would potentially improve the sensitivity to electrical stimulation, channel selectivity and even temporal properties of cochlear implant stimulation.
2. Rationale/impetus for enhancing SGC survival and inducing nerve fiber regeneration
Despite significant advances in cochlear implant outcomes, the rate of improvement has slowed over the past decade(O’Leary et al., 2009), and there remain limitations in the benefit of cochlear implantation, particularly in restoring hearing in noise and in music appreciation. The concept behind the design of multichannel cochlear implants is the stimulation of a discrete neural population by each electrode along the length of the cochlea, allowing the specific and tonotopic stimulation of the auditory nerve fibers and spatial separation of frequency-specific temporal envelope information. If each electrode stimulates a specific and discrete neural population, each electrode should correspond to a specific sound percept and render a distinct perceptual channel (O’Leary et al., 2009). Currently, despite most devices having over 20 electrodes in the array, the actual number of perceptual channels achieved is closer to 8 (Friesen et al., 2001). This is likely due to current spread between electrodes and longitudinally along the cochlea via the perilymph, thereby causing non-specific stimulation of neurons adjacent to the target neuronal population. A greater number of effective perceptual channels are needed: as many as 20 to hear well in noise (Dorman et al., 1998) and over 30 to appreciate music and lexical tone (Kong et al., 2004; Xu et al., 2003). Not only are a greater number of perceptual channels necessary to appreciate music, but also they must function according to a specific and finely tuned temporal and spectral scale (Kong et al., 2004). In order to accomplish this, the implant’s electrode array must stimulate a more discrete population of auditory neuronal fibers, and it must be able to do so in a way that retains the fine spatiotemporal cues of the original acoustic signal.
Various methods of accomplishing this goalhave been proposed, including bringing the electrodes and peripheral neuronal elements into closer approximation. The greater the distance of the electrode from the target neural element, the greater current is needed to activate the target. With greater current, there is greater spread of the electric signal via the surrounding perilymph (Snyder et al., 2008); thus, to avoid overlapping signals, fewer more widely spaced electrodes must be used. In addition, because current spreading also causes the strength of the signal to decay exponentially as a function of distance (Black et al., 1981; Black et al., 1983), more power must be used to stimulate those broader, more distant targets. If electrodes are brought into closer contact with the neural elements, a smaller current is needed to activate the neurons, and less current spread will occur (Snyder et al., 2008). Presently, this goal is accomplished by the use of perimodiolar electrode arrays. These arrays are designed such that, rather than lying along the outer cochlear bony wall as with traditional straight arrays, they curve to approximate the modiolus. Furthermore, the electrode contacts are isolated to the medial surface of the array. Studies of perimodiolar devices have demonstrated a significantly lower threshold, increased dynamic range, increased specificity of neural excitation, and a maintained tonotopic map along the length of the array (Briaire et al., 2006; Cohen et al., 2006; Frijns et al., 2001; Snyder et al., 2008). However, these improvements have not translated to improved speech perception outcomes (Cohen et al., 2003; Cohen et al., 2005; Fitzgerald et al., 2007; Hughes et al., 2006), let alone improved music appreciation abilities.
One potential limitation of perimodiolar arrays, as demonstrated in computational models of the human cochlea, is the potential to stimulate across cochlear turns in the apex, leading to a false pitch percept (Briaire et al., 2006; Frijns et al., 2001), although little evidence of this has been found in human perimodiolar implant recipients (Cohen et al., 2006). Additional possible reasons for the limited efficacy of the perimodiolar array in eliciting improved speech perception is that the electrodes may still be too far from their targets and there is still significant volume of intervening perilymph allowing the longitudinal spread of current (O’Leary et al., 2009). Neuronal fiber regeneration may bring the target peripheral fibers in closer proximity to the electrode, thereby allowing more specific neural activation at lower thresholds (Staecker et al., 2010).
3. Normal innervation of the cochlea
The sensory epithelium of the inner ear is composed by a mosaic of sensory and non-sensory cells. Sensory hair cells transduce vibratory mechanical energy into neuronal impulses which are then conveyed by SGCs to the brain and produce auditory perception. SGCs are bipolar nerve cells with central axons that connect with the auditory brain stem and peripheral fibers that synapse with the hair cells (Fig. 1A) (Anniko, 1983; Raphael et al., 2003; Spoendlin, 1981; Webster et al., 1981). The larger Type I afferents synapse with inner hair cells and the smaller Type II afferents synapse with outer hair cells (Kiang et al., 1982; Liberman et al., 1990; Spoendlin, 1969). Efferent peripheral nerve fibers arise from the lateral superior olive in the auditory brain stem (Warr et al., 1979). Efferent fibers synapse with outer hair cells and with Type I afferent nerve fibers (before they reach the inner hair cells) (Liberman, 1980; Liberman et al., 1986; Smith et al., 1963). Myelination of SGCs and nerve fibers is provided by Schwanncells. The extent and areas of myelin presence vary between species (Romand et al., 1990; Romand et al., 1982). In the osseous spiral lamina, one Schwann cell encloses each nerve fiber, but afferent nerves lose their myelin sheaths just before entering the habenula perforata. Once in the habenula perforata, extensions of a single satellite cell surround a nerve axon. Past the habenula, axons continue as unmyelinated fibers (Spoendlin, 1969). It is possible that maintenance and support of these fibers within the auditory epithelium is provided by the supporting cells in the sensory epithelium (Spoendlin, 1966).
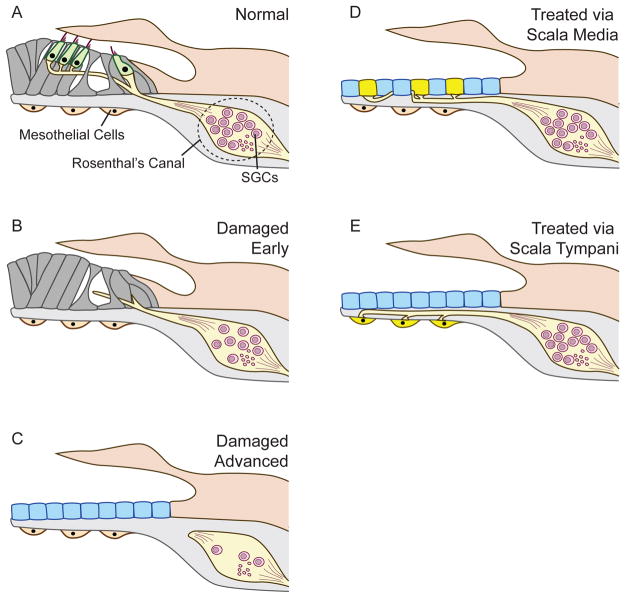
Figure 1. Schematic figures depictingauditory epithelium and nerve in normal versus deafened organ of Corti (A through C) and Ad. neurotrophin treated auditory epithelium via scala media (D) or scala tympani (E).
Inner and outer hair cells are innervated by peripheral nerve fibers extending from the SGC in the Rosenthal’s canal (A). Shortly after an insult, the hair cells have degenerated and supporting cells form pharyngeal scars. The peripheral nerve fibers become disorganized and regress back to the SGC soma. SGC loss begins subsequently (B). In severe cases, the organ of Corti is replaced by single layer of cuboidal cells, the “flat epithelium”. The peripheral nerve fibers have regressed completely and SGC loss is more substantial (C). Treatment of the flat epithelium treated with viral vectors carrying neurotrophic factors via scala media leads to secretion of neurotrophins by cells of the flat epithelium. Nerve fibers re-sprout and grow towards the source of the neurotrophin, such that the epithelial cells serve as hot spots and attract peripheral nerve fibers. SGC survival is also enhanced (D). When the neurotrophin expressing viral vector is placed in scala tympani, the mesothelial cells are transduced and serve as hot spots that attract peripheral fibers to the basilar membrane area (E).
4. Hair cell loss and SGC degeneration
Various etiologies, hereditary, environmental or combined, can result in sensorineural hearing loss. Regardless of the causes of sensorineural hearing loss, the typical pathological outcome usually involves hair cell degeneration. Once lost, hair cells in the mammalian cochlea are not spontaneously replaced (Hawkins, 1973; Roberson et al., 1994). Once hair cells are lost, they are replaced by phalangeal scars generated by supporting cells (Forge, 1985; Raphael et al., 1991).
With the degeneration of the hair cells, the afferent fibers lose their target and eventually start to die back (Fig. 1B). Their regression leaves the SGCs with a short peripheral process that does not reach the BMA. In some cases, the peripheral process dies back all the way to the SGC soma leaving the cell body and central process intact and functional (Hardie et al., 1999; Spoendlin, 1975). In addition to regression from the BMA nerve fibers usually also undergo demyelination in the osseous spiral lamina, starting from the distal end (Dodson et al., 2000; Lawner et al., 1997b). The central connections of the SGCs in the cochlear nucleus are maintained, although the characteristics of this connection change in deafened ears (Bledsoe et al., 1995; Ryugo et al., 1998; Zeng et al., 2009).
The SGC bodies can survive for many years in human ears devoid of hair cells, although degeneration can take place at a slow or even rapid pace. In animal models of severe hair cell lesions, SGCs degenerate in a faster pace than in human ears. The exact mechanism of SGC death is not fully understood, although earlier SGCs death after intracochlear injection of gentamicin is thought to be caused by glutamate toxicity (Dodson, 1997), and later SGC degeneration may be due to depletion of neurotrophic factors or lack of activity in the SGC, which normally would be provided by the hair cells. Early changes in the synaptic region after noise exposure, observed without hair cell loss, may also result on SGC degeneration (Kujawa et al., 2009). Studies detecting the precise signaling cascades that promote SGC injury and death will be valuable for future therapies that enhance SGC survival.
5. Animal models for auditory nerve degeneration and regeneration
Examining the pathological process of auditory nerve degeneration requires a variety of models that encompass the different conditions and time points of auditory nerve degeneration. Both moderate and severe SGC lesions in animal models can be created by systemic or local application of various ototoxic reagents, including but not limited to aminoglycosides, loop diuretics, carboplatin, and ouabain. Aminoglycosides can cause partial to complete degeneration of the organ of Corti and secondary degeneration of the SGCs (Johnsson, 1974; Leake et al., 1988). Systemic application of kanamycin and ethacrynic acid or local application of neomycin via perilymph injection can cause severe hair cell and SGC lesions (Raphael et al., 1991; Shibata et al., 2010; Wise et al., 2010). Carboplatin can induce a lesion restricted to inner hair cells in chinchilla (Sugawara et al., 2005; Wang et al., 2003). Ouabain, a cardiac glycoside, attenuates the action of Na+-K+ ATPase and leads to selective degeneration Type I SGCs in gerbils and mice (Lang et al., 2010; Matsuoka et al., 2007; Schmiedt et al., 2002). Mechanical damage caused by traumatic acoustic overstimulation can create a varying degree of hair cell and/or nerve degeneration (Abrashkin et al., 2006; Bohne et al., 2000; Borg et al., 1983). Axotomy of the VIIIth nerve in the inner auditory meatus causes a secondary degeneration of the majority of the afferent nerve fibers and most of the efferent nerve fibers (Spoendlin, 1979). Sectioning the nerve fibers at the osseous spiral lamina can cause degeneration of auditory nerve fibers with an intact organ of Corti (Spoendlin, 1979; Sugawara et al., 2005).
If the insult is mild or recent, the supporting cells can retain their normal morphological characters. In cases where the insult is severe or a prolonged period of time has passed since the insult, the supporting cells may degenerate as well and form or are replaced by a flat layer of polymorphic cuboidal cells, which we refer to as the “flat epithelium” (Fig. 1C) (Kim et al., 2007). The flat epithelium can be observed frequently as the outcome of severe hearing loss caused by various etiologies including hereditary deafness (Pawlowski et al., 2006), aminoglycoside insult in guinea pigs (Izumikawa et al., 2008; Shibata et al., 2010), and in humans with prolonged history of deafness (Nadol et al., 2006). This highly degenerated condition of sensory epithelium that remains in the deaf ears will likely be the substrate to first receive any of the future therapies.
The time course of secondary neuronal degeneration subsequent to hair cell loss appears to vary between species. In mice, rat, and guinea pigs the loss of SGCs can occur in weeks or months (Bichler et al., 1983; Dodson et al., 2000; Staecker et al., 1998) while in humans the process is much slower and can take years (Nadol, 1997; Nadol et al., 1989). Postmortem human temporal bone studies have shown that in some cases, many years after a severe cochlear lesion leaving a flat epithelium, the SGCs are still intact in both numbers and morphology (Nadol et al., 2006; Nadol et al., 1989). It is not clear whether this is a pure species-dependent phenomenon or a manifestation of the sub-optimal animal models where the initial lesion is often very severe. Currently there is no ideal animal model which would mimic the same degeneration process seen in humans. As we argued in our KHRI 50th anniversary paper written by Dr. Pfingst, perhaps the histological picture we create by deafening and providing neurotrophins (with better SGC survival) will more closely resemble human cochlear implant users, especially now that guidelines for cochlear implant use have broadened.
Supporting cells can play an important role in the survival of peripheral nerve fibers. In a lesion where the supporting cells remain, degeneration of SGCs may proceed much slower than in the absence of supporting cells (Sugawara et al., 2005). These results suggest that neuronal degeneration may be a complex process that depends on more factors than just hair cell survival. In addition to supporting cells, the roles of other non-sensory cells in the inner ear should be assessed, including the mesothelium and Schwann cells.
The survival of SGCs and other morphological features related to SGC survival has been correlated with several measures of cochlear implant function. Thus, maintaining healthy and functional population of SGC bodies and associated structures would seem to be crucial for the performance of the cochlear implant (Chikar et al., 2008; Hartshorn et al., 1991; Kang et al., 2010; Pfingst et al., 1981; Shepherd et al., 1997). As such, studies which investigate the factors that contribute to slowing down or preventing SGC degeneration are likely to enhance outcome of the cochlear implant therapy in the future. The functional outcomes of enhanced SGC survival on cochlear implant stimulation in animals are discussed in detail in the review by Pfingst et al., in this issue of Hearing Research.
6. Spontaneous regeneration of the cochlear nerve
Spontaneous resprouting of SGC nerve fibers has been seen following the induction of various inner ear lesions, including severing of the eighth cranial nerve at the internal acoustic meatus (Spoendlin et al., 1976), aminoglycoside toxicity (Johnsson et al., 1972; Terayama et al., 1977; Terayama et al., 1979; Webster et al., 1982), and noise induced hearing loss (Bohne et al., 1992; Lawner et al., 1997a; Strominger et al., 1995). After severing the eighth cranial nerve there is initially a near total loss of SGCs and their associated peripheral processes (Spoendlin et al., 1976). However, months following this injury Spoendlin and Suter found many new large fibers in the region between the habenula perforata and inner hair cells. They noted that this occurred in the absence of a corresponding increase in SGC bodies and concluded that these new fibers were likely sprouting from the few surviving neurons (Spoendlin et al., 1976).
Regenerated nerve fibers were found in the auditory epithelium in chinchillas exposed to a severe acoustic trauma, leading to elimination of hair cells and damage to supporting cells (Bohne et al., 1992; Lawner et al., 1997a; Strominger et al., 1995). Presence of relatively well preserved areas of the organ of Corti adjacent to the focal lesion appears to correlate with the presence of regenerated fibers. The spontaneous regeneration of nerve fibers in the traumatized cochleae was limited to the area adjacent to the habenula perforata (Bohne et al., 1992; Lawner et al., 1997a; Spoendlin et al., 1976; Terayama et al., 1977; Terayama et al., 1979). Similarly, more recent studies of the guinea pig ear following neomycin deafening demonstrated that a few looping neuronal fibers remain in the BMA close to the habenula perforata (Fig. 2A) (Shibata et al., 2010). The looping fibers exit and return back to the habenula perforata while weaving their way between the cuboidal cells of the flat epithelium. Similar results have been observed in other reports in the deafened inner ear (Wise et al., 2005). These results suggest that the nerve fibers may be sensitive to gradients of neurotrophin concentrations in the flat epithelium. This lack of significant nerve fiber regeneration in the weeks following aminoglycoside toxicity is likely related to the complete absence of hair cells and supporting cells to provide structural and neurotrophic support to the residual SGCs. In the longer term, most SGCs in severely deafened animals degenerate so fiber regeneration is not expected to occur.
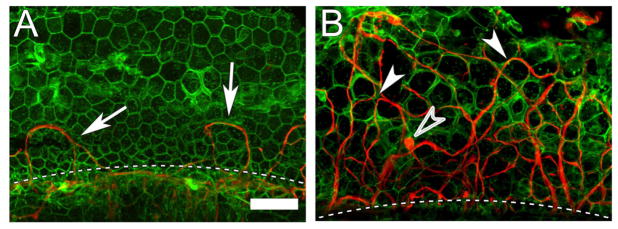
Figure 2. Whole mount of a flat epithelium of a guinea pig cochlea with (B) or withoutAd.BDNFtreatment (A), stained for actin (phalloidin, green) and neurofilaments (red).
The flat epithelium, in the area where the organ of Corti used to reside, is composed of polymorphic cuboidal cells. The intercellular adherens junctions are actin rich. Nerve fibers are mostly absent, except for a few looping fibers (arrow) next to the habenula perforata (dashed line) (A). In a cochlea with flat epithelium treated with Ad.BDNFa large number of re-grown peripheral nerve fibers is present. The fibers appear to traverse the epithelium between the cuboidal cells (arrowheads). Fibers are of different diameter and their orientation in the flat epithelium varies from longitudinal to radial. Some bulging regions that resemble terminals are seen (open arrowhead) (B). Scale bar = 50 μm, for (A) and (B).
7. Neurotrophic factors for inner ear treatment
Different molecules have been employed to prevent SGC degeneration after an insult. Neurotrophins, glia derived neurotrophic factor (GDNF), and fibroblast growth factors (FGF) were found to be potent. Neurotrophins play multiple roles in the mammalian CNS and PNS, such as promotion of cell survival, neurogenesis, maintenance of neurons and synaptogenesis. The family of neurotrophins includes nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3), and neurotrophin 4 (NT-4). Neurotrophin function is regulated by intercellular signaling pathways which are activated by the membrane receptors which include the tyrosine kinase receptor family (TrkA, B, and C) or p75 neurotrophin receptors. During inner ear development, BDNF and NT-3 and their respective receptors TrkB and C are necessary for the differentiation and survival of the cochlea and vestibular neurons. BDNF and/or NT-3 are secreted from hair cells and are thought to play a crucial role in synaptogenesis of these cells (Despres et al., 1994; Medd et al., 2000). BDNF or NT-3 knockout mice exhibit partial or complete loss of afferent and efferent neurons in the cochlea (Ernfors et al., 1995; Fritzsch et al., 1997; Fritzsch et al., 2004). Additionally, neurotrophins have been employed in regenerative studies in other systems of the CNS and PNS (Bellamkonda, 2006; Di Polo et al., 1998; Tuszynski et al., 2005). Therefore, attempting to deliver neurotrophins into the inner ear fluids to prevent SGC degeneration was a logical choice. Indeed, exogenous application of neurotrophins (e.g. BDNF, NGF and NT-3) into the cochlear fluids have proven to be beneficial in neuro-protection against insults (Glueckert et al., 2008; Hildebrand et al., 2008; Kanzaki et al., 2002; Nakaizumi et al., 2004; Staecker et al., 1996; Wise et al., 2005). GDNF and FGF have been demonstrated to be equally neuro-protective in the inner ear (Glueckert et al., 2008; Shibata et al., 2007; Yagi et al., 1999).
Delivery of trophic factors such as neurotrophins into the inner ear has been accomplished by several methods. Studies have shown enhanced survival of neurons by delivering neurotrophins via mini-osmotic pumps (Glueckert et al., 2008; Miller et al., 1997; Wise et al., 2005) or employing forced expression of neurotrophins by introducing the genes for these growth factors into cells that line the perilymph or endolymph (Bowers et al., 2002; Miller et al., 1997; Nakaizumi et al., 2004; Shibata et al., 2010; Staecker et al., 1998; Wise et al., 2010). Both methods have been shown to significantly improve SGC survival against ototoxic drugs or traumatic noise exposure. However, SGC degeneration is thought to be a continuous process after an initial insult, thus, sustained levels of neurotrophins are required throughout life. The withdrawal of exogenous neurotrophins can lead to a rapid degeneration of SGCs (Gillespie et al., 2003; Shepherd et al., 2008). However, when brief electrical stimulation for eABR recording is provided, the effects of neurotrophin withdrawal are less traumatizing (Agterberg et al., 2009). Thus, selecting the method for delivery of neurotrophins may depend on additional considerations and improvements of the technology.
Several lines of evidence have shown that electrical stimulation can promote enhanced spiral ganglion survival (Miller, 2001; Miller et al., 1995; Mitchell et al., 1997) and thus when combined with cochlear implant, a sustained application of neurotrophins may not be required, although this notion is still controversial (Agterberg et al., 2010; Chen et al., 2010; Li et al., 1999; Shepherd et al., 1994). If indeed prolonged administration of neurotrophins is required, gene transfer technology seems to be a promising option given its efficacy in the inner ear. Some viral vectors such as adenovirus provide gene expression lasting for a month or two (Lalwani et al., 1998; Li Duan et al., 2002; Praetorius et al., 2002; Yagi et al., 2000) but pose the potential risks of cytotoxicity and immunogenicity, such that risks of their use in the ear outweighs their benefits. However advancement in viral vectors such as the adeno-associated virus, which has few side effects and induces gene expression lasting many years (Bankiewicz et al., 2006) may increase the clinical feasibility of gene transfer technology.
8. Peripheral nerve fiber re-growth
Neurotrophins are useful not only for SGC body protection but also for inducing the outgrowth of peripheral nerve fibers, as initially shown in culture condition (Avila et al., 1993; Van De Water et al., 1996). Re-growth of peripheral nerve fibers in a deaf ear may provide several benefits. First it may enhance SGC survival, which would be a benefit by itself. Second, peripheral fiber regrowth may improve cochlear implant outcomes if it can be directed into the BMA and the underlying connective tissue and remain there in the long term; this would place neurons closer to the cochlear implant electrodes and enhance the spatial selectivity of stimulation. Third, such regrowth would provide similar enhancement of the functional outcomes of other restorative methods, including such novel techniques as cell replacement by stem cell therapy.
Several groups have investigated whether enhanced re-growth of peripheral nerve fibers could be induced by osmotic pump injection of neurotrophins in guinea pigs with deafened ears (Glueckert et al., 2008; Wise et al., 2005). Injection of neurotrophin proteins into the perilymph resulted in a disorganized fashion of peripheral nerve fiber re-growth, probably because the highest concentration of neurotrophins was in the perilymph at the tip of the cannula. For the re-grown nerve fibers to be meaningful, they need to be distributed preferentially in the BMA, in a tonotopic fashion. Ectopic nerve fibers may be counter-productive and diminish the cochlear implant performance outcome.
We investigated whether it is possible to direct nerve fibers more locally into the BMA of the deaf epithelium via forced expression of transgenic BDNF in cells lining the cochlear fluids. We determined that robust growth of fibers into the deafened epithelium occurred after inoculating with either adenovirus carrying BDNF reporter gene insert (Ad.BDNF) or adeno-associated virus carrying GFP-BDNF reporter gene insert (AAV.GFP-BDNF) (Shibata et al., 2010). In ears treated via scala media or scala tympani with Ad.BDNF, a robust regrowth of nerve fibers was observed in the BMA of the deaf epithelium (Fig. 1D, 1E and 2B). Furthermore, in ears inoculated with AAV.GFP-BDNF, we were able to visualize cells that were transduced and expressed GFP, which served as focal targets for nerve fibers growth. Similarly, Wise et al. have shown localized re-growth of nerve fibers in deafened cochlear tissue using adenovirus with GFP-BDNF or GFP-NTF-3 reporter gene inserts (Wise et al., 2010). Thus, gene transfer technology to force neurotrophin transgene expression in the cells lining the cochlear fluids, allows us to guide re-growth of peripheral nerve fibers into the BMA of the deaf epithelium. Further research is needed on the safety of this method, long term survival of the regenerated fibers, the identification of the type of nerve fibers that grow into the BMA and the outcome on the performance of the cochlear implant. Although many questions remain concerning neural regeneration in the inner ear, its potential clinical applications are promising and exciting, and research is ongoing.
9. Potential role of Schwann cells in inner ear axonal re-growth
Recently, there has been growing interest in the role of Schwann cells in the cochlea (Bohne et al., 1992; Glueckert et al., 2008; Hansen et al., 2001; Morris et al., 2006; Whitlon et al., 2009; Wise et al., 2005). Schwann cells can exist in one of two phenotypes; in the immature state they proliferate, but do not myelinate axons, whereas in the mature state they are myelinating but do not undergo mitosis. Connexin-29 has been identified as a marker expressed exclusively in myelinating Schwann cells, in their cell body and along the myelin sheath surrounding the neural fibers (Eiberger et al., 2006; Tang et al., 2006), and has been harnessed as a tool for investigating the importance of the Schwann cells and myelination. In the Connexin-29 null mouse, Tang and colleagues found delayed hearing development, increased sensitivity to otoacoustic trauma, and prolonged ABR latencies in spite of normal hair cell morphology, attributable to defects in myelination of SGC bodies (Tang et al., 2006). These findings can be explained by the role of Schwann cells in providing myelin layers that increase the speed of propagation of action potentials, as well as their putative role in energy conservation, neurotrophin support, and tonotopic organization via facilitation of path finding.
Schwann cells are also essential to creating an environment favorable to nerve fiber regrowth (Jessen et al., 2005). Recent studies using Schwann cells co-cultured with neurons and neurotrophic factors by Whitlon and colleagues revealed the intimate relationship between neurite growth and Schwann cell proliferation. Growth cones were absent in cultures without Schwann cells, and Schwann cells only appeared to migrate to and around areas of neurite growth, suggesting an important reciprocal relationship between these two phenomena (Whitlon et al., 2009). Schwann cells certainly provide their own neurotrophic factors, but Wise suggested that Schwann cells also bind exogenously delivered neurotrophins, thus providing a neurotrophin-laden pathway for neurite growth (Wise et al., 2005). This role for Schwann cells in regeneration should be considered when designing methods for neurotrophin delivery and peripheral fiber regeneration in the deafened cochlea.
Multiple studies have looked at the ultrastructure of SGCs, peripheral processes and their myelin sheaths after noise-induced and ototoxic damage to the cochlea. In the chinchilla, spontaneous nerve regeneration after noise exposure has been observed, and included re-growth of myelin sheaths, but the pattern and thickness of those myelin sheaths differed from normal (Bohne et al., 1992; Lawner et al., 1997b). In these cases, Schwann cell-like cells were seen extending through the habenula and into the basilar membrane, in association with regenerating nerve fibers. In the guinea pig, it has been shown that after ototoxic damage, there is widespread hair cell loss and subsequent degeneration of SGCs. The remaining neurons have fewer mitochondria, decreased cell circularity, fewer layers of myelin, and resemble a gestational state of Type I SGCs (Agterberg et al., 2008; Dodson et al., 2000). In cases of VII nerve transection, Schwann cells dedifferentiate into their immature phenotype allowing for proliferation and migration along with axons during the process of regeneration (Cheng et al., 2002).
The recent efforts to regenerate SGCs using neurotrophic factors such as BDNF and NT-3 are explained in section 7 above. In one such study by Wise, guinea pigs were deafened with systemic kanamycin and furosemide, and subsequently implanted with a mini-osmotic pump for BDNF delivery. Results at one month suggest BDNF helps preserve SGCs, increasing numbers of Schwann cells and peripheral fibers (Wise et al., 2005). However Agterberg and colleagues used comparable methods as Wise, and examined specimens under the electron microscope, noting altered pattern and reduced thickness of myelin surrounding surviving neurons compared to normal, contributable either to the initial damage, or the transient nature of the neurotrophin delivery (Agterberg et al., 2008). It is likely that improved pattern and thickness of myelination in regenerated nerve fibers would enhance action potential propagation and SGC functioning in ears that receive cochlear implant.
10. Nerve fiber regeneration versus auditory neuropathy
While the ultimate goal of regenerative medicine in the field of neurotology is the restoration of hearing via regeneration of sensory hair cells and auditory nerve fibers in the deafened human cochlea, nerve fiber regeneration has the potential for more immediate use in the clinical setting. One potential application for isolated auditory neuronal fiber regeneration is in the treatment of auditory neuropathy (AN), which is found in approximately 8% of the sensorineural hearing loss population (Madden et al., 2002). AN is a relatively recently recognized clinical entity in which the outer hair cells remain intact and functional until late in the disease process, and the hearing loss is secondary to pathology at the level of the synapse of the inner hair cell and auditory neuronal fiber, or in the auditory nerve itself (Starr et al., 1996; Worthington et al., 1980). Audiologic findings include an absent or abnormal auditory brainstem response (ABR), intact otoacoustic emissions (OAE), variable results on pure tone audiometry and disproportionately poor speech understanding, particularly in noise (Kraus et al., 2000; Starr et al., 1996). This spectrum of findings has been attributed to abnormal temporal processing along the auditory neuronal pathway in these patients (Kraus et al., 2000; Zeng et al., 1999).
Hearing aids frequently are not significantly beneficial to this patient population, and currently the best method of auditory rehabilitation for AN patients is in the form of cochlear implantation (Buss et al., 2002; Madden et al., 2002; Mason et al., 2003; Shallop et al., 2001; Trautwein et al., 2000). However, significant variability in cochlear implant therapy outcomes remains in this population, and this likely is related to inter-patient variability in the exact site of the pathologic lesion (Madden et al., 2002; Mason et al., 2003; Rance et al., 2008; Rance et al., 2009; Teagle et al., 2010; Trautwein et al., 2000). Some data have suggested that a normal appearing electrically evoked ABR may predict improved outcomes following cochlear implantation in this population (Gibson et al., 2007), while cochlear nerve deficiency may predict poorer performance (Teagle et al., 2010; Walton et al., 2008). These findings suggest that restoration of a functional auditory nerve is key to the optimal hearing rehabilitation in auditory neuropathy.
In cases of AN, the sensory hair cells function normally and serve as a prime target for regenerated functional auditory neurons. Animal models for the study of auditory neuropathy include various genetic defects such as mutations inotoferlin, pejvakin, and diaphanous homolog 3 (Delmaghani et al., 2006; Grati et al., 2009; Kim et al., 2004; Romanos et al., 2009; Schoen et al., 2010; Starr et al., 2004; Varga et al., 2003). Animal models have also been created with the use of ouabain, a Na/K ATPase pump toxin that selectively kills Type I afferent neurons and preserves the hair cells (Lang et al., 2005; Lang et al., 2010; Schmiedt et al., 2002). Development of these animal models will allow further research into the role of regenerative technologies in the treatment of this disorder.
11. Conclusions
Means of improving the efficacy of the cochlear prosthesis are being investigated.
Hair cell loss and SGC degeneration can occur in varying levels and rates pertaining to the various insults and species.
Limited spontaneous regeneration of nerve fibers can be seen after insults.
Neurotrophic factors have been demonstrated to induce peripheral nerve fiber re-growth in animal models in parallel with enhanced survival of SGCs.
Neuronal fiber regeneration may bring the target peripheral fibers in closer proximity to the cochlear implant electrode, thereby allowing more specific neural activation at lower thresholds.
Schwann cells may play a potential role in designing methods for nerve fiber regeneration.
Neuronal fiber regeneration may be a potential approach for treatment in isolated cases of AN.
Research emphasizing neuronal re-growth will likely be beneficial for future clinical goals.
Research Highlights.
Nerve fibers regress from the sensory epithelium after hair cell death
Transgene expression of BDNF or NTF-3 in the basilar membrane area rescues spiral ganglion cell bodies and promotes outgrowth of peripheral nerve fiberss
Acknowledgments
We thank Donald Swiderski and Hiu Tung Wong for images and helpful comments. Our work is supported by the A. Alfred Taubman Medical Research Institute, the Berte and Alan Hirschfield Foundation, the R. Jamison and Betty Williams Professorship, and NIH/NIDCD Grants R01-DC01634, R01 DC007634, T32DC005356and P30-DC05188.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrashkin KA, Izumikawa M, Miyazawa T, Wang CH, Crumling MA, Swiderski DL, Beyer LA, Gong TW, Raphael Y. The fate of outer hair cells after acoustic or ototoxic insults. Hear Res. 2006 doi: 10.1016/j.heares.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Agterberg MJ, Versnel H, de Groot JC, van den Broek M, Klis SF. Chronic electrical stimulation does not prevent spiral ganglion cell degeneration in deafened guinea pigs. Hear Res. 2010;269:169–79. doi: 10.1016/j.heares.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Agterberg MJ, Versnel H, de Groot JC, Smoorenburg GF, Albers FW, Klis SF. Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear Res. 2008;244:25–34. doi: 10.1016/j.heares.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Agterberg MJH, Versnel H, van Dijk LM, de Groot JCMJ, Klis SFL. Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs. J Assoc Res Otolaryngol. 2009;10:355–67. doi: 10.1007/s10162-009-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anniko M. Early development and maturation of the spiral ganglion. Acta Otolaryngol (Stockh) 1983;95:263–76. doi: 10.3109/00016488309130943. [DOI] [PubMed] [Google Scholar]
- Avila MA, Varela-Nieto I, Romero G, Mato JM, Giraldez F, Van De Water TR, Represa J. Brain-derived neurotrophic factor and neurotrophin-3 support the survival and neuritogenesis response of developing cochleovestibular ganglion neurons. Dev Biol. 1993;159:266–75. doi: 10.1006/dbio.1993.1239. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, Herscovitch P, Carson RE, Eckelman W, Reutter B, Cunningham J. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–70. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Bellamkonda RV. Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy. Biomaterials. 2006;27:3515–8. doi: 10.1016/j.biomaterials.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Bichler E, Spoendlin H, Rauchegger H. Degeneration of cochlear neurons after amikacin intoxication in the rat. Arch Otorhinolaryngol. 1983;237:201–8. doi: 10.1007/BF00453725. [DOI] [PubMed] [Google Scholar]
- Black RC, Clark GM, Patrick JF. Current distribution measurements within the human cochlea. IEEE Trans Biomed Eng. 1981;28:721–5. doi: 10.1109/TBME.1981.324668. [DOI] [PubMed] [Google Scholar]
- Black RC, Clark GM, Tong YC, Patrick JF. Current distributions in cochlear stimulation. Ann N Y Acad Sci. 1983;405:137–45. doi: 10.1111/j.1749-6632.1983.tb31626.x. [DOI] [PubMed] [Google Scholar]
- Bledsoe SC, Jr, Nagase S, Miller JM, Altschuler RA. Deafness-induced plasticity in the mature central auditory system. Neuroreport. 1995;7:225–9. [PubMed] [Google Scholar]
- Bohne BA, Harding GW. Neural regeneration in the noise-damaged chinchilla cochlea. Laryngoscope. 1992;102:693–703. doi: 10.1288/00005537-199206000-00017. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Harding GW. Degeneration in the cochlea after noise damage: primary versus secondary events. Am J Otol. 2000;21:505–9. [PubMed] [Google Scholar]
- Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Skene JH. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci. 2001;4:38–43. doi: 10.1038/82881. [DOI] [PubMed] [Google Scholar]
- Borg E, Engstrom B. Damage to sensory hairs of inner hair cells after exposure to noise in rabbits without outer hair cells. Hear Res. 1983;11:1–6. doi: 10.1016/0378-5955(83)90040-0. [DOI] [PubMed] [Google Scholar]
- Bowers WJ, Chen X, Guo H, Frisina DR, Federoff HJ, Frisina RD. Neurotrophin-3 transduction attenuates Cisplatin spiral ganglion neuron ototoxicity in the cochlea. Mol Ther. 2002;6:12–8. doi: 10.1006/mthe.2002.0627. [DOI] [PubMed] [Google Scholar]
- Briaire JJ, Frijns JHM. The consequences of neural degeneration regarding optimal cochlear implant position in scala tympani: a model approach. Hear Res. 2006;214:17–27. doi: 10.1016/j.heares.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Gerber J, Kessens P, Chen YG, Royall RM. Contributions of pathway and neuron to preferential motor reinnervation. J Neurosci. 1998;18:8674–81. doi: 10.1523/JNEUROSCI.18-21-08674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss E, Labadie RF, Brown CJ, Gross AJ, Grose JH, Pillsbury HC. Outcome of cochlear implantation in pediatric auditory neuropathy. Otol Neurotol. 2002;23:328–32. doi: 10.1097/00129492-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988a;106:1281–8. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988b;1:85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- Chen I, Limb CJ, Ryugo DK. The effect of cochlear-implant-mediated electrical stimulation on spiral ganglion cells in congenitally deaf white cats. J Assoc Res Otolaryngol. 2010;11:587–603. doi: 10.1007/s10162-010-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Zochodne DW. In vivo proliferation, migration and phenotypic changes of Schwann cells in the presence of myelinated fibers. Neuroscience. 2002;115:321–9. doi: 10.1016/s0306-4522(02)00291-9. [DOI] [PubMed] [Google Scholar]
- Chikar JA, Colesa DJ, Swiderski DL, Polo AD, Raphael Y, Pfingst BE. Over-expression of BDNF by adenovirus with concurrent electrical stimulation improves cochlear implant thresholds and survival of auditory neurons. Hear Res. 2008;245:24–34. doi: 10.1016/j.heares.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LT, Richardson LM, Saunders E, Cowan RSC. Spatial spread of neural excitation in cochlear implant recipients: comparison of improved ECAP method and psychophysical forward masking. Hear Res. 2003;179:72–87. doi: 10.1016/s0378-5955(03)00096-0. [DOI] [PubMed] [Google Scholar]
- Cohen LT, Saunders E, Knight MR, Cowan RSC. Psychophysical measures in patients fitted with Contour and straight Nucleus electrode arrays. Hear Res. 2006;212:160–75. doi: 10.1016/j.heares.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Cohen LT, Lenarz T, Battmer RD, Bender von Saebelkampf C, Busby PA, Cowan RSC. A psychophysical forward masking comparison of longitudinal spread of neural excitation in the Contour and straight Nucleus electrode arrays. Int J Audiol. 2005;44:559–66. doi: 10.1080/14992020500258743. [DOI] [PubMed] [Google Scholar]
- Delmaghani S, del Castillo FJ, Michel V, Leibovici M, Aghaie A, Ron U, Van Laer L, Ben-Tal N, Van Camp G, Weil D, Langa F, Lathrop M, Avan P, Petit C. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat Genet. 2006;38:770–8. doi: 10.1038/ng1829. [DOI] [PubMed] [Google Scholar]
- Despres G, Leger GP, Dahl D, Romand R. Distribution of cytoskeletal proteins (neurofilaments, peripherin and MAP-tau) in the cochlea of the human fetus. Acta Otolaryngol. 1994;114:377–81. doi: 10.3109/00016489409126073. [DOI] [PubMed] [Google Scholar]
- Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998;95:3978–83. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson HC. Loss and survival of spiral ganglion neurons in the guinea pig after intracochlear perfusion with aminoglycosides. J Neurocytol. 1997;26:541–56. doi: 10.1023/a:1015434524040. [DOI] [PubMed] [Google Scholar]
- Dodson HC, Mohuiddin A. Response of spiral ganglion neurones to cochlear hair cell destruction in the guinea pig. J Neurocytol. 2000;29:525–37. doi: 10.1023/a:1007201913730. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Loizou PC, Fitzke J, Tu Z. The recognition of sentences in noise by normal-hearing listeners using simulations of cochlear-implant signal processors with 6–20 channels. J Acoust Soc Am. 1998;104:3583–5. doi: 10.1121/1.423940. [DOI] [PubMed] [Google Scholar]
- Eiberger J, Kibschull M, Strenzke N, Schober A, Bussow H, Wessig C, Djahed S, Reucher H, Koch DA, Lautermann J, Moser T, Winterhager E, Willecke K. Expression pattern and functional characterization of connexin29 in transgenic mice. Glia. 2006;53:601–11. doi: 10.1002/glia.20315. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Kucera J, Lee KF, Loring J, Jaenisch R. Studies on the physiological role of brain-derived neurotrophic factor and neurotrophin-3 in knockout mice. Int J Dev Biol. 1995;39:799–807. [PubMed] [Google Scholar]
- Fitzgerald MB, Shapiro WH, McDonald PD, Neuburger HS, Ashburn-Reed S, Immerman S, Jethanamest D, Roland JT, Svirsky MA. The effect of perimodiolar placement on speech perception and frequency discrimination by cochlear implant users. Acta Otolaryngol. 2007;127:378–83. doi: 10.1080/00016480701258671. [DOI] [PubMed] [Google Scholar]
- Forge A. Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hear Res. 1985;19:171–82. doi: 10.1016/0378-5955(85)90121-2. [DOI] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110:1150–63. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Frijns JH, Briaire JJ, Grote JJ. The importance of human cochlear anatomy for the results of modiolus-hugging multichannel cochlear implants. Otol Neurotol. 2001;22:340–9. doi: 10.1097/00129492-200105000-00012. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago II, Bianchi LM, Farinas II. Effects of neurotrophin and neurotrophin receptor disruption on the afferent inner ear innervation. Semin Cell Dev Biol. 1997;8:277–284. [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–78. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Gibson WPR, Sanli H. Auditory neuropathy: an update. Ear Hear. 2007;28:102S–106S. doi: 10.1097/AUD.0b013e3180315392. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects. J Neurosci Res. 2003;71:785–90. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- Glueckert R, Bitsche M, Miller JM, Zhu Y, Prieskorn DM, Altschuler RA, Schrott-Fischer A. Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. J Comp Neurol. 2008;507:1602–21. doi: 10.1002/cne.21619. [DOI] [PubMed] [Google Scholar]
- Grati FR, Lesperance MM, De Toffol S, Chinetti S, Selicorni A, Emery S, Grimi B, Dulcetti F, Malvestiti B, Taylor J, Milani S, Ruggeri AM, Maggi F, Simoni G. Pure monosomy and pure trisomy of 13q21.2–31.1 consequent to a familial insertional translocation: exclusion of PCDH9 as the responsible gene for autosomal dominant auditory neuropathy (AUNA1) Am J Med Genet A. 2009;149A:906–13. doi: 10.1002/ajmg.a.32754. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Vijapurkar U, Koland JG, Green SH. Reciprocal signaling between spiral ganglion neurons and Schwann cells involves neuregulin and neurotrophins. Hear Res. 2001;161:87–98. doi: 10.1016/s0378-5955(01)00360-4. [DOI] [PubMed] [Google Scholar]
- Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128:147–65. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Hartshorn DO, Miller JM, Altschuler RA. Protective effect of electrical stimulation in the deafened guinea pig cochlea. Otolaryngol Head Neck Surg. 1991;104:311–9. doi: 10.1177/019459989110400305. [DOI] [PubMed] [Google Scholar]
- Hawkins JE., Jr Comparative otopathology: aging, noise, and ototoxic drugs. Adv Otorhinolaryngol. 1973;20:125–41. doi: 10.1159/000393093. [DOI] [PubMed] [Google Scholar]
- Hildebrand MS, Newton SS, Gubbels SP, Sheffield AM, Kochhar A, de Silva MG, Dahl HH, Rose SD, Behlke MA, Smith RJ. Advances in molecular and cellular therapies for hearing loss. Mol Ther. 2008;16:224–36. doi: 10.1038/sj.mt.6300351. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Abbas PJ. Electrophysiologic channel interaction, electrode pitch ranking, and behavioral threshold in straight versus perimodiolar cochlear implant electrode arrays. J Acoust Soc Am. 2006;119:1538–47. doi: 10.1121/1.2164969. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008;240:52–6. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–82. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Johnsson LG. Sequence of degeneration of Corti’s organ and its first-order neurons. Ann Otol Rhinol Laryngol. 1974;83:294–303. doi: 10.1177/000348947408300303. [DOI] [PubMed] [Google Scholar]
- Johnsson LG, Hawkins JE. Strial atrophy in clinical and experimental deafness. The Laryngoscope. 1972;82:1105–25. doi: 10.1288/00005537-197207000-00002. [DOI] [PubMed] [Google Scholar]
- Kang SY, Colesa DJ, Swiderski DL, Su GL, Raphael Y, Pfingst BE. Effects of hearing preservation on psychophysical responses to cochlear implant stimulation. J Assoc Res Otolaryngol. 2010;11:245–65. doi: 10.1007/s10162-009-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454:350–60. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- Kiang NY, Rho JM, Northrop CC, Liberman MC, Ryugo DK. Hair-cell innervation by spiral ganglion cells in adult cats. Science. 1982;217:175–7. doi: 10.1126/science.7089553. [DOI] [PubMed] [Google Scholar]
- Kim TB, Isaacson B, Sivakumaran TA, Starr A, Keats BJB, Lesperance MM. A gene responsible for autosomal dominant auditory neuropathy (AUNA1) maps to 13q14–21. J Med Genet. 2004;41:872–6. doi: 10.1136/jmg.2004.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Raphael Y. Cell division and maintenance of epithelial integrity in the deafened auditory epithelium. Cell Cycle. 2007;6:612–9. doi: 10.4161/cc.6.5.3929. [DOI] [PubMed] [Google Scholar]
- Kong YY, Cruz R, Jones JA, Zeng FG. Music perception with temporal cues in acoustic and electric hearing. Ear Hear. 2004;25:173–85. doi: 10.1097/01.aud.0000120365.97792.2f. [DOI] [PubMed] [Google Scholar]
- Kraus N, Bradlow AR, Cheatham MA, Cunningham J, King CD, Koch DB, Nicol TG, Mcgee TJ, Stein LK, Wright BA. Consequences of neural asynchrony: a case of auditory neuropathy. J Assoc Res Otolaryngol. 2000;1:33–45. doi: 10.1007/s101620010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalwani A, Walsh B, Reilly P, Carvalho G, Zolotukhin S, Muzyczka N, Mhatre A. Long-term in vivo cochlear transgene expression mediated by recombinant adeno-associated virus. Gene Ther. 1998;5:277–81. doi: 10.1038/sj.gt.3300573. [DOI] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Schmiedt RA. Ouabain induces apoptotic cell death in type I spiral ganglion neurons, but not type II neurons. J Assoc Res Otolaryngol. 2005;6:63–74. doi: 10.1007/s10162-004-5021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Li M, Kilpatrick LA, Zhu J, Samuvel DJ, Krug EL, Goddard JC. Sox2 Up-regulation and Glial Cell Proliferation Following Degeneration of Spiral Ganglion Neurons in the Adult Mouse Inner Ear. JARO. 2010:1–21. doi: 10.1007/s10162-010-0244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawner BE, Harding GW, Bohne BA. Time course of nerve-fiber regeneration in the noise-damaged mammalian cochlea. Int J Dev Neurosci. 1997a;15:601–17. doi: 10.1016/s0736-5748(96)00115-3. [DOI] [PubMed] [Google Scholar]
- Lawner BE, Harding GW, Bohne BA. Time course of nerve-fiber regeneration in the noise-damaged mammalian cochlea. Int J Dev Neurosci. 1997b;15:601–17. doi: 10.1016/s0736-5748(96)00115-3. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33:11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Li Duan M, Bordet T, Mezzina M, Kahn A, Ulfendahl M. Adenoviral and adeno-associated viral vector mediated gene transfer in the guinea pig cochlea. Neuroreport. 2002;13:1295–9. doi: 10.1097/00001756-200207190-00016. [DOI] [PubMed] [Google Scholar]
- Li L, Parkins CW, Webster DB. Does electrical stimulation of deaf cochleae prevent spiral ganglion degeneration? Hear. Res. 1999;133:27–39. doi: 10.1016/s0378-5955(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Efferent synapses in the inner hair cell area of the cat cochlea: an electron microscopic study of serial sections. Hear Res. 1980;3:189–204. doi: 10.1016/0378-5955(80)90046-5. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res. 1986;24:17–36. doi: 10.1016/0378-5955(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J Comp Neurol. 1990;301:443–60. doi: 10.1002/cne.903010309. [DOI] [PubMed] [Google Scholar]
- Madden C, Hilbert L, Rutter M, Greinwald J, Choo D. Pediatric cochlear implantation in auditory neuropathy. Otol Neurotol. 2002;23:163–8. doi: 10.1097/00129492-200203000-00011. [DOI] [PubMed] [Google Scholar]
- Mason JC, De Michele A, Stevens C, Ruth RA, Hashisaki GT. Cochlear implantation in patients with auditory neuropathy of varied etiologies. The Laryngoscope. 2003;113:45–9. doi: 10.1097/00005537-200301000-00009. [DOI] [PubMed] [Google Scholar]
- Matsuoka AJ, Kondo T, Miyamoto RT, Hashino E. Enhanced survival of bone-marrow-derived pluripotent stem cells in an animal model of auditory neuropathy. Laryngoscope. 2007;117:1629–35. doi: 10.1097/MLG.0b013e31806bf282. [DOI] [PubMed] [Google Scholar]
- Medd AM, Bianchi LM. Analysis of BDNF production in the aging gerbil cochlea. Exp Neurol. 2000;162:390–3. doi: 10.1006/exnr.2000.7353. [DOI] [PubMed] [Google Scholar]
- Miller AL. Effects of chronic stimulation on auditory nerve survival in ototoxically deafened animals. Hear Res. 2001;151:1–14. doi: 10.1016/s0378-5955(00)00226-4. [DOI] [PubMed] [Google Scholar]
- Miller JM, Altschuler RA. Effectiveness of different electrical stimulation conditions in preservation of spiral ganglion cells following deafness. Ann Otol Rhinol Laryngol Suppl. 1995;166:57–60. [PubMed] [Google Scholar]
- Miller JM, Chi DH, O’Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–43. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Miller JM, Finger PA, Heller JW, Raphael Y, Altschuler RA. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear Res. 1997;105:30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- Morris JK, Maklad A, Hansen LA, Feng F, Sorensen C, Lee KF, Macklin WB, Fritzsch B. A disorganized innervation of the inner ear persists in the absence of ErbB2. Brain Res. 2006;1091:186–99. doi: 10.1016/j.brainres.2006.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB., Jr Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117:220–8. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Eddington DK. Histopathology of the inner ear relevant to cochlear implantation. Adv Otorhinolaryngol. 2006;64:31–49. doi: 10.1159/000094643. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–6. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- Nakaizumi T, Kawamoto K, Minoda R, Raphael Y. Adenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damage. Audiol Neurootol. 2004;9:135–43. doi: 10.1159/000077264. [DOI] [PubMed] [Google Scholar]
- O’Leary SJ, Richardson RR, McDermott HJ. Principles of design and biological approaches for improving the selectivity of cochlear implant electrodes. J Neural Eng. 2009;6:055002. doi: 10.1088/1741-2560/6/5/055002. [DOI] [PubMed] [Google Scholar]
- Pawlowski KS, Kikkawa YS, Wright CG, Alagramam KN. Progression of inner ear pathology in Ames waltzer mice and the role of protocadherin 15 in hair cell development. J Assoc Res Otolaryngol. 2006;7:83–94. doi: 10.1007/s10162-005-0024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Sutton D, Miller JM, Bohne BA. Relation of psychophysical data to histopathology in monkeys with cochlear implants. Acta Otolaryngol (Stockh) 1981;92:1–13. doi: 10.3109/00016488109133232. [DOI] [PubMed] [Google Scholar]
- Praetorius M, Knipper M, Schick B, Tan J, Limberger A, Carnicero E, Alonso MT, Schimmang T. A novel vestibular approach for gene transfer into the inner ear. Audiol Neurootol. 2002;7:324–34. doi: 10.1159/000066157. [DOI] [PubMed] [Google Scholar]
- Rance G, Barker EJ. Speech perception in children with auditory neuropathy/dyssynchrony managed with either hearing AIDS or cochlear implants. Otol Neurotol. 2008;29:179–82. doi: 10.1097/mao.0b013e31815e92fd. [DOI] [PubMed] [Google Scholar]
- Rance G, Barker EJ. Speech and language outcomes in children with auditory neuropathy/dys-synchrony managed with either cochlear implants or hearing aids. Int J Audiol. 2009;48:313–320. doi: 10.1080/14992020802665959. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Scar formation after drug-induced cochlear insult. Hear Res. 1991;51:173–83. doi: 10.1016/0378-5955(91)90034-7. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Structure and innervation of the cochlea. Brain Res Bull. 2003;60:397–422. doi: 10.1016/s0361-9230(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Rubel EW. Cell division in the gerbil cochlea after acoustic trauma. Am J Otol. 1994;15:28–34. [PubMed] [Google Scholar]
- Romand MR, Romand R. Development of spiral ganglion cells in mammalian cochlea. J Electron Microsc Tech. 1990;15:144–54. doi: 10.1002/jemt.1060150206. [DOI] [PubMed] [Google Scholar]
- Romand R, Romand MR. Myelination kinetics of spiral ganglion cells in kitten. J Comp Neurol. 1982;204:1–5. doi: 10.1002/cne.902040102. [DOI] [PubMed] [Google Scholar]
- Romanos J, Kimura L, Fávero ML, Izarra FAR, de Mello Auricchio MTB, Batissoco AC, Lezirovitz K, Abreu-Silva RS, Mingroni-Netto RC. Novel OTOF mutations in Brazilian patients with auditory neuropathy. J Hum Genet. 2009;54:382–5. doi: 10.1038/jhg.2009.45. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Rosenbaum BT, Kim PJ, Niparko JK, Saada AA. Single unit recordings in the auditory nerve of congenitally deaf white cats: morphological correlates in the cochlea and cochlear nucleus. J Comp Neurol. 1998;397:532–48. doi: 10.1002/(sici)1096-9861(19980810)397:4<532::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Savio T, Schwab ME. Rat CNS white matter, but not gray matter, is nonpermissive for neuronal cell adhesion and fiber outgrowth. J Neurosci. 1989;9:1126–33. doi: 10.1523/JNEUROSCI.09-04-01126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Okamura HO, Lang H, Schulte BA. Ouabain application to the round window of the gerbil cochlea: a model of auditory neuropathy and apoptosis. J Assoc Res Otolaryngol. 2002;3:223–33. doi: 10.1007/s1016200220017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–3. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Schoen CJ, Emery SB, Thorne MC, Ammana HR, Sliwerska E, Arnett J, Hortsch M, Hannan F, Burmeister M, Lesperance MM. Increased activity of Diaphanous homolog 3 (DIAPH3)/diaphanous causes hearing defects in humans with auditory neuropathy and in Drosophila. Proc Natl Acad Sci U S A. 2010;107:13396–401. doi: 10.1073/pnas.1003027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Monnier PP, Schluesener HJ, Conrad S, Beschorner R, Chen L, Meyermann R, Mueller BK. Central nervous system injury-induced repulsive guidance molecule expression in the adult human brain. Arch Neurol. 2005;62:1561–8. doi: 10.1001/archneur.62.10.1561. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci. 1985;5:2415–23. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallop JK, Peterson A, Facer GW, Fabry LB, Driscoll CL. Cochlear implants in five cases of auditory neuropathy: postoperative findings and progress. The Laryngoscope. 2001;111:555–62. doi: 10.1097/00005537-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res. 1997;108:112–44. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res. 2008;242:100–9. doi: 10.1016/j.heares.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Matsushima J, Martin RL, Clark GM. Cochlear pathology following chronic electrical stimulation of the auditory nerve: II. Deafened kittens Hear Res. 1994;81:150–66. doi: 10.1016/0378-5955(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Shibata SB, Cortez SR, Beyer LA, Wiler JA, Di Polo A, Pfingst BE, Raphael Y. Transgenic BDNF induces nerve fiber regrowth into the auditory epithelium in deaf cochleae. Exp Neurol. 2010;223:464–72. doi: 10.1016/j.expneurol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata SB, Osumi Y, Yagi M, Kanda S, Kawamoto K, Kuriyama H, Nishiyama T, Yamashita T. Administration of amitriptyline attenuates noise-induced hearing loss via glial cell line-derived neurotrophic factor (GDNF) induction. Brain Res. 2007;1144:74–81. doi: 10.1016/j.brainres.2007.01.090. [DOI] [PubMed] [Google Scholar]
- Smith CA, Rasmussen GL. Recent observations on the olivo-cochlear bundle. Ann Otol Rhinol Laryngol. 1963;72:489–506. doi: 10.1177/000348946307200218. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Middlebrooks JC, Bonham BH. Cochlear implant electrode configuration effects on activation threshold and tonotopic selectivity. Hear Res. 2008;235:23–38. doi: 10.1016/j.heares.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soileau LC, Silberstein L, Blau HM, Thompson WJ. Reinnervation of muscle fiber types in the newborn rat soleus. J Neurosci. 1987;7:4176–94. doi: 10.1523/JNEUROSCI.07-12-04176.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H. The organization of the cochlear receptor. Fortschr Hals Nasen Ohrenheilkd. 1966;13:1–227. [PubMed] [Google Scholar]
- Spoendlin H. Innervation patterns in the organ of corti of the cat. Acta Otolaryngol (Stockh) 1969;67:239–54. doi: 10.3109/00016486909125448. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Retrograde degeneration of the cochlear nerve. Acta Otolaryngol. 1975;79:266–75. doi: 10.3109/00016487509124683. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Neural connections of the outer haircell system. Acta Otolaryngol (Stockh) 1979;87:381–7. doi: 10.3109/00016487909126437. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Differentiation of cochlear afferent neurons. Acta Otolaryngol (Stockh) 1981;91:451–6. doi: 10.3109/00016488109138527. [DOI] [PubMed] [Google Scholar]
- Spoendlin H, Suter R. Regeneration in the VIII nerve. Acta Otolaryngol (Stockh) 1976;81:228–36. doi: 10.3109/00016487609119954. [DOI] [PubMed] [Google Scholar]
- Staecker H, Garnham C. Neurotrophin therapy and cochlear implantation: Translating animal models to human therapy. Experimental Neurology. 2010 doi: 10.1016/j.expneurol.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Staecker H, Gabaizadeh R, Federoff H, Van De Water TR. Brain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell loss. Otolaryngol Head Neck Surg. 1998;119:7–13. doi: 10.1016/S0194-5998(98)70194-9. [DOI] [PubMed] [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889–94. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119 (Pt 3):741–53. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Starr A, Isaacson B, Michalewski HJ, Zeng FG, Kong YY, Beale P, Paulson GW, Keats BJB, Lesperance MM. A dominantly inherited progressive deafness affecting distal auditory nerve and hair cells. J Assoc Res Otolaryngol. 2004;5:411–26. doi: 10.1007/s10162-004-5014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger RN, Bohne BA, Harding GW. Regenerated nerve fibers in the noise-damaged chinchilla cochlea are not efferent. Hear Res. 1995;92:52–62. doi: 10.1016/0378-5955(95)00196-4. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6:136–47. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zhang Y, Chang Q, Ahmad S, Dahlke I, Yi H, Chen P, Paul DL, Lin X. Connexin29 is highly expressed in cochlear Schwann cells, and it is required for the normal development and function of the auditory nerve of mice. J Neurosci. 2006;26:1991–9. doi: 10.1523/JNEUROSCI.5055-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teagle HFB, Roush PA, Woodard JS, Hatch DR, Zdanski CJ, Buss E, Buchman CA. Cochlear implantation in children with auditory neuropathy spectrum disorder. Ear Hear. 2010;31:325–35. doi: 10.1097/AUD.0b013e3181ce693b. [DOI] [PubMed] [Google Scholar]
- Terayama Y, Kaneko Y, Kawamoto K, Sakai N. Ultrastructural changes of the nerve elements following disruption of the organ of Corti. I. Nerve elements in the organ of Corti. Acta Otolaryngol. 1977;83:291–302. doi: 10.3109/00016487709128848. [DOI] [PubMed] [Google Scholar]
- Terayama Y, Kaneko K, Tanaka K, Kawamoto K. Ultrastructural changes of the nerve elements following disruption of the organ of Corti. II. Nerve elements outside the organ of Corti. Acta Otolaryngol. 1979;88:27–36. doi: 10.3109/00016487909137136. [DOI] [PubMed] [Google Scholar]
- Torvik A, Soreide AJ. The perineuronal glial reaction after axotomy. Brain Res. 1975;95:519–29. doi: 10.1016/0006-8993(75)90125-0. [DOI] [PubMed] [Google Scholar]
- Trautwein PG, Sininger YS, Nelson R. Cochlear implantation of auditory neuropathy. J Am Acad Audiol. 2000;11:309–15. [PubMed] [Google Scholar]
- Tuszynski MH, Thal L, Pay M, Salmon DP, UHS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–5. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- Van De Water TR, Staecker H, Ernfors P, Moonen G, Lefebvre PP. Neurotrophic factors as pharmacological agents for the treatment of injured auditory neurons. [Review] [38 refs] Ciba Found Symp. 1996;196:149–62. doi: 10.1002/9780470514863.ch11. discussion 162–6. [DOI] [PubMed] [Google Scholar]
- Varga R, Kelley PM, Keats BJ, Starr A, Leal SM, Cohn E, Kimberling WJ. Non-syndromic recessive auditory neuropathy is the result of mutations in the otoferlin (OTOF) gene. J Med Genet. 2003;40:45–50. doi: 10.1136/jmg.40.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J, Gibson WPR, Sanli H, Prelog K. Predicting cochlear implant outcomes in children with auditory neuropathy. Otol Neurotol. 2008;29:302–9. doi: 10.1097/MAO.0b013e318164d0f6. [DOI] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Carboplatin-induced early cochlear lesion in chinchillas. Hear Res. 2003;181:65–72. doi: 10.1016/s0378-5955(03)00176-x. [DOI] [PubMed] [Google Scholar]
- Warr WB, Guinan JJ., Jr Efferent innervation of the organ of corti: two separate systems. Brain Res. 1979;173:152–5. doi: 10.1016/0006-8993(79)91104-1. [DOI] [PubMed] [Google Scholar]
- Webster DB, Webster M. Multipolar spiral ganglion neurons following organ of Corti loss. Brain Res. 1982;244:356–9. doi: 10.1016/0006-8993(82)90097-x. [DOI] [PubMed] [Google Scholar]
- Webster M, Webster DB. Spiral ganglion neuron loss following organ of Corti loss: a quantitative study. Brain Res. 1981;212:17–30. doi: 10.1016/0006-8993(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Tieu D, Grover M, Reilly B, Coulson MT. Spontaneous association of glial cells with regrowing neurites in mixed cultures of dissociated spiral ganglia. Neuroscience. 2009;161:227–35. doi: 10.1016/j.neuroscience.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O’Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–65. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE, O’Leary SJ, Shepherd RK, Richardson RT. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther. 2010;18:1111–22. doi: 10.1038/mt.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington DW, Peters JF. Quantifiable hearing and no ABR: paradox or error? Ear Hear. 1980;1:281–5. doi: 10.1097/00003446-198009000-00009. [DOI] [PubMed] [Google Scholar]
- Xu L, Pfingst BE. Relative importance of temporal envelope and fine structure in lexical-tone perception. J Acoust Soc Am. 2003;114:3024–7. doi: 10.1121/1.1623786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M, Lee J, Magal E, Sheng Z, Raphael Y. Rescue of hair cells from aminoglycoside ototoxicity by adenoviral-mediated overexpression of GDNF. Midwinter Meeting of the Association for Research in Otolaryngology; St. Petersburg, FL. 1999. [Google Scholar]
- Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, Sheng J, Raphael Y. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000;1:315–25. doi: 10.1007/s101620010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Nannapaneni N, Zhou J, Hughes LF, Shore S. Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus. J Neurosci. 2009;29:4210–7. doi: 10.1523/JNEUROSCI.0208-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG, Oba S, Garde S, Sininger Y, Starr A. Temporal and speech processing deficits in auditory neuropathy. Neuroreport. 1999;10:3429–35. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]